Bayesian Maximum-A-Posteriori Approach with Global and Local Regularization to Image Reconstruction Problem in Medical Emission Tomography
Abstract
:1. Introduction
2. Theory
2.1. Bayesian Approach for Solving Image Reconstruction Problems in Nuclear Medicine
2.2. Maximum-Likelihood-Based Image Reconstruction Method (ML)
2.3. Bayesian Image Reconstruction Method MAP-GIBBS
2.3.1. Gibbs Prior Based on Image Properties
2.3.2. Gibbs a Priori Probability Based on a Closed System Model
2.4. Bayesian Image Reconstruction Method MAP-ENT
2.4.1. Entropy a Priori Probability Based on Image Properties
2.4.2. Entropy Prior Based on the Boltzmann Isolated System Model
2.4.3. Entropy Prior Based on Open System Model
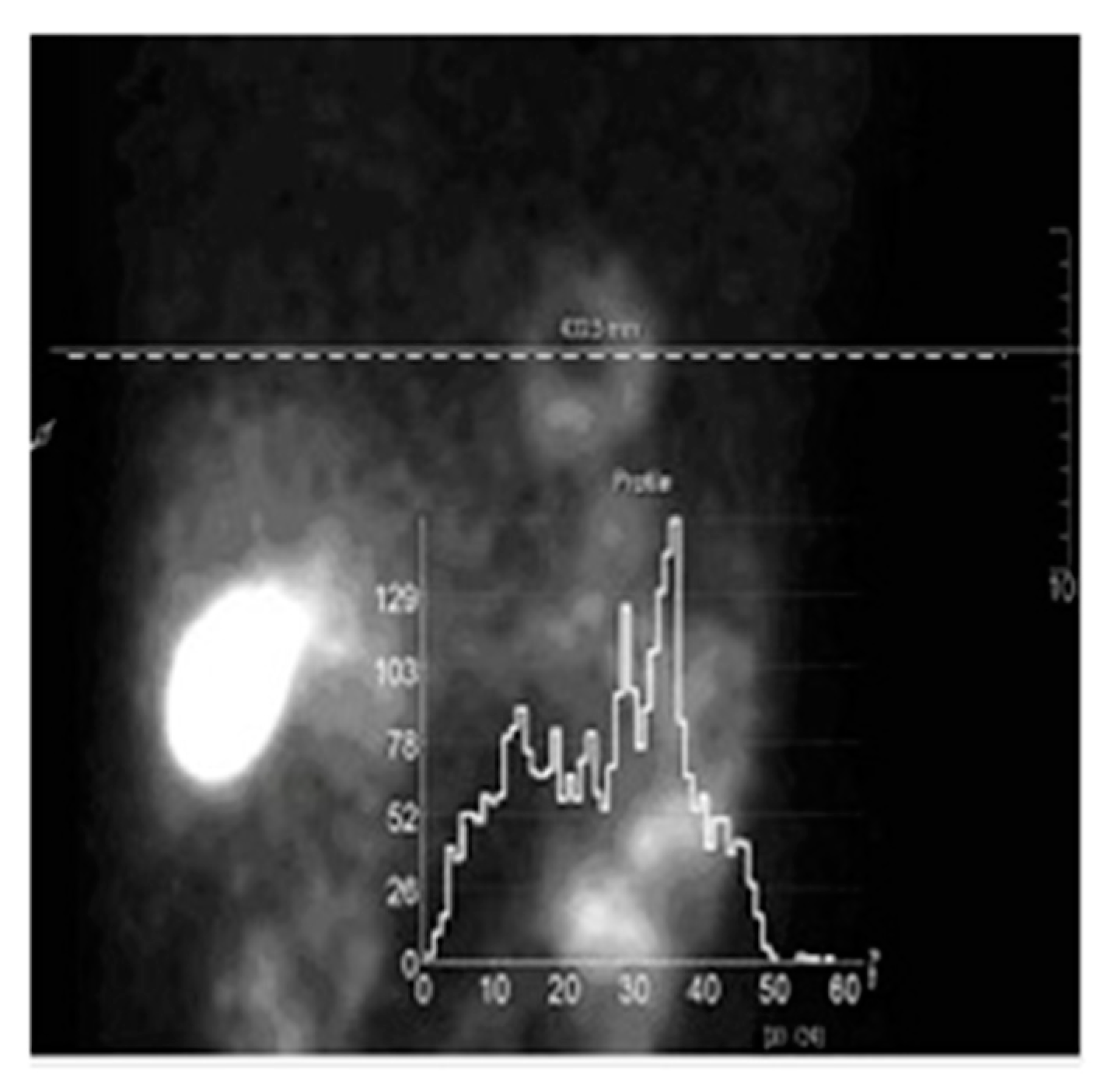
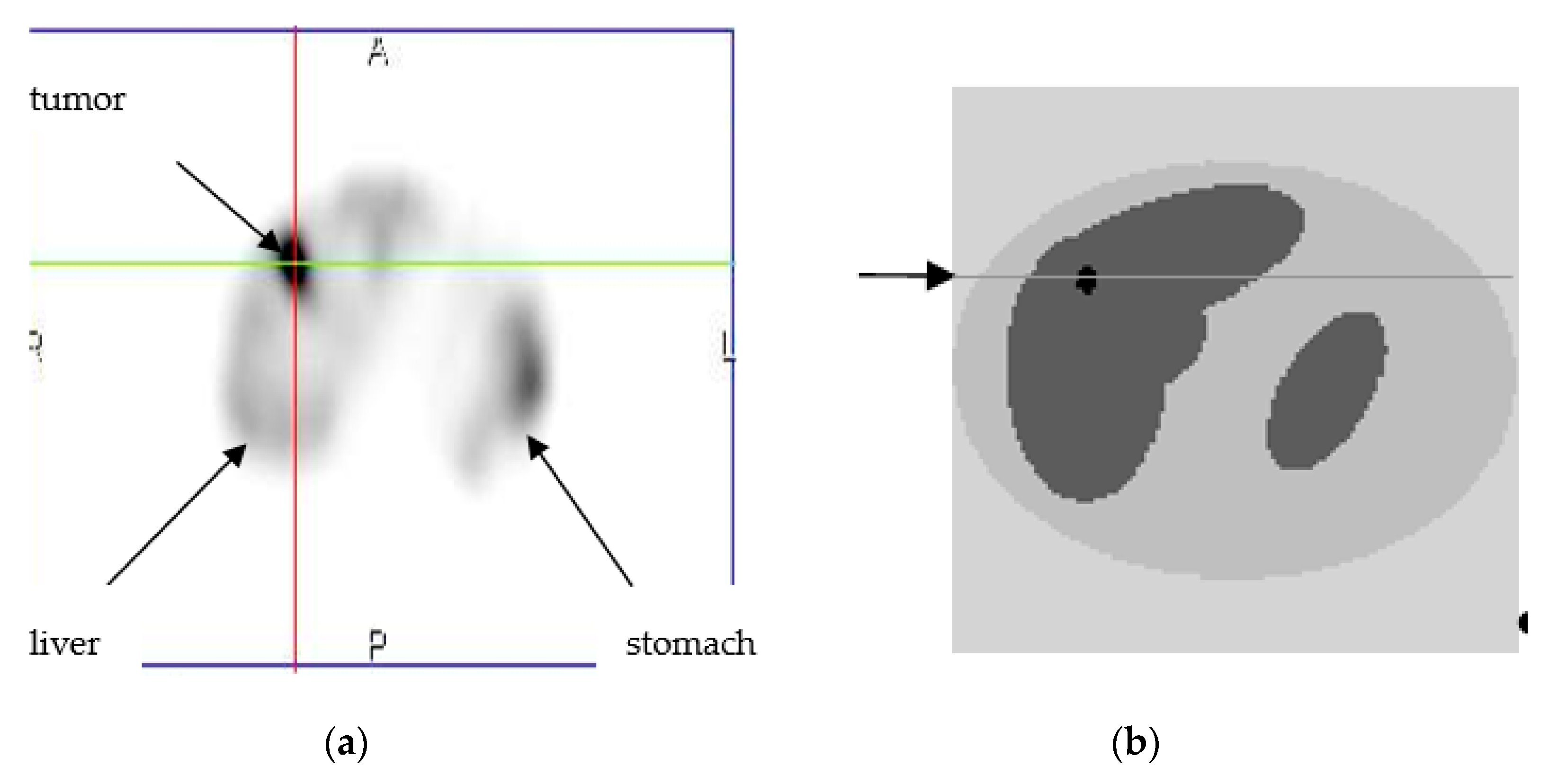
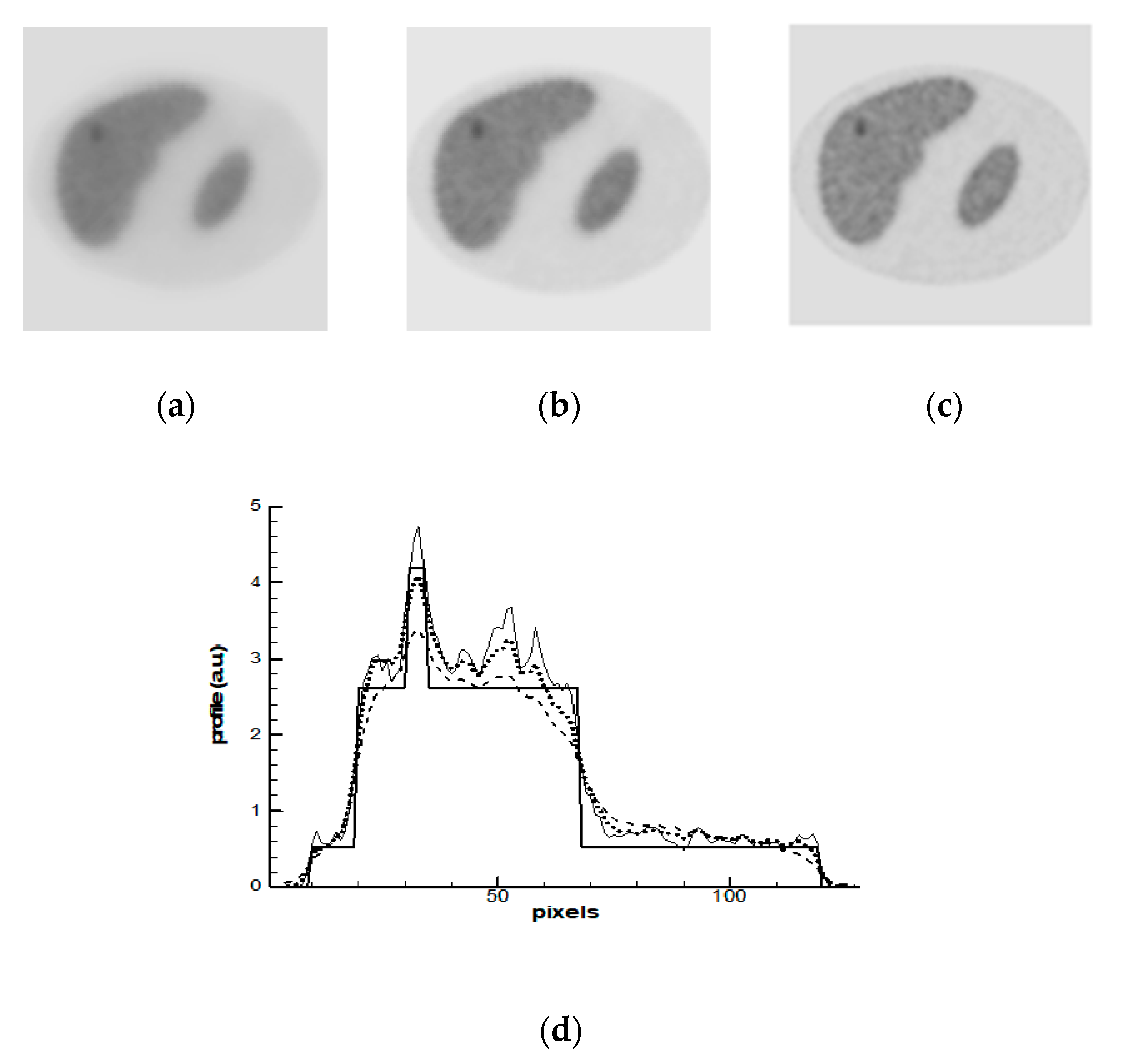
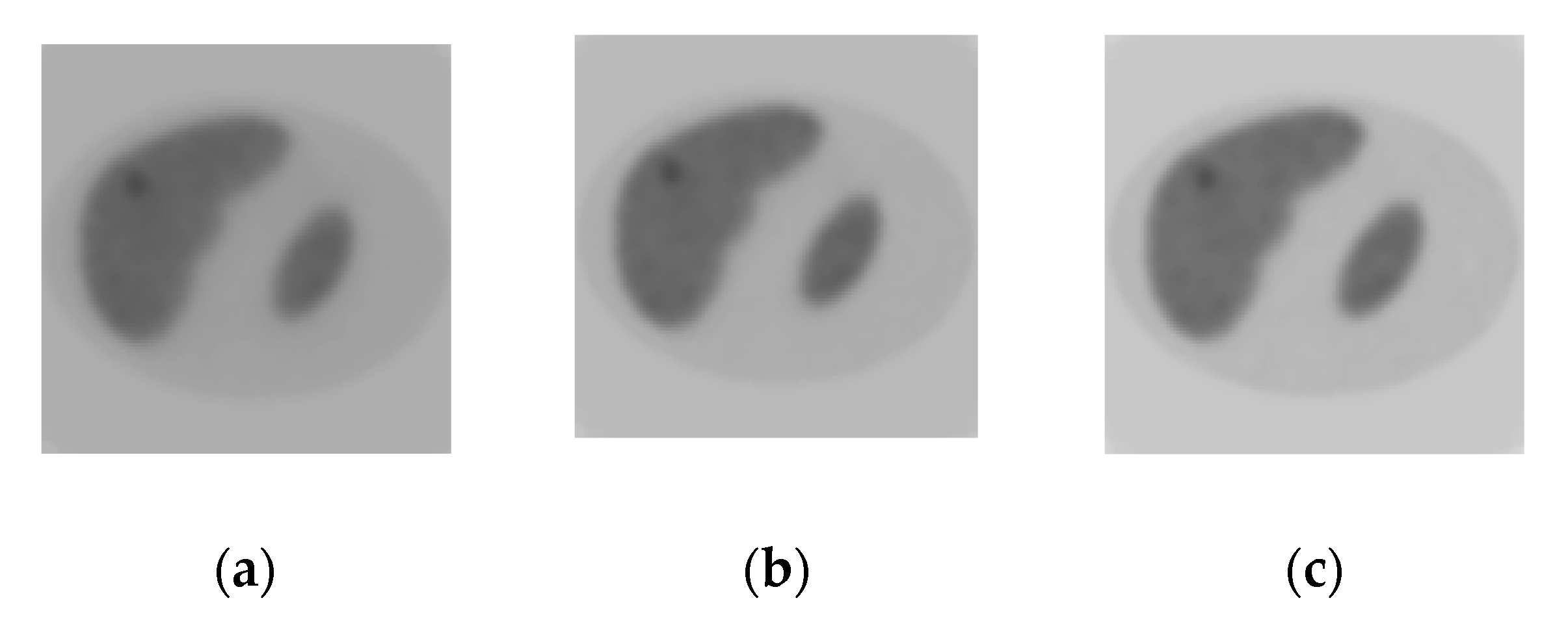
3. Numerical Simulations
- OSEM—the standard non-regularized algorithm (9) applied on the most SPECT and PET systems;
- MAP-GIBBS—the Bayesian algorithm Maximum a Posteriori (MAP) with prior model based on the Gibbs distribution (14), global regularization;
- MAP-ENT—the MAP algorithm with prior model based on the entropy functional (34), global regularization; and,
- MAP-ENT-LOC—the MAP algorithm with prior model based on the entropy functional for open system (43), local regularization.
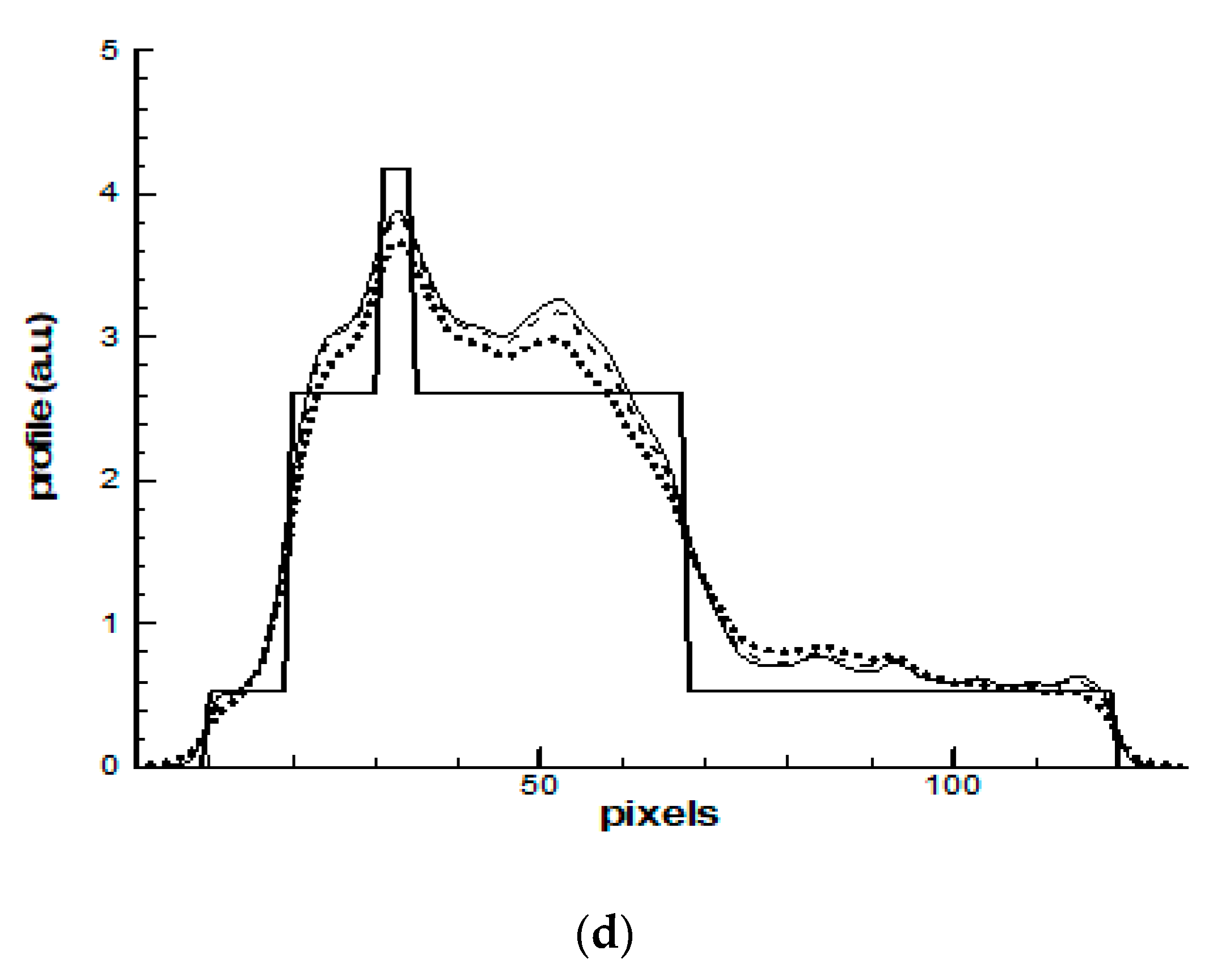
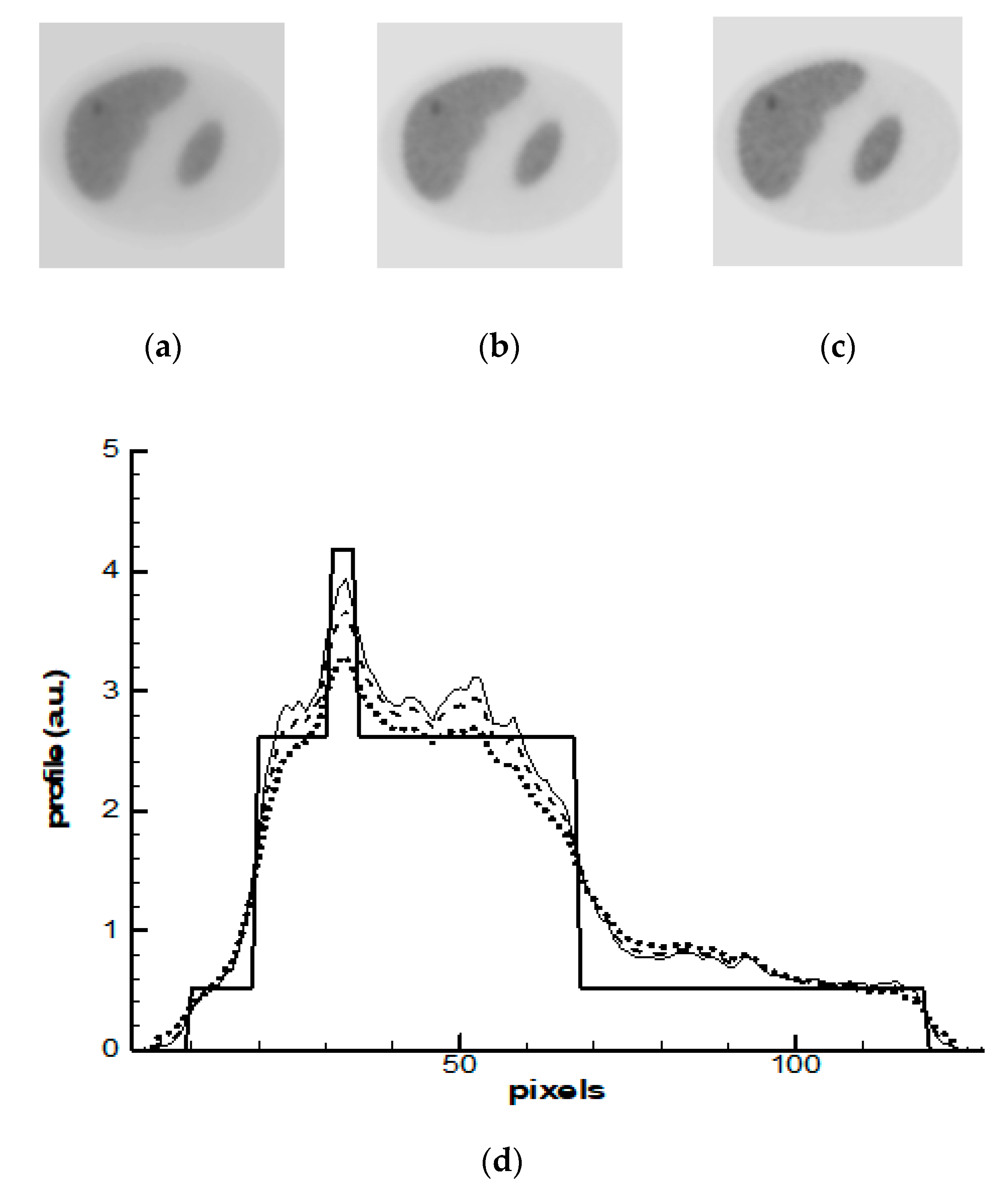
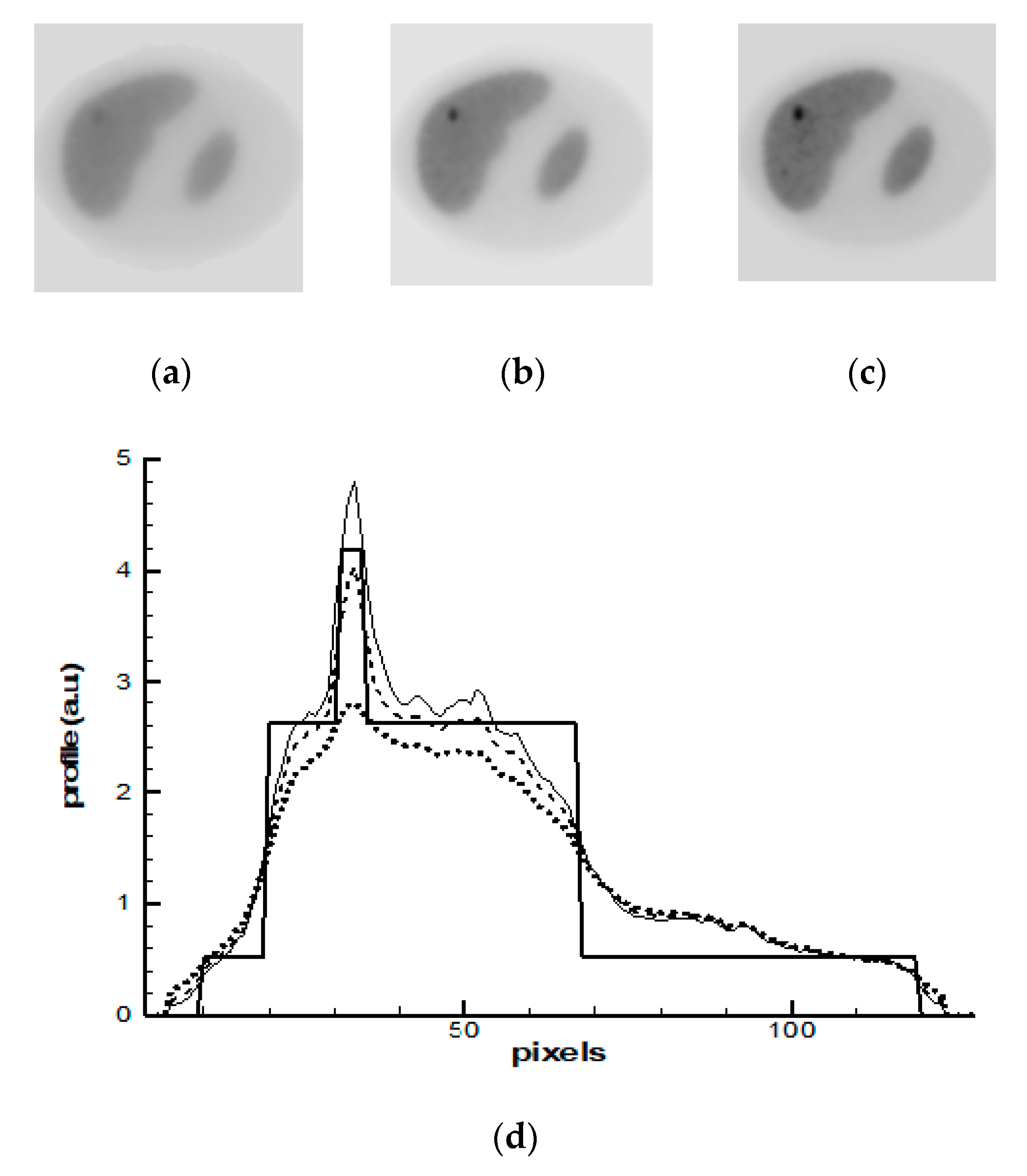
4. Results and Discussions
5. Conclusions
Funding
Conflicts of Interest
References
- Hadamard, J. Le Probleme de Cauchy et les Equations aux Derives Partielles Lineares Hyperboliques; Hermann: Paris, France, 1932. [Google Scholar]
- Tikhonov, A.N. Solution of incorrectly formulated problems and the regularization method. Sov. Math. Doklady 1963, 4, 1035–1038. [Google Scholar]
- Arsenin, V.J.; Timonov, A.A. Building on the basis of local regularization of algorithms of finding approximated resolutions integral equations of the first kind of the convolution type with application of additional information. Prepr. Keldysh Inst. Appl. Math. 1983, 40, 25. [Google Scholar]
- Tikhonov, A.N.; Arsenin, V.J.; Rubashev, I.B.; Timonov, A.A. The first soviet computer tomography. Priroda 1984, 4, 11–21. [Google Scholar]
- Turchin, V.F. A solution of Fredholm equation in statistical ensemble of smooth functions. J. Comput. Math. Math. Phys. 1967, 6, 1270. [Google Scholar]
- Turchin, V.F.; Koslov, V.P.; Malkevich, M.S. The use of mathematical-statistics methods in the solution of incorrectly posed problems. Sov. Phys. Uspekhi 1971, 13, 681–703. [Google Scholar] [CrossRef]
- Jaynes, E. Information theory and statistical mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Jaynes, E. Information theory and statistical mechanics II. Phys. Rev. 1958, 108, 171–190. [Google Scholar] [CrossRef]
- Jaynes, E. Prior Probabilities. IEEE Trans. Syst. Sci. Cybern. 1968, 4, 227–241. [Google Scholar] [CrossRef]
- Frieden, B.R. Restoring with maximum likelihood and maximum entropy. J. Opt. Soc. Am. 1972, 62, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Gull, S.F.; Daniell, G.J. Image reconstruction from incomplete and noisy data. Nature 1978, 272, 686. [Google Scholar] [CrossRef]
- Skilling, J. The Axioms of Maximum Entropy in Maximum Entropy and Bayesian Methods in Science and Engineering 1; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 173–187. [Google Scholar]
- Minerbo, G. MENT: A Maximum Entropy Algorithm for Reconstructing a Source from Projection Data. Comp. Graph. Image Process. 1979, 10, 48–68. [Google Scholar] [CrossRef]
- Von der Linden, W. Maximum Entropy data analysis. Appl. Phys. 1995, 60, 155. [Google Scholar] [CrossRef]
- Denisova, N. A maximum a posteriori reconstruction method for plasma tomography. Plasma Sources Sci. Technol. 2004, 13, 531–536. [Google Scholar] [CrossRef]
- Denisova, N.V. Bayesian Reconstruction in SPECT with Entropy Prior and Iterative Statistical Regularization. IEEE Trans. Nucl. Sci. 2004, 51, 136–141. [Google Scholar] [CrossRef]
- Besag, J. Spatial interaction and the statistical analysis of lattice systems. J. R. Stat. Soc. B 1974, 36, 192–236. [Google Scholar] [CrossRef]
- Geman, S.; Geman, D. Stochastic Relaxation, Gibbs Distributions, and the Bayesian restoration of Images. IEEE Trans. Pattern Anal. Mach. Intell. 1984, 6, 721–741. [Google Scholar] [CrossRef] [PubMed]
- Geman, S.; McClure, D. Bayesian image analysis: An application to single photon emission. Amer. Statist. Assoc. 1985, 12–18. Available online: http://www.dam.brown.edu/people/geman/Homepage/Image%20processing,%20image%20analysis,%20Markov%20random%20fields,%20and%20MCMC/1985GemanMcClureASA.pdf (accessed on 12 November 2019).
- Nuyts, J.; Fessler, J.A. A penalized-likelihood image reconstruction method for emission tomography, compared to post-smoothed maximum-likelihood with matched spatial resolution. IEEE Trans. Med. Imaging 2003, 22, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Denisova, N.V.; Terekhov, I.N. A study of myocardial perfusion SPECT imaging with reduced radiation dose using maximum likelihood and entropy-based maximum a posteriori approaches. Biomed. Phys. Eng. Express 2016, 3, 055015. [Google Scholar] [CrossRef]
- Shepp, L.A.; Vardi, Y. Maximum Likelihood Reconstruction for Emission Tomography. IEEE Trans. Med. Imaging 1982, 1, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hammersly, J.; Clifford, P. Hammersly-Clifford theorem. 1971; Unpublished work. [Google Scholar]
- Hebert, T.; Gopal, S. The GEM MAP Algorithm with 3D SPECT System Response. IEEE Trans Med. Imaging 1992, 11, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Leahy, R.M. Iterative reconstruction techniques in emission computed tomography. Phys. Med. Biol. 2006, 51, R541. [Google Scholar] [CrossRef] [PubMed]
- Landau, L.D.; Lifshitz, E.M. Statistical Physics v.5; Nauka: Moscow, Russia, 1964. [Google Scholar]
- Gull, S.F.; Skilling, J. Maximum Entropy method in image processing. IEE Proc. 1984, 131, 646–659. [Google Scholar] [CrossRef]
- Skilling, J.; Bryan, R.K. Maximum Entropy image reconstruction: General algorithm. Mon. Not. R. Astron. Soc. 1984, 211, 11–124. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denisova, N. Bayesian Maximum-A-Posteriori Approach with Global and Local Regularization to Image Reconstruction Problem in Medical Emission Tomography. Entropy 2019, 21, 1108. https://doi.org/10.3390/e21111108
Denisova N. Bayesian Maximum-A-Posteriori Approach with Global and Local Regularization to Image Reconstruction Problem in Medical Emission Tomography. Entropy. 2019; 21(11):1108. https://doi.org/10.3390/e21111108
Chicago/Turabian StyleDenisova, Natalya. 2019. "Bayesian Maximum-A-Posteriori Approach with Global and Local Regularization to Image Reconstruction Problem in Medical Emission Tomography" Entropy 21, no. 11: 1108. https://doi.org/10.3390/e21111108
APA StyleDenisova, N. (2019). Bayesian Maximum-A-Posteriori Approach with Global and Local Regularization to Image Reconstruction Problem in Medical Emission Tomography. Entropy, 21(11), 1108. https://doi.org/10.3390/e21111108




