1. Introduction
When optimizing a thermal plant, using a heat driven power cycle or a heat pump, practical experience indicates that one seldom have the luxury of choosing the components in any of the systems considered. Instead, the plant designer often needs to choose between preexisting, industrially standardized machines. Such preexisting thermal systems will have characteristics almost according to the designer’s preferences, but seldom exactly. Each of the potential thermal system will respond differently to optimizations of the plant. Unless the providers make a complete and unique model available of each potential thermal system, plant optimization has to rely heavily on assumptions. Since such unique models have a tendency to become biased, the problem remains regardless. We therefore propose a different approach to comparing thermal systems on a global level. Global in this approach means that the power cycle or heat pump is treated as a “black box” with global efficiency defined by the real boundary conditions dictated by the plant in which the “black box” operates.
A sound comparison of energy efficiency of thermal systems performing almost similar duty benefits not only from a “black box” approach, but also from a non-dimensional scale and an accurate definition of the reversible energy efficiency of each system. In this article, “thermal systems” means heat engines and heat pumps.
Black box approaches can be defined as independent of technology used. The importance of the black box approach is determined by its purpose. When comparing thermal systems using different cycles, there is no benefit in separating losses internal to the cycle from losses external to the cycle. By cycle design typical external losses, such as pinch-effects and impact of limited heat exchanger inventories, can be mitigated and are therefore linked to internal cycle losses in various ways. If the purpose instead is to study possible improvement potential of a particular cycle, then conventional second law analysis is highly effective. In such a case, there is no need for a black box approach.
In most practical cases, heat sources as well as heat sinks are finite. Therefore, exit temperatures from the thermal system vary depending on its energy efficiency. A small heat engine, relative to the apparent thermal capacity of source and sink, will operate at a larger temperature difference compared to a large system. Therefore, a comparison between thermal systems of different magnitude requires a measure indicating how small or large each system is relative to the boundary conditions of the heat source and sink. Furthermore, any variation in temperature difference means that an immediate comparison of energy efficiency becomes ambiguous. Instead comparison of energy efficiencies should relate to the reversible energy efficiency of each system, implicitly therefore also to the exit temperatures of the source and sink that would be obtainable using a thermodynamically perfect cycle.
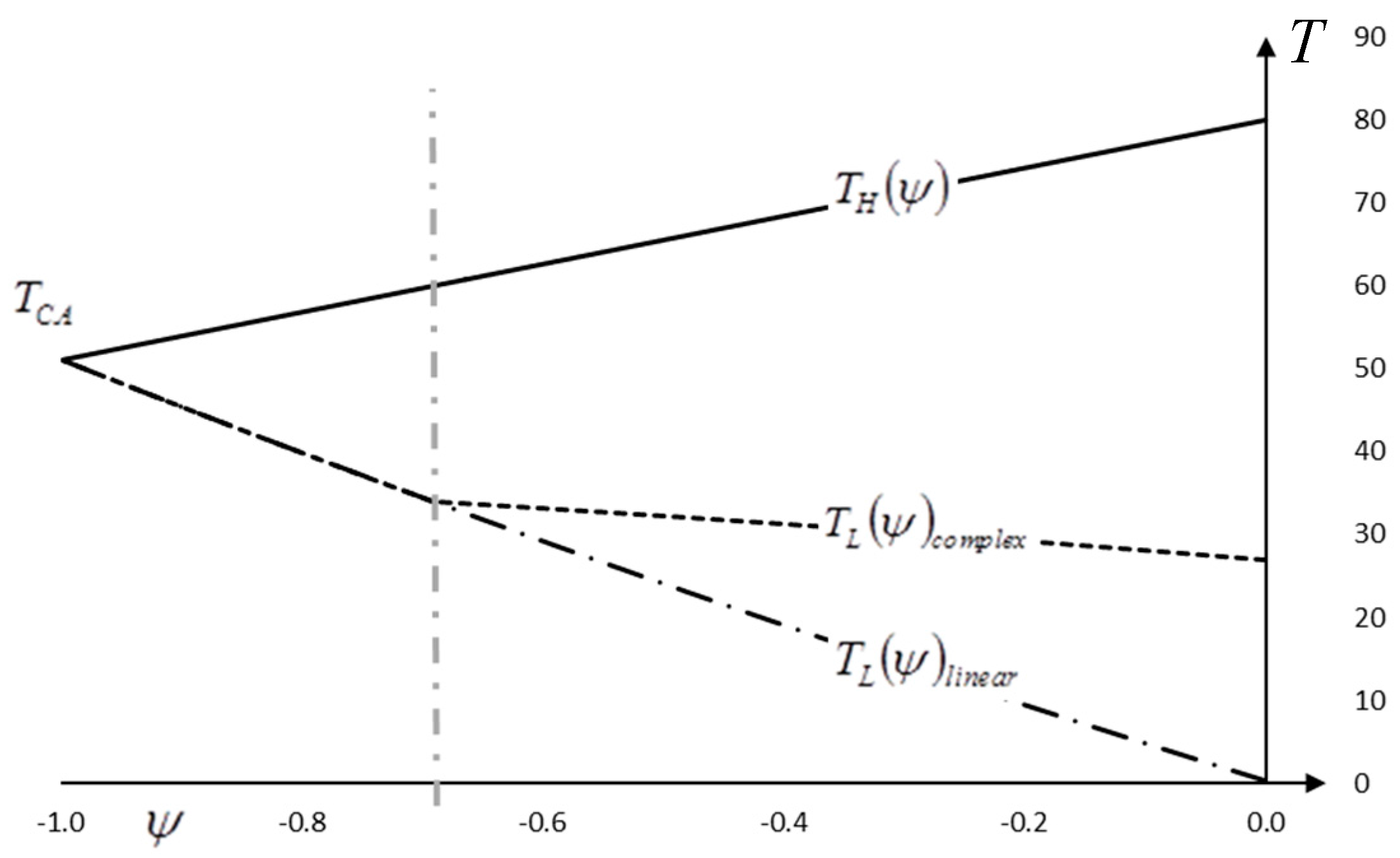
Complexity is added by the fact that many thermal systems operate in environments where apparent heat capacity of heat source and/or heat sink are functions of temperature. We call them “complex”, or non-linear. If the apparent heat capacity is constant we call them “linear”. In the latter case, analytical formulas can be derived by defining reversible exit temperatures of source and sink as well as reversible energy efficiency. In the complex or non-linear cases, we need a numerical approach.
In the following, we will refer to methods of defining reversible energy efficiency of heat engines and we will propose the same method for comparison of heat pumps. Obviously, refrigeration systems can be compared in a similar manner as heat pumps.
Lorenz [
1] defined a type of reversible cycles specifying how the temperatures in finite source and sink changed with transportation of heat through it. With such definition, reversible energy efficiency can be found analytically if the polytropes of the Lorenz cycle constitute equations suitable to integration. In many applications, for example, a heat engine using waste heat recovery of a diesel engine, however the polytropes become difficult to integrate.
Ibrahim & Klein [
2] showed a numerical definition of extractable work from a reversible cycle named the “max power cycle”. They used multiple, small, Carnot cycles in consecutive order relative to source and sink. In Öhman & Lundqvist [
3] the concept of “Integrated Local Carnot Efficiency”
was defined as the reversible thermal efficiency of a max power cycle. Using
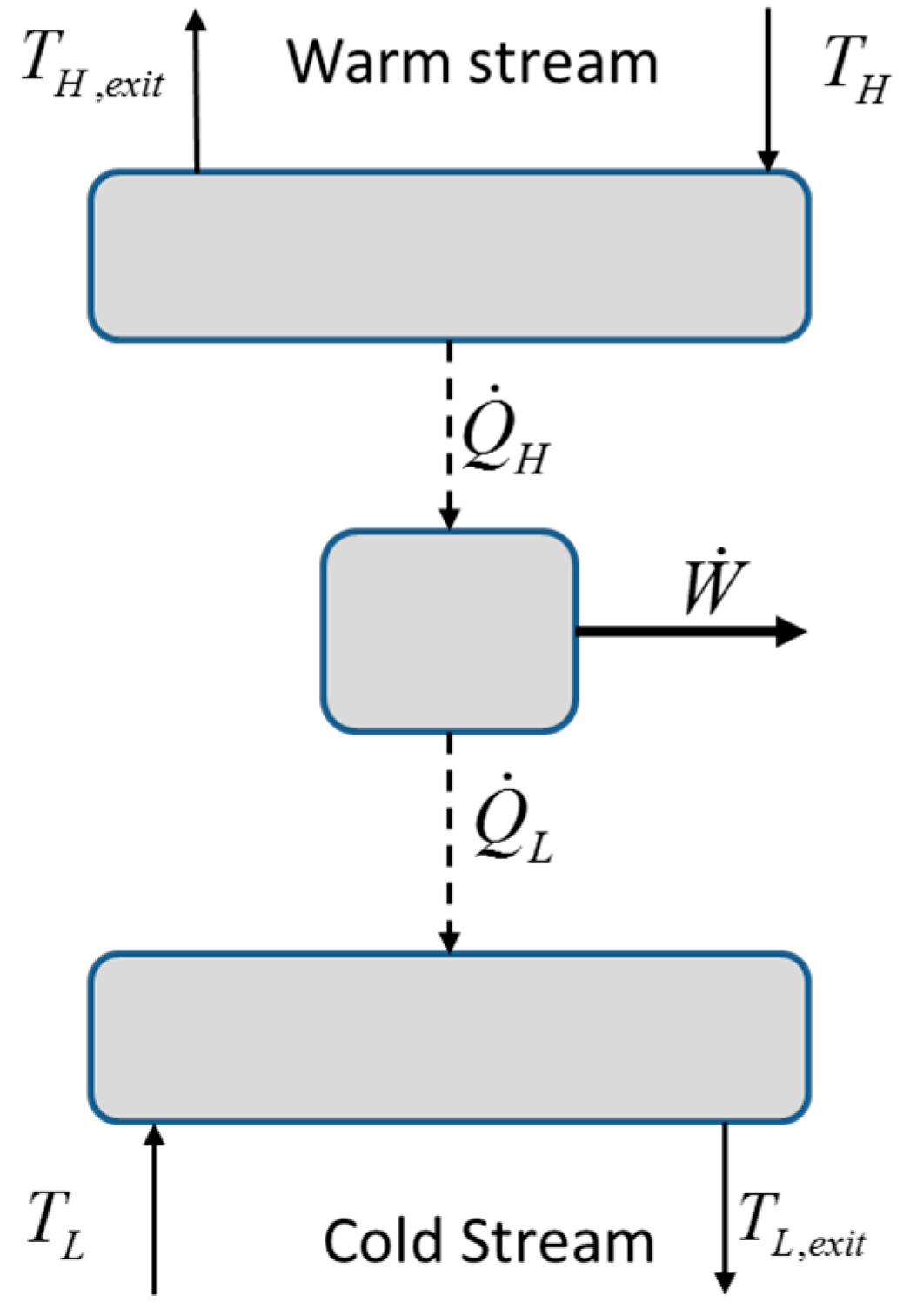
Figure 1 we can extend the approach to also comprise heat pumps.
Equation (1) shows a definition of energy efficiency, or thermal efficiency, of a reversible heat engine.
Consequently, we may define energy efficiency of a reversible heat pump in Equation (2), operating at the same temperatures.
As explained in Öhman & Lundqvist [
4] we may use the approximation of Curzon–Ahlborn efficiency as of Curzon & Ahlborn [
5] in Equation (3), to define the temperature that a source and a sink would equalize to by using a reversible heat engine as in Equation (4), the Curzon–Ahlborn temperature
. Note however that, as explained in Öhman [
6], using Curzon–Ahlborn efficiency limits the use of Equation (4) to applications with a linear source and sink. A generalization to complex sources and sinks requires the numerical solution of the max power cycle to determine this temperature.
We wish to stress that the use of Equation (3) does not imply that the method described is developed according to the tradition of finite-time thermodynamics, FFT, or endo-reversible thermodynamics. It is only used as a simplification used to derive Equation (4) and only applicable to linear boundary conditions. Öhman [
6] explains that Equation (3) is incorrect, however the error is small enough to allow its use in Equation (4) for determining Equation (5).
FTT, influenced by Curzon & Ahlborn [
5] is a related field of science often focusing on cycle optimization and heat transfer in finite environments. Dong et al. [
7] explain a general method to obtain optimal operating points for endo-reversible and irreversible heat engines. Ge et al. [
8] explain the advances in finite-time thermodynamics for internal combustion cycle optimization. Feidt [
9] explains the development in some traditions of thermodynamic analysis and that FTT is based on the idea of reversible cycle operating with irreversible heat exchange. Feidt [
10] explains thermodynamic analysis of reverse cycles, clearly showing that FTT focuses on studying effects on thermal efficiency caused by explicit losses emanating from technical limitations. The black box approach focuses on thermal efficiency as a function of the magnitude of a thermal system relative to the source and sink, without assumptions on specific losses. From that reason, it is natural to use that approach also to study effects of complex boundary conditions. The method explained in this article is explicitly designed for simplified communication of the findings to practitioners.
2. Method
Using Equation (1) and the definition of inverse apparent heat capacity in the source as
and in the sink as
, Öhman & Lundqvist [
4] derived Equation (4) as follows. (Note the printing error in the equation in the reference.)
As extensively explained in Öhman [
6], we can now define a dimensionless scale, named “utilization” and defined in Equation (5), on which to project the energy efficiency of a heat engine.
Note that is only determined by the nature of the source and sink, while is the actual rate of heat entering the heat engine.
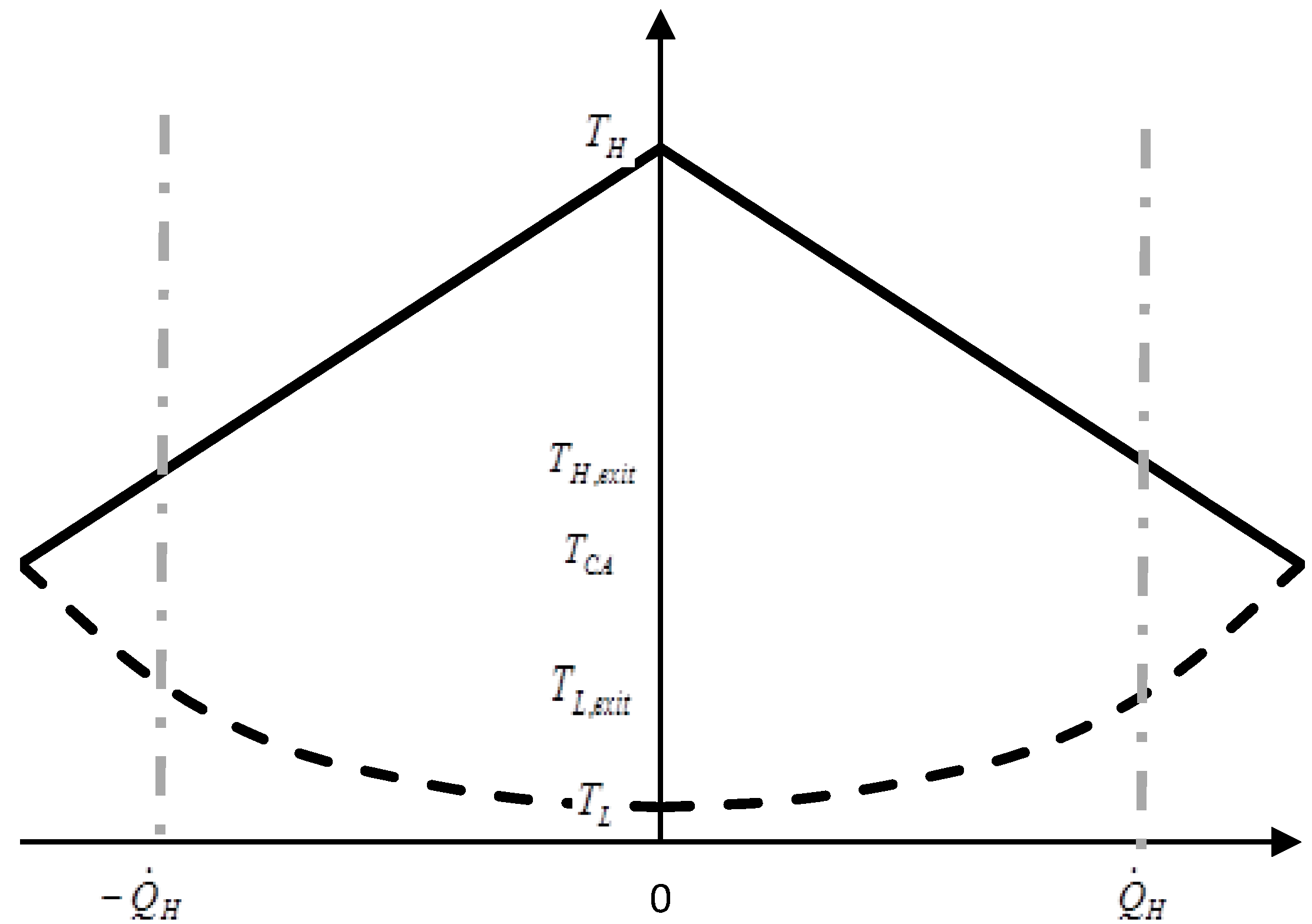
Now we can construct a diagram by plotting various characteristic data vs. “utilization” in a dimensionless way, thereby allowing comparison of heat engines of different magnitude relative to the finiteness of heat source and sink. This approach is suggested in Öhman [
6] for the comparison of the global energy efficiency of different real heat engines. For the correlation of global energy efficiency of a real heat engine, it is possible to define a “Fraction of Carnot” (
FoC) as of Equation (6). The
FoC can be explained as measured, or simulated, using thermal efficiency divided by the ideally possible at the given utilization
, where
.
Note that, by referring to boundary conditions of the reversible thermal system,
FoC is not equivalent to common definitions of exergy efficiency. Note also that
, determined by the numerical max power cycle approach, can be easily validated for linear boundary conditions using equations available in standard literature. Appendix A in Öhman [
6] provides explicit expressions for such validation.
4. Discussion
The explained method uses conventional thermodynamic entities to create a dimensionless comparative method for black box energy efficiency. It comprises first as well as second law effects. Other methods can be used but are likely to become more complicated. The numerical approach of the max power cycle provides the benefit of detailed information about local temperatures in source and sink and thereby greatly simplifies the understanding of pinch points and similar effects.
The use of detailed exergy destruction analysis is constructive in identifying and evaluating irreversibility. However, when determining reversible thermal efficiency exergy efficiency becomes meaningless. The approach of Integrated Local Carnot efficiency is a direct implementation of multiple Carnot cycles hence it can be directly derived by assuming zero increase of entropy.
With a similar, systematic black box approach, for heat engines and heat pumps, experiences from one type of thermal system may be useful to understand effects of another.
A particularly interesting question arises when using the concept of utilization for heat engines and heat pumps in a symmetric manner, such as in
Figure 2. A research question arising from this discussion is if a correlation, similar to Equation (8) could be found for heat pumps or cooling systems. We suggest researching real heat pump thermal efficiency for further studies using the method described in this article.