Abstract
The evaluation of the variety suitability regarding each appellation’s specificities should be a strategy for maximizing the varieties’ oenological potential while contributing to the sustainable production of quality wines, keeping their typicity and rationalizing winemaking costs. Thus, the combination of several grape physicochemical attributes, modulated by climate and vineyard characteristics, providing knowledge for each grape variety’s oenological potential, is a relevant and reliable support for winemakers’ decisions. To prove this hypothesis, six mature grape varieties from three harvests, each one from three vineyard parcels with different topographical conditions from Bairrada Appellation (Portugal), were studied using analysis of variance–simultaneous components analysis (ASCA). The effects of harvest year and parcel on grape berry weight, pH, titratable acidity, total sugars, total phenolics, antiradical activity, and volatile composition in free and glycosidically-linked forms were analyzed. The compositional plasticity of autochthonous varieties (white Arinto and Bical and red Baga, Castelão, and Touriga Nacional) was observed. Sauvignon Blanc grape composition was significantly modulated by harvest. This study represents an important contribution for the maintenance of varieties’ biodiversity while contributing to establishing their peculiarities. Autochthonous varieties, if accurately exploited, can provide higher characteristic diversity than worldwide used varieties, an aspect to be more objectively taken into consideration by winemakers.
1. Introduction
Sustainable viticulture has appeared as a breakthrough approach that globally aims to promote an integrated and efficient use of non-renewable natural resources, such as grape varieties, for quality wine production while keeping their typicity and safety and promoting economically viable production. Thus, the detailed knowledge of each grape variety’s suitability regarding the natural specificities of each appellation is crucial for sustainability in the viticulture sector [1,2]. Grape constitutes the raw material for producing wines. Its quality traits reflect the outcome of complex physiological and biochemical interactions between the grape variety and its environmental conditions (vineyard soil type and topography and climate, among others) [1], known to influence the expression of grape characteristics [3,4], and thus impacting wine quality [5]. This capacity of a grape variety to be modulated under variable environmental conditions is referred to as metabolic plasticity [6]. Climate elements, such as temperature, sunlight exposure, and precipitation, can affect grapes’ growth and ripening and, therefore, mature berry composition, influencing the levels of sugars, acids, and secondary metabolites of major oenological significance, such as phenolics and volatiles [7,8]. Grape composition is also modulated by a vineyard characteristic, namely soil type, that can act as a regulator of the climate elements, due to its water-retaining (affecting water and nutrient availability to the plant), heat-retaining, and light-reflecting capacities (affecting microclimate) and penetrability (affecting the root growth) [9,10]. Additionally, vineyard topographical factors, such as altitude and slope, modulate grape composition, influencing grapevine vigor and grape ripening, and are directly associated with the vineyards’ resulting humidity, surrounding vegetation, sunlight exposure and shadow, orientation, and trellising [1,9].
To understand the grape variety’s oenological potential under the uncontrollable harvest climatic conditions and vineyard’s characteristics, the application of chemometric tools to obtain fast and reliable information that can help the winemakers’ decision is crucial. Robust multivariate analysis of the long-term data series may represent a key tool to reduce the environmental impact on wine quality by assisting in the implementation of adequate winemaking practices and technologies, while contributing to reducing wine production costs. Over many years, research has been aimed at developing a simple model or method that could define and/or predict grape and wine quality. In fact, different models constructed by the application of multivariate methods have already been developed [11,12,13,14,15]. For instance, a Pearson’s correlation model was developed to predict Sauvignon Blanc wine quality, correlating microclimatic data (temperature and radiation) with volatile concentration and wine sensory parameters [11]. Geo-spatial modelling and partial least squares (PLS) were also used to assess the spatial behavior of three grape varieties (Cabernet Sauvignon, Syrah, and Merlot) according to quality zone delineation [12]. Although contributing to the understanding of the relationships that may occur between factors, such as climate, viticulture techniques, vineyard ecosystems, and grape composition, this kind of model is restricted in space and in the number of parameters evaluated, thus limiting its prediction power.
Analysis of variance–simultaneous component analysis (ASCA) was revealed to be particularly helpful for the analysis of the effects in complex data sets, where large number of variables have been measured. ASCA has been already applied to evaluate the effects of pressure (250–650 MPa) and pressure holding time (15–120 min) on the phenolic content of Nero D’avola Syrah red wine [16] to evaluate the effects of age and variety on the electronic tongue response and wine composition with respect to the organic acids, phenolics, and furanic derivatives, using HPLC data [17] to evaluate how storage temperature and time (as well as their interaction) influence cheddar cheese ripening, combining Raman and Mid-InfraRed (MIR) spectroscopy data [18], and to investigate the effects of CO2 and soil–water levels on 1H NMR-based metabolic fingerprints of Arabica coffee beans [19]. ASCA is a merging of analysis of variance (ANOVA) and principal component analysis (PCA) that allows the overcoming of the drawbacks of using both methods individually. ANOVA fails in cases when the number of the variables exceeds the number of samples or when dependent variables are correlated. On the other hand, PCA does not consider the experimental design, which means that the different contributions to the variation caused by the experimental design are confounded in the model [20,21]. The ASCA method consists of partitioning the original data matrix into a set of matrices corresponding to the different factors of the experimental design and, subsequently, subjecting each one of these matrices to a PCA. The ANOVA-like model is constructed from the PCA models of all effects and interactions. Since loadings are calculated for each sub-model independently, the contribution of the variables to every source of variation can be identified. The significance of each factor can be assessed using a permutation test that consists of randomly changing the order of the rows in the data set [22,23,24].
The present research study aims to evaluate the reliability of a multivariate statistical tool (ASCA) to combine grape physicochemical attributes (berry weight, pH, titratable acidity, total sugars, total phenolics, antiradical activity, and free and glycosidically linked volatile composition) to evaluate the impact of harvest year conditions and vineyard parcel characteristics on grapes’ oenological potential. For this, mature grapes belonging to five Vitis vinifera autochthonous varieties (Arinto, Bical, Baga, Castelão, and Touriga Nacional) and a worldwide cultivated one (Sauvignon Blanc) from Bairrada Appellation (Portugal) were selected as a case study. Each variety was collected in three consecutive harvests and in three vineyard parcels with different topographical characteristics.
2. Materials and Methods
2.1. Samples, Vineyards and Harvests Characteristics, and Sampling
2.1.1. Samples
Healthy-state Vitis vinifera L. cv. Arinto, Bical, Sauvignon Blanc (white varieties) and Baga, Castelão, and Touriga Nacional (red varieties) grapes, from three consecutive harvests (2010–2012), were collected, at technological maturity, in São Mateus (SM) (110 ha; 40°26’56″ N; 8°29’20″ W) and in Vale de Azar (VA) vineyards (60 ha; 40°25’50″ N; 8°26’54″ W), located at Manuel dos Santos Campolargo, Herdeiros company, in Bairrada Appellation (Portugal), each one from three vineyard parcels (Figure 1).
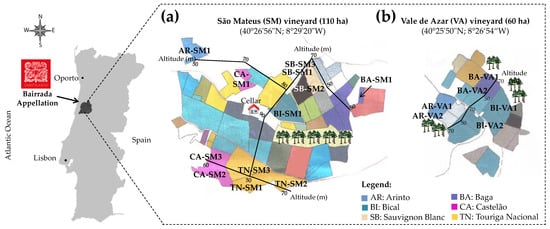
Figure 1.
Representative scheme of the location of the vineyards of (a) São Mateus (SM) and (b) Vale de Azar (VA) at Manuel dos Santos Campolargo, Herdeiros company, in Bairrada Appellation (Portugal), highlighting the varieties (Vitis vinifera L cv. Arinto (AR), Bical (BI), Sauvignon Blanc (SB), Baga (BA), Castelão (CA), and Touriga Nacional (TN)), altitude (ca. 50 to 90 m), and vineyard parcels (SM1 to SM3 and VA1 and VA2) under study.
To help defining the technological maturity state of each variety on the different conditions under study, grapes were collected weekly from half-véraison (when 50% of the grapes were in turning color in red varieties or with a translucent skin in white ones) to maturity and, if grapes were available, also to the post-maturity state (Figure S1). Although sugar content and titratable acidity were commonly used to define grapes’ maturity state (sugar content tends to increase while acidity tends to decrease, and then both stabilize when maturity is reached) [25], the berry weight and pH where also considered as indicators of berry development and to assess changes occurred in the berry, such as dehydration or microbiological contamination, respectively (Figures S2–S7).
2.1.2. Vineyard Parcels Characteristics
Table 1 summarizes the main characteristics of the vineyard parcels under study, such as soil type (defined based on its texture), row orientation, the presence or not of pine trees (which influences vineyards sunlight, shadow, and wind exposures), and altitude (determined with a global positioning system (GPS) apparatus). All these vineyard parcel conditions were defined and selected by Manuel dos Santos Campolargo, Herdeiros company.

Table 1.
Main characteristics of the vineyard parcels where the six Vitis vinifera varieties were collected.
Although grape quality also depends on agricultural practices [1], in this, the same agricultural conditions were observed: parcels were not irrigated, thinning was not performed, no kinds of mulches were added to the soil, and the bilateral cordon trellising system was used in all vineyard parcels.
2.1.3. Harvest Weather Conditions
The harvest year weather information (mean precipitation and mean temperature) for a ten-year period (2010–2020) was obtained from the local meteorological station (type: EMA II climatologic station, number 705), from IPMA (Instituto Português do Mar e da Atmosfera, IP, https://www.ipma.pt/pt/ (accessed on 1 April 2021)), located in the municipality of Anadia (Portugal).
As can be observed in Figure 2a, the weather conditions of Bairrada Appellation have not changed significantly in the last decade. Looking for the weather conditions of 2010, 2011, and 2012 (Figure 2b), the harvests object of this study, the precipitation amount of this appellation is irregular throughout the year, attaining its maximum in November/December. Although the occurrence of moderate precipitation in spring is common, in 2012, an unusual precipitation amount was observed, contrasting with its also rare dry winter season (Figure 2b). Moreover, the 2010 harvest was considered an equilibrated year, with moderate temperatures during spring and a moderate precipitation amount, which were considered suitable for the maturation process by the winemaker involved in this work. On the other hand, except in July and August, 2011 exhibited an unusual hot spring (only similar to the one observed in 2015), while 2012 exhibited lower temperatures throughout the year (except in March and September), thus being fresher and rainier (Figure 2b), which contributed to accelerating or delaying the grapes’ maturation process, respectively (Figure S1).
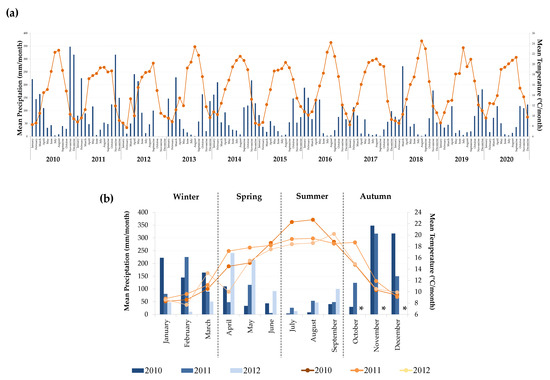
Figure 2.
Meteorological data available for Bairrada Appellation, expressed as mean precipitation (blue colors) and temperature (orange colors): (a) data from the last decade (2010–2020) and (b) zoom of the three harvests under study (2010–2012). * Precipitation data were not available at the meteorological station from October to December of 2012 harvest.
2.1.4. Sampling
For each variety and condition under study, ca. 1000 g of grape berries was picked randomly throughout the vineyard parcels, following a z-shaped pattern to avoid edge and center effects, considering the number of berries per bunch and the balance between shadow and sun exposure. To obtain random samples and avoid piking samples from other vineyard environments, every vine in the vineyards was previously marked, contributing to the understanding of the intrinsic and natural variability of the fruit and allowing the validation of the data obtained. Samples were transported immediately under refrigeration (ca. 4 °C) to the laboratory where the grapes’ classical physicochemical parameters were promptly determined. The remaining grapes were stored at −20 °C for a maximum period of six months until the remaining characterization analyses.
2.2. Grapes Physicochemical Parameters Determination
2.2.1. Classical Physicochemical Parameters
Two hundred grape berries from each variety and condition under study were randomly selected. After determining their weight, the grape berries were crushed, and the juice was obtained by filtration. The pH was measured using a pH meter (micropH 2002, Crison, Barcelona, Spain). Sugar content was established through the determination of alcoholic degree using a refractometer (Fabre réfractomètres, Sarl Germain, France). The titratable acidity was measured by titrimetry using NaOH 0.1 M (Panreac, Barcelona, Spain) and bromothymol blue as the indicator. All measurements were made in triplicate, each one corresponding to a different aliquot.
2.2.2. Total Phenolic Content
Briefly, 0.125 mL of grape juice (diluted five times) was mixed with 0.5 mL of distilled water and 0.125 mL of Folin–Ciocalteu reagent (react for 5 min). Then, 1.250 mL of Na2CO3 (75 g L−1) and 1.0 mL of distilled water were added (vortex, 90 min, room temperature). The absorbance was measured at 760 nm in a spectrophotometer (6405 Jenway UV–vis spectrophotometer, UK). The calibration curve of gallic acid (10.0–200.0 mg L−1) was obtained as described for the samples. The total phenolic content was expressed as milligrams of gallic acid equivalents per liter (mg GAE L−1). All measurements were made in triplicate, each one corresponding to a different aliquot. Analytical grade reagents and high-purity standards were used.
2.2.3. Antiradical Activity
Briefly, 0.1 mL of grape juice was added to 3.9 mL of 60 μM DPPH● methanol solution. The absorbance was read after 30 min, at 515 nm, on the UV–visible light spectrophotometer (6405 Jenway UV–vis spectrophotometer, UK) against blank (distilled water), at room temperature. The percentage of the remaining DPPH● (% DPPHrem) was calculated as:
where Asample and Ablank are the absorbance of each sample and blank, respectively, and the % DPPHrem of the blank was considered to be 100%.
The analyses were made in three independent replicates (n = 3) and analytical grade reagents and high-purity standards were used.
2.3. Grapes Volatile Composition Determination
2.3.1. Determination of Grapes Free Volatile Profiles–HS-SPME Procedure
The HS-SPME experimental parameters were adopted from a methodology previously developed [26]. Briefly, 4 g of each grape sample was crushed and inserted into a 20 mL glass vial with 5 mL of ultra-pure water, 2 g of sodium chloride, and stirring (400 rpm). The vial was capped with a PTFE septum and an aluminum cap (Chromacol, Hertfordshire, UK) and placed in a thermostated bath (60 ± 0.1 °C, 5 min). The 1 cm DVB/CAR/PDMS SPME fiber (50/30 μm divinylbenzene-carboxen-polydimethylsiloxane, Supelco, Aldrich, Bellefonte, PA, USA) was inserted into the vial headspace for 20 min. Prior to use, the SPME fiber was conditioned according to the manufacturer’s recommendations. Three independent replicates for each assayed sample were made.
2.3.2. Determination of Grapes’ Glycosidically Linked Profiles—SPE Procedure
The experimental procedure for determination of glycosidically linked volatiles was adopted from a methodology previously developed [27]. Briefly, 350 g of grape berries from each sample were crushed and centrifuged (3000 rpm, 25 min, 4 °C). Then, each supernatant (75 mL) was submitted to solid-phase extraction (SPE) using Amberlite XAD-2 column resin (20–60 mesh, Supelco, Inc., Bellefont, PA), where the volatile compounds were eluted with ethyl acetate (50 mL). Ultra-pure water (15 mL) was used to remove water-soluble compounds, and the retained glycosidically linked fraction was eluted with methanol (75 mL). This methanolic extract was evaporated under vacuum until dryness and then dissolved in phosphate–citrate buffer (0.1 M, 10 mL) in ultra-pure water (250 mL, pH 5.0). To release the aglycones from the glycosidically linked compounds, a commercial enzyme mixture (ProZym® Aroma M, Proenol) was used (100 mg L−1, 42 h, 35 °C). Then, generated free volatiles were extracted with ethyl acetate (75 mL), and 3-octanol (8.72 μg L−1) was used as the internal standard. The extracts were cooled to −20 °C and dried over anhydrous sodium sulphate. The excess of low-boiling solvent was removed using a liquid nitrogen trap. The obtained concentrate (ca. 1 mL) was stored in a glass screw-top vial at −20 °C until analysis. Three independent replicates (n = 3) were done for each assayed sample. All solvents were analytical grade with high purity (≥99%).
2.3.3. GC×GC-ToFMS Analysis
The analysis of free and glycosidically linked volatile fractions was carried out based on a previous work using LECO Pegasus 4D (LECO, St. Joseph, MI, USA) GC×GC-ToFMS system [26]. For free volatile compounds, the SPME coating fiber was manually introduced into the GC×GC–ToFMS injection port at 250 °C for 3 min. For glycosidically linked volatile compounds, 0.5 μL of extract was injected into GC×GC–ToFMS injection port (250 °C), turning the detector off during 150 s. More details were given in the Supplementary Materials.
Contour plots were used to evaluate the general separation quality and for manual peak identification. For identification purposes, the mass spectrum of each compound was compared to those in mass spectral libraries, including an in-house library of standards and two commercial databases (Wiley 275 and US National Institute of Science and Technology (NIST) V.2.0—Mainlib and Replib). Additionally, the experimentally determined retention index (RI) values were compared, when available, with RI values reported in the literature for chromatographic columns like those used in this work [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. The RI values were determined using a C8-C20 n-alkanes series and calculated according to the van den Dool and Kratz equation [45]. The majority (>85%) of the identified compounds presented similarity matches ≥850 (850/1000). Due to matrix complexity, the GC×GC peak areas data of free volatile compounds were used as an approach to estimate the relative content of each component and for glycosidically linked compounds, the data were expressed as μg L−1 of 3-octanol equivalents.
2.4. Data Processing
ASCA was applied to the data set (a total of six varieties, each one from three vineyard parcels, three harvests (2010–2012), a total of 137 parameters, and three independent replicates) and calculated for each grape variety including two main effects, harvest year and vineyard parcel, and their interaction. The significance of the effects was assessed using a permutation test using 2000 permutation and the percentage of the variance explained by each sub-model in the total model as a quality-of-fit criterion [22,23]. ASCA and a permutation test were implemented in MATLAB R2020b using the algorithms already described [20,23].
Principal component analysis (PCA) was performed using MetaboAnalyst 5.0 (web interface—https://www.metaboanalyst.ca/ (accessed on 1 April 2021)), displaying pairwise scores’ plot for the top three PCs, joining the data for the six grape varieties and conditions under study.
3. Results and Discussion
3.1. Grapes Physicochemical Composition Evaluation
Berry weight (an indicator of berry development or dehydration), pH and titratable acidity (to estimate wine acidity and indirectly taste and microbial stability) [46,47], and sugar content (to estimate wine alcohol content) [47], were used to evaluate the oenological potential of the six grape varieties under study, considering the three harvests (2010–2012) and the set of vineyard parcels (Table 2).

Table 2.
Synoptic table of the physicochemical and glycosidically linked content of V. vinifera cv. Arinto (AR), Bical (BI), Sauvignon Blanc (SB), Baga (BA), Castelão (CA), and Touriga Nacional (TN) grapes, each one from three harvests and three vineyard parcels.
Considering all the assayed samples, berry weight ranged from 1.0 to 2.1 g, tending to be higher in red varieties (1.6–2.1 g), except for BA-VA1 grapes from 2010 harvest, than in the white ones (1.0–1.7 g), also except for BI-SM1 (2012) and SB-SM2 (2011 and 2012) grapes. Although within a tiny range (ca. 1.5–1.6 g) compared to the one determined in this work (1.3–2.1 g) (Table 2), similar values were already reported for Baga variety [25]. The pH values were very similar among the studied V. vinifera varieties, harvests, and vineyard parcel conditions (2.7–3.3) (Table 2). However, for Sauvignon Blanc (3.0–3.2) (Table 2), these were like the ones reported for this white variety (3.06–3.14) collected in a similar period (2011–2013) in New Zealand [48].
For all assayed samples, the titratable acidity ranged from 4.1 to 7.9 g tartaric acid L−1, and the sugar content ranged from 163.8 to 254.2 g L−1 (Table 2). These parameters were very variable among the six varieties, harvests, and parcel conditions. Globally, Sauvignon Blanc and Castelão grape varieties (5.2–7.6 and 5.2–7.5 g tartaric acid L−1, respectively) exhibited the higher acidity values, while Touriga Nacional exhibited the lower ones (4.2–5.8 g tartaric acid L−1). Additionally, Sauvignon Blanc and Touriga Nacional had higher sugar content (201.7–247.6 and 184.7–254.2 g L−1, respectively), while the lower one was determined for Baga (163.8–214.8 g L−1) (Table 2). The lower sugar content of Baga grapes from Bairrada Appellation was already reported [25].
The phenolic content and antiradical activity were also considered indicators of the wines’ color and astringency and the bioactive potential [49] of the grape varieties under study. The phenolic content ranged from 197.5 to 1341.3 mg GAE L−1, being higher in red varieties (494.1–1341.3 mg GAE L−1) than in the white ones (197.5–467.6 mg GAE L−1). Phenolic compounds are well known for their antiradical properties [49]; thus, as expected, higher activity was determined in red varieties (51.9% to 89.6 % DPPHrem) when compared to the white ones (62.7% to 89.9 % DPPHrem). Moreover, Touriga Nacional, which is characterized by high phenolic content (501.6–1341.3 mg GAE L−1), was already reported as a source of phenolics, such as anthocyanins and proanthocyanidins, which contribute to the color, astringency, and bitterness of the resulting wines [50,51].
3.2. Grapes’ Volatile Profile Evaluation
Mature grapes contain free and glycosidically linked forms of volatiles, which are accumulated in the berries during maturation. Free forms are volatile compounds directly involved in aroma, playing a key role in the quality and peculiar aroma of wines. Additionally, glycosidically linked forms, non-volatile and odorless, can be released during the winemaking process through the action of β-glucosidases [52], giving rise to odorant compounds that play a role in the final wine aroma. Based on this, both forms of volatiles, determined by GC×GC-ToFMS, were considered in this study for the assayed grape varieties. An example of the obtained chromatograms was shown in Figure 3.
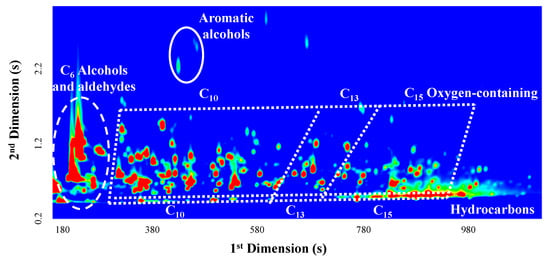
Figure 3.
Blow-up of a GC×GC-ToFMS chromatogram contour plot obtained in full-scan mode for AR-VA2 grapes collected in 2010. The chromatographic spaces corresponding to C6 alcohols and aldehydes, aromatic alcohols, mono (C10) and sesquiterpenic (C15) compounds, and C13 norisoprenoids were highlighted.
A total of 95 free volatile compounds, including varietal and pre-fermentative ones, grouped by chemical families, have been determined in the six grape varieties obtained from the three harvest years and from different parcels of São Mateus and Vale de Azar vineyards (Table 3): seven C6 compounds (alcohols and aldehydes), three aromatic alcohols, 17 norisoprenoids (1 C9 and 16 C13 norisoprenoids), and 69 terpenic compounds (47 mono-, 21 sesqui-, and one diterpenic compounds). Varietal volatile composition offers a means of evaluating the aroma potential of each variety, and herbaceous notes of C6 alcohols and aldehydes are appreciated in some wines by the consumers [53]. Likewise, in the glycosidically linked fractions, mono- and sesquiterpenic compounds and C13 norisoprenoids were the selected chemical families due to their considerable significance to the varietal aroma of V. vinifera varieties [54]. In these fractions, a total of 36 terpenic compounds (25 mono- and seven sesquiterpenic ones) and four C13 norisoprenoids were determined (Table 3). Detailed information related to the volatile compounds of each grape variety is shown in Supplementary Tables S1–S12.

Table 3.
Volatile components (free and glycosidically linked ones) determined at technological maturity for Vitis vinifera cv. Arinto (AR), Bical (BI), Sauvignon Blanc (SB), Baga (BA), Castelão (CA), and Touriga Nacional, for the three consecutive harvests and three vineyard parcels under study.
The varietal volatile compounds present in higher number, such as free or glycosidically linked forms, were the terpenic compounds (mono- followed by sesquiterpenic ones). Their sensory thresholds are rather low (a few hundred micrograms per liter); thus, even in small amounts, they contribute significantly to the aroma potential of the varieties with characteristic fruity, citric, and floral notes [55]. However, the term ‘varietal’ did not imply that each variety has specific and exclusive varietal volatile compounds. In fact, the same volatile compounds were found in different varieties (Table 3). This discloses that the individual aroma potential of these grape varieties is related to the infinite combinations, concentrations, and/or synergetic effects of the various volatile compounds [55], which are modulated by environmental characteristics.
Similar to terpenic compounds, C13 norisoprenoids (Table 3) have very low sensory thresholds (e.g., 0.05 μg L−1 for β-damascenone, a compound determined in all samples), contributing to floral, fruity, and sweet grape aroma notes [55]. Moreover, three aromatic alcohols were determined in the assayed grape samples (Table 3). Although they are mainly produced during the yeast fermentation process, aromatic alcohols are an important chemical family that contribute floral and sweet notes to the wines [56,57]. Additionally, pre-fermentative compounds, such as six-carbon alcohols and aldehydes, were also determined (Table 3). These compounds can result from mechanical and/or technological operations (transport, crushing, maceration, and clarification) performed before the beginning of the fermentation process, being associated to herbaceous notes that, although deleterious, consumers appreciate in some wines [53].
Based on the volatile data (Tables S1–S12), differences can be noticed in relation to the volatile composition of each grape variety. Globally, the relative total free volatiles amount ranged from 12,656.8 to 33,529.7 × 104 (a.u.), of which from 1958.6 to 12,815.1 × 104 (a.u.) were varietal ones. In particular, the total varietal content was higher in the Sauvignon Blanc white variety (3196.8 to 12,815.1 × 104) and the Touriga Nacional red one (4073.2 to 7038.4 × 104) and lower in Bical (1958.6 to 6232.2 × 104) and Baga (2779.9 to 5855.5 × 104), a white and a red variety, respectively. Geraniol isomers (1 and 2) and linalool were the major monoterpenols determined in grapes from Sauvignon Blanc and Touriga Nacional varieties, respectively, accounting for 18–35% and 20–27% of the total monoterpenic GC×GC peak areas, respectively (Tables S5 and S11). These compounds have low sensory perception limits, being important to the general enhancement of the fruity, floral, and citric aromas of the resulting wines [58]. Moreover, previous studies also identified linalool as a major monoterpenic compound determined in Touriga Nacional wines, being considered an important varietal compound in the aroma of its wines [59]. However, the potential contribution of each grape volatile component to the aroma properties of the final wines is a combination of free and glycosidically linked forms. Highly variable amounts of the varietal glycosidically linked fractions were determined in the assayed varieties, being higher in white varieties (35.6–278.6 μg L−1), mainly in Sauvignon Blanc (91.2–278.6 μg L−1) followed by Bical (69.3–185.5 μg L−1), than in red ones (22.3–67.8 μg L−1), whose amount was quite similar among Baga and Castelão and a little higher in Touriga Nacional (34.6–67.8 μg L−1) (Table 2). The different volatile composition herein determined may result in different aroma sensory attributes of each variety, following the trend already described for the aroma of wines produced from these set of Bairrada grape varieties [60].
3.3. Statistic Tools to Evaluate Each Variety Oenological Potential
Following the individual parameters characterization previously described, the overall profiles were analyzed by applying a comprehensive approach that allowed the simultaneously verification of the potential effects of harvest and vineyard parcel characteristics on the oenological potential of the six assayed grape varieties. This was achieved by ASCA and applied for each variety to the set of parameters measured at technological maturity. The significance of these factors (harvest, parcel, and their interaction) was assessed using a permutation test (2000 permutations), and the resulting p-values are shown in Table 4.

Table 4.
Significance testing of factors harvest and parcel, for V. vinifera cv. Arinto, Bical, Sauvignon Blanc, Baga, Castelão, and Touriga Nacional varieties, determined at technological maturity.
According to Table 4, the harvest year effect was significant for all varieties under study (p-value < 0.0005), explaining ca. 54–68% of the total data set variance. Except for the Sauvignon Blanc variety, the vineyard parcel effect was also significant for the varieties under study (p-value < 0.05), explaining ca. 15–19% of the total data set variance, while the interaction effect was not statistically significant (p > 0.05; data not shown).
The harvest year and its weather conditions represent the largest source of the data variability (Table 4). As can be seen in Figure 2, the weather conditions of Bairrada Appellation have not changed significantly from 2010–2012 when compared to the last 10 years (2010–2020), which reinforces this finding. This was already expected, since the weather conditions of each harvest year were similar among the vineyard parcels present in the same area. Additionally, it was already shown that the formation of phenolic and volatile compounds, an object of study in this research work, result from a plant secondary metabolism in response to stress conditions caused by harvest weather changes [1].
- Arinto
For Arinto grapes, the effect of the harvest year accounted for 62.2% of total data set variance (Table 4). The first component of the scores plot separates the 2010 harvest (placed in PC1 positive) from the others (placed in PC1 negative), while the second component separates the 2011 (PC2 negative) and 2012 (PC2 positive) harvests (Figure 4a).
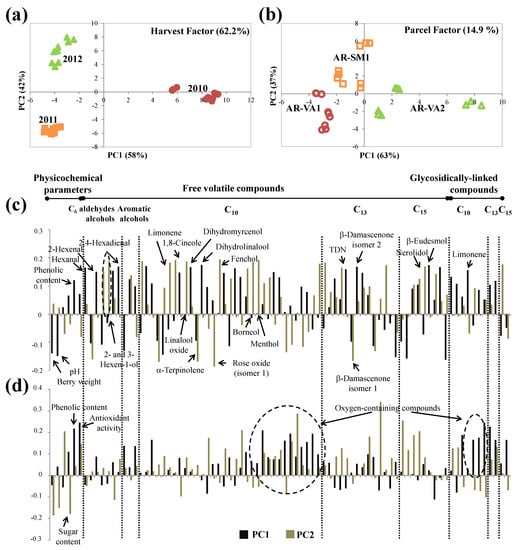
Figure 4.
ASCA scores plot for (a) harvest and (b) parcel factors, and the corresponding variable loadings plot (c,d), respectively, obtained for Arinto, at technological maturity (significance test reported on Table 4). Each variable is normalized separately by dividing by its standard deviation value.
According to the separation along PC1, 2010 grapes had lower berry weight and pH and higher phenolic content and higher diversity and number of volatiles (Figure 4c). The latter comprised mono- and sesquiterpenic oxygen-containing compounds, such as dihydromyrcenol, dihydrolinalool, nerolidol, and β-eudesmol, and C13 norisoprenoids, mainly TDN and β-damascenone (isomer 2). Additionally, the separation along PC2 of grapes from 2011 and 2012 harvests was mostly due to the higher content of six monoterpenic compounds (limonene, 1,8-cineole, linalool oxide, fenchol, borneol, and menthol) in grapes from 2012, while α-terpinolene and rose oxide (isomer 1) were higher in 2011 grapes. Varietal compounds are secondary plant metabolites modulated by the temperature and precipitation amount [61]. These results revealed that 2010 weather conditions seem to favor the Arinto varietal compound formation, suggesting that grapes from the 2010 harvest have higher aroma potential compared to the other two harvests. Additionally, three C6 aldehydes (hexanal, 2-hexenal, and 2,4-hexadienal) were determined in higher amounts in grapes from 2010, while higher content of two C6 alcohols (2- and 3-hexen-1-ol) was determined in grapes from 2012. The formation of C6 aldehydes and their reduction to the corresponding alcohols depends on the content of unsaturated lipids present in grapes and on the activities of lipoxygenase and alcohol dehydrogenase enzymes [61]. All these observations suggested that both the dry and warm conditions of 2011 and the fresher and rainier ones observed in 2012 (Figure 2) diminished Arinto grapes’ content of phenolics and volatiles. Previous works showed that high temperatures promoted significant reductions of the phenolic content of Cabernet-Sauvignon [62] and Merlot [63] grape varieties, as well as aroma quality losses [55], while higher precipitation promotes a decrease in the concentration of phenolics [64] and volatiles [9].
The effect of parcel characteristics on Arinto grapes accounted for 14.9% of the total data set variance (Table 4). Scores and loading plots are shown in Figure 4b,d, respectively. PC1 distinguishes AR-VA2, placed on the positive side of PC1, from the other two parcels, placed on the negative side of PC1 (AR-VA1) or near to the origin (AR-SM1) (Figure 4b). The same number of volatile compounds was found in grapes from all parcels (a total of 66 individual compounds); thus, the separation observed in the loadings plot (Figure 4d) was due to the different amounts determined for most of the varietal volatile compounds, in particular, monoterpenic ones, including free and glycosidically linked fractions, as well as total phenolic content and antiradical activity: Arinto grapes from AR-VA2 with higher contents were placed in PC1 positive, and grapes from AR-VA1, with lower ones, were placed in PC1 negative. Additionally, sugar content also allowed the distinguishing of grapes from AR-SM1 from the other two parcels, these grapes having lower sugar content. According to the main parcel characteristics (Table 1), the observed differences may be related to parcels’ soil type: soils with lower (clay–sandy soil: AR-VA2) and middle (clay–calcareous soil: AR-SM1) water-holding seem to favor formation of phenolics, varietal volatile compounds, and antiradical activity (Table 2, Tables S1 and S2). It was already shown that clay–calcareous and sandy soils increased the varietal content of Bairrada Fernão-Pires sparkling wines [3].
As the oenological potential of Arinto variety was different from one parcel to another, it is expected that Arinto grapes from AR-VA2 may have higher aroma potential, while AR-VA1 grapes may have the lowest one and AR-SM1 grapes with lower sugar content may produce wines with lower alcohol content. This revealed the metabolites’ plasticity of this autochthonous variety, which should be considered and exploited to produce a higher diversity of Arinto wines.
- Bical
The harvest effect on Bical grapes composition accounted for 53.8% of total data set variance (Table 4). According to the scores plot, all three harvests can be distinguished: the 2010 harvest was placed in PC1 positive, and the other two harvests were placed in PC1 negative (2011), near to the origin (2012) (Figure 5a).
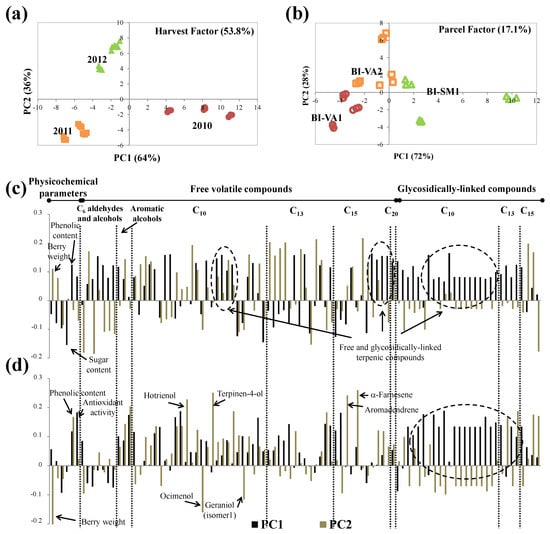
Figure 5.
ASCA scores plot for (a) harvest and (b) parcel factors, and the corresponding variable loadings plot (c,d), respectively, obtained for Bical, at technological maturity (significance test reported on Table 4). Each variable is normalized separately by dividing by its standard deviation value.
According to the loadings plot (Figure 5c), the separation of grapes from the 2010 harvest (PC1 positive) was mainly related to the lower sugar content, higher total phenolic content, and higher contents of mono- and sesquiterpenic compounds determined in the free fraction and to the monoterpenic compounds determined in the glycosidically linked fraction. On the other hand, lower amounts of these compounds were observed for grapes from 2011 harvest, which explains their position in PC1 negative. Additionally, higher sugar content was determined in grapes from the 2011 harvest and higher berry weight in grapes from 2012. The Bical variety was demonstrated to be very sensitive to different weather conditions; thus. higher aroma potential was expected for 2010 grapes and the opposite for 2011 grapes. Additionally, higher alcohol content was expected for grapes from 2011.
The scores plot obtained for the parcel factor accounting for 17.1% of variance (Table 4) is shown in Figure 5b, where parcels were distributed along the PC1 with BI-SM1 in PC1 positive and BI-VA1 in PC1 negative, and BI-VA2 was near to origin. Analysis of loadings (Figure 5d) showed that this distribution along PC1 was related to the content of monoterpenic compounds determined in free and glycosidically linked fractions and aromatic alcohols, and phenolic compounds and antiradical activity: higher amounts were determined for grapes from BI-SM1, while the opposite was observed for grapes from BI-VA1. Grapes from BI-SM1 not only exhibited higher content of free volatile monoterpenic compounds, which are very important for grape aroma and quality, but also higher amounts of these compounds in the glycosidically linked fraction. This suggests higher aroma potential for grapes from this parcel. The distribution observed along PC2 allowed distinguishing BI-VA2 (PC2 positive) from BI-VA1 (PC2 negative) (Figure 5b). According to the loadings plot (Figure 5d), this distinction was mainly due to the lower berry weight and higher amounts of hotrienol, terpinene-4-ol, aromadendrene, and α-farnesene in BI-VA2 grapes, while BI-VA1 exhibited higher amounts of ocimenol and geraniol (isomer 1).
Grapes from BI-SM1 and BI-VA2 parcels exhibited higher phenolic and volatile contents, as well as higher antiradical activity. Similarly to Arinto, the observed differences between vineyards may be related to the soil type: clay–sandy (BI-SM1) and clay–calcareous (BI-VA2) soils seem to favor the formation of varietal volatile and phenolic compounds and antiradical activity of Bical grapes. Additionally, the presence of pine trees at the East side of BI-VA1 parcel (Table 1) may reduce the sunlight exposure of grapes in the first hours of the morning, modulating BI-VA1 grapes phenolics and volatiles, affecting these grapes’ oenological potential mainly in terms of astringency and aroma properties. Lower sun exposure inhibits the synthesis and accumulation of terpenic compounds and C13 norisoprenoids in berries [65,66], while higher sunlight exposure leads to the higher levels of total phenolics [67]. This comprehensive approach demonstrated that Bical grapes exhibit a degree of plasticity with respect to their secondary metabolites and respond physiologically to the soil type and sunlight.
- Sauvignon Blanc
The comprehensive approach performed for Sauvignon Blanc grapes revealed that harvest and parcel factors explained ca. 68% and 12% of the total data set variance, respectively. However, only the effect of harvest was found to be significant (p-value < 0.0005), (Table 4, Figure 6). A recent study performed with New Zealand Sauvignon Blanc juices and wines also demonstrated that harvest year variations were more effectual than vineyard conditions [48], thus corroborating lesser compositional plasticity of this worldwide variety regarding vineyard parcel characteristics.
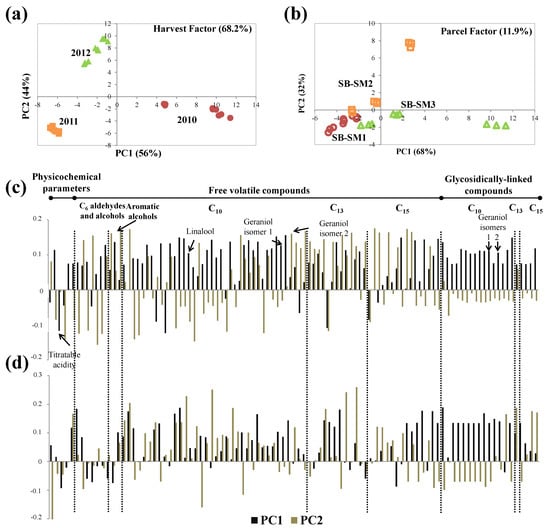
Figure 6.
ASCA scores plot for (a) harvest factor and (b) parcel factors, and the corresponding variable loadings plot (c,d), respectively, obtained for Sauvignon Blanc, at technological maturity (significance test reported on Table 4). Each variable is normalized separately by dividing by its standard deviation value.
According to the scores plot (Figure 6a), samples were distributed along PC1: the 2010 harvest was placed in PC1 positive, and the 2011 and 2012 harvests were placed in PC1 negative and near to origin, respectively. According to the loadings plot (Figure 6b), practically all measured parameters contributed to the sample separation along the PC1: 2010 grapes had the highest content of volatile compounds, while 2011 grapes had higher titratable acidity and lower volatile content, principally varietal one.
- Baga
The effect of the harvest year accounted for 61.4% of total data set variance (Table 4). The first component of scores plot separates the 2010 harvest (PC1 positive) from the others (PC1 negative), while the second component separates 2011 and 2012 harvests (Figure 7a). The loadings plot (Figure 7c) showed that 2010 was differentiated by the higher content of monoterpenic compounds. From these, β-ocimene, α-terpinolene, rose oxide, 1-terpineol, nerol oxide, digydrocarvone, menth-1-en-9-al, and carvone were detected only in 2010 grapes. The higher content of C13 norisoprenoids and sesquiterpenic compounds was responsible for the separation of the 2012 harvest from 2011 along the PC2. Additionally, grapes from 2011 (PC2 negative) also exhibited higher titratable acidity and sugar content compared to the other harvests. The obtained results highlighted the sensitivity of Baga grapes to weather conditions, which affect their oenological potential, mainly in terms of the final wines’ alcohol content, astringency, and aroma profile.
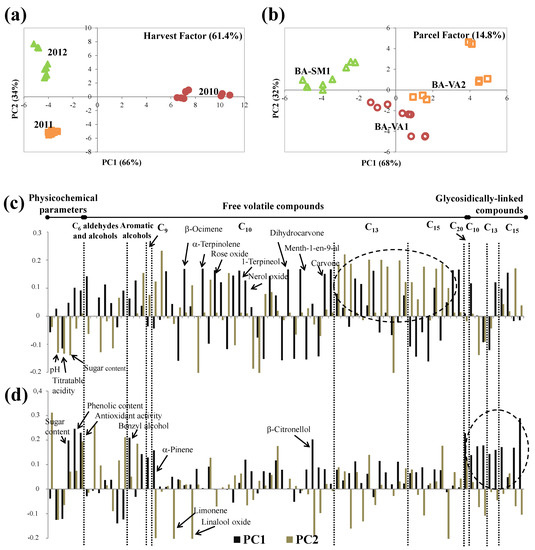
Figure 7.
ASCA scores plot for (a) harvest and (b) parcel factors, and the corresponding variable loadings plot (c,d), respectively, obtained for Baga, at technological maturity (significance test reported on Table 4). Each variable is normalized separately by dividing by its standard deviation value.
The effect of parcels on Baga grapes accounted for 14.8% of total data set variance (Table 4). Scores and loading plots are shown in Figure 7b,d, respectively. PC1 distinguishes BA-VA1, placed on the positive side of PC1, from BA-SM1 placed in PC1 negative, while BA-VA2 was near to the origin, but in PC1 positive. The corresponding loadings plot (Figure 7d) showed that this separation was due to the higher sugar and phenolic contents and antiradical activity, as well as higher varietal grapes components determined on the glycosidically linked fraction of BA-VA2 grapes compared to BA-SM1 grapes, suggesting higher aroma potential of BA-VA2 [61].
Moreover, PC2 allowed distinguishing BA-VA1 (PC2 negative) from BA-VA2 (near to origin) (Figure 7b). The loadings plot (Figure 7d) showed that this differentiation along the PC2 was mainly related to the higher BA-VA1 monoterpenic content, including α-pinene, limonene, linalool oxide, and β-citronellol. These compounds can contribute characteristic notes: α-pinene has fresh and citrus notes; limonene has lemon, orange, and sweet notes; linalool has fruity, floral, and rosy notes; and β-citronellol exhibits rose and lemon notes [58].
Considering the characteristics of Baga parcels (Table 1), soil type is the main factor that influences Baga grapes’ composition, which is strongly associated with the water status [9]: clay–calcareous (BA-VA2) and clayey (BA-VA1) soils seem to favor the formation of the varietal volatile and phenolic compounds and also the increase of the antiradical activity. It was already shown that soils with higher water-holding capacity than sand-based ones increased Baga sparkling wines volatiles [3] and Agiorgitiko oenological potential by increasing the concentration of anthocyanins and total phenolics in the berries [68].
Taken together, these data provide evidence that Baga variety exhibits a degree of plasticity within its secondary metabolites and responds physiologically to the soil type by modulating metabolites with potential aroma, color, astringency, and antioxidant properties.
- Castelão
The effect of the harvest year on Castelão grape composition accounted for 66.7% of the total data set variance (Table 4). According to the scores plot (Figure 8), first PC allowed distinguishing the 2010 harvest, placed in PC1 positive, from the other two harvests (PC1 negative). The corresponding loadings plot (Figure 8c) showed that the separation of 2010 grapes was related to the higher pH and phenolic content of these grapes and, principally, with the higher volatile content, especially the monoterpenic ones, which represented ca. 20% of the total volatiles, while in 2011 and 2012, this chemical family represented ca. 10% and 13%, respectively. Furthermore, PC2 allowed distinguishing 2012 (PC2 positive) from 2011 (PC2 negative) (Figure 8a). The loadings plot (Figure 8c) showed that the projection of 2011 in PC2 negative was mainly related to the lower values of almost all determined parameters compared to 2012, essentially oxygen-containing monoterpenic compounds (both free and glycosidically linked fractions). Additionally, 2012 grapes (PC2 positive) exhibited higher titratable acidity and lower sugar content. These results revealed the high sensitivity of the Castelão variety to the different weather conditions of each year: 2010 moderate weather conditions (Figure 2) seem to be proper for Castelão development, thus potentiating aroma and astringency to the final wines. The opposite was observed for 2011 grapes.
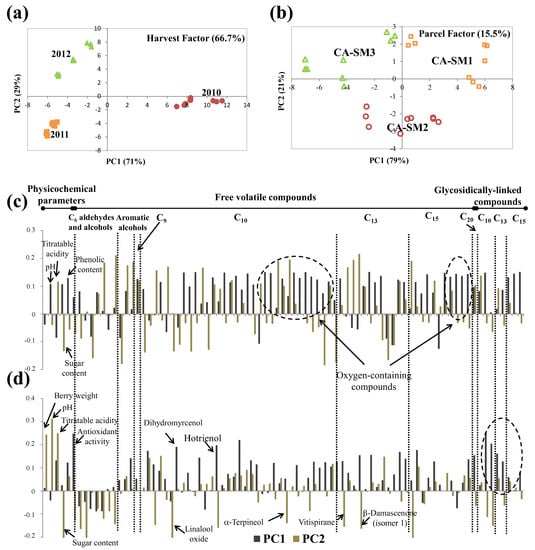
Figure 8.
ASCA scores plot for (a) harvest and (b) parcel factors, and the corresponding variable loadings plot (c,d), respectively, obtained for Castelão, at technological maturity (significance test reported on Table 4). Each variable is normalized separately by dividing by its standard deviation value.
The scores plot for the factor parcel accounted for 15.5% of the total data set variance (Table 4, Figure 8b). The different parcels were distributed along the PC1 with CA-SM1 situated in PC1 positive and CA-SM3 in PC1 negative. Analysis of loadings (Figure 8d) showed that this distribution along PC1 was related to the higher titratable acidity, antiradical activity, and higher content of some oxygen-containing monoterpenic compounds (dihydromyrcenol, hotrienol, and nerol oxide) and varietal glycosidically linked compounds of CA-SM1 grapes compared to CA-SM3 grapes. Additionally, along PC2, it was possible to distinguish CA-SM2 from the other parcels (PC2 negative), which were characterized by lower berry weight and pH; a higher amount of sugar, C6 alcohols, and aldehydes; and some varietal compounds such as linalool oxide, hotrienol, α-terpineol, vitispirane, and β-damascenone.
The differences between Castelão grapes’ composition were related to the parcels’ soil type (Table 1): clay–calcareous (CA-SM1) and clayey (CA-SM2) soils seem to favor the formation of the higher primary and secondary metabolites of Castelão grapes, revealing their high compositional sensitivity when exposed to different vineyard soils.
- Touriga Nacional
For Touriga Nacional variety, harvest year accounted for 59.3% of the total data set variance (Table 4). The PC1 of the scores plot allowed the separation of the 2010 harvest (PC1 positive) from the 2012 and 2011 harvests placed in PC1 negative side and near to the origin, respectively (Figure 9a).
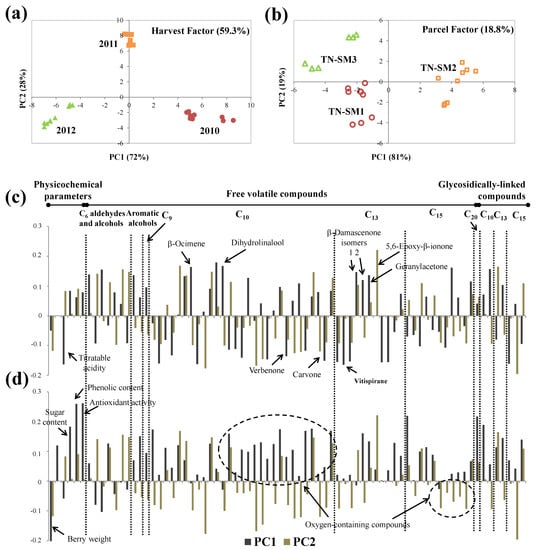
Figure 9.
ASCA scores plot for (a) harvest and (b) parcel factors, and the corresponding variable loadings plot (c,d), respectively, obtained for Touriga Nacional, at technological maturity (significance test reported on Table 4). Each variable is normalized separately by dividing by its standard deviation value.
The loadings plot (Figure 9c) showed that essentially aromatic alcohols, C13 norisoprenoids and monoterpenic compounds both in the free and glycosidically linked forms, contributed to the harvest differentiation along PC1. Grapes from the 2010 harvest had higher content of aromatic alcohols accounting for ca. 4% of the total GC×GC areas, but only for ca. 2.5% in grapes from 2011 and 2012. Higher amounts of two isomers of β-damascenone and geranylacetone were also found in 2010 grapes. Additionally, β-ocimene and dihydrolinalool were only determined in this harvest. In contrast, higher titratable acidity and lower amounts of monoterpenic compounds were determined in grapes from the 2012 harvest. Touriga Nacional has a late maturation (Figure S1) requiring good sunlight exposure for a long period to mature and develop its volatile characteristics. As 2012 was fresh and rainy, this may explain the lower volatile content and higher titratable acidity determined in 2012 grapes (Table 2, Tables S11 and S12). It was already shown that higher precipitation amounts may decrease malic acid respiration, resulting in higher acidity of the berries [69,70].
The effect of the parcel on Touriga Nacional grapes accounted for 18.8% of the total data set variance (Table 4). According to the scores plot, PC1 distinguishes TN-SM2 (PC1 positive) from the other two parcels, TN-SM1 and TN-SM3, both placed in PC1 negative (Figure 9a). The corresponding loadings plot (Figure 9c) showed that grapes from TN-SM2 (PC1 positive) had lower berry weight and higher content of sugars, phenolic and monoterpenic compounds, mainly the oxygen-containing ones, and higher antiradical activity. Additionally, PC2 distinguishes TN-SM1 (PC2 negative) from TN-SM3, which was characterized by higher content of varietal compounds, including mono- and sesquiterpenic compounds, and C13 norisoprenoids. Linalool, the major monoterpenic compound determined in all parcels, accounted for ca. 27% and 25% of the total monoterpenic GC×GC peak areas in grapes from TN-SM2 and TN-SM1, respectively, and only for ca. 14% in TN-SM3 grapes. According to the characteristics of these parcels (Table 1), higher altitude (ca. 70 m) and clay–calcareous soil of the TN-SM2 parcel seem to be beneficial for the higher varietal aroma potential of the Touriga Nacional red variety, as well as higher phenolic content and antiradical activity.
Despite the relevance of the ASCA analyses to evaluate each variety’s oenological potential under different weather and vineyard environmental conditions, to easily compare the varieties compositional behavior and biodiversity, a PCA was built displaying pairwise scores plot for the top three PCs joining the data of the six grape varieties and conditions under study (Figure 10a). Considering the parameters under study, Figure 10a shows that the Sauvignon Blanc variety is distinct from the five Bairrada Appellation autochthonous varieties, also revealing a higher similarity between the last ones. This comprehensive analysis also reveals that the compositional variability of Sauvignon Blanc grapes was more modulated by the harvest weather conditions than by the vineyards environment, corroborating the ASCA results (Figure 6).
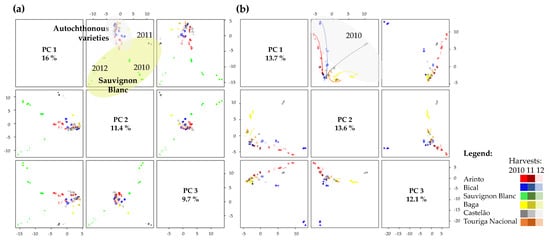
Figure 10.
Pairwise scores plot for the top three PCs for joined data of (a) the six varieties under study and (b) for the five autochthonous Bairrada Appellation. Each grape variety was identified with a different color and the color gradient code corresponds to the different harvests under study. For each vineyard parcel, a different shape code was automatically displayed by the MetaboAnalyst software.
Considering the pairwise scores plot only for the autochthonous varieties (Figure 10b), a distinction, among the first three PCs, were observed for the five varieties under study due to their different compositional profiles, which contributed to highlighting the peculiarities and typicity of each variety. This comprehensive analysis also confirms the results observed for the ASCA data processing of each variety, revealing the impact of the harvest on the variety composition, and the fact that samples collected in 2010 are more distinct, with those from 2011 and 2012 being more similar to each other.
4. Conclusions
The comprehensive approach herein used provides an overview of the oenological potential of different grape varieties based on their metabolic responses to different Bairrada environmental conditions. The composition of each autochthonous variety is dependent on the effect of the climatic conditions related to the harvest, as well as of vineyard characteristics. The composition of grapes from Sauvignon Blanc, a worldwide cultivated variety, seems to be mainly modulated by harvest year. Therefore, ASCA was revealed to be a valuable tool to underlie edaphoclimatic-dependent quality traits in different grape varieties, improving the interpretation of compositional plasticity of autochthonous grape ones. In this context, this research contributes to the understanding of the different varieties’ interaction with the environmental conditions and effects of the latter on the development of primary (sugars and organic acids) and secondary (phenolic and volatile compounds) grapes metabolites. This knowledge represents an important contribution for the maintenance of biodiversity and sustainability of the grape varieties, while helping to establish the typicity of autochthonous varieties. Additionally, the information generated under this research study can assist in the development of strategies to better exploit the autochthonous varieties in the production of a wide range of wines with different characteristics that can be produced from the same variety, which represent a step forward for the sustainability in the viticulture sector that should be extended to other wine regions worldwide.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11094003/s1: Figure S1: Sampling period performed for the six varieties under study, during maturation. For each variety, the sampling period was organized for each parcel (SM1 to SM3, VA1 and VA2) where the three harvest years (2010 to 2012) were represented. The first point for each variety, indicate grapes collection at half-véraison and * refers to technological maturity state. Figure S2: Berry weight, pH, sugar content, and titratable acidity of V. vinifera cv. Arinto, obtained during maturation, on the three parcels and three consecutive harvests. Technological maturity is indicated with a dash line. Figure S3: Berry weight, pH, sugar content, and titratable acidity of V. vinifera cv. Bical, obtained during maturation, on the three parcels and three consecutive harvests. Technological maturity is indicated with a dash line. Figure S4: Berry weight, pH, sugar content, and titratable acidity of V. vinifera cv. Sauvignon Blanc, obtained during maturation, on the three parcels and three consecutive harvests. Technological maturity is indicated with a dash line. Figure S5: Berry weight, pH, sugar content, and titratable acidity of V. vinifera cv. Baga, obtained during maturation, on the three parcels and three consecutive harvests. Technological maturity is indicated with a dash line. Figure S6: Berry weight, pH, sugar content, and titratable acidity of V. vinifera cv. Castelão, obtained during maturation, on the three parcels and three consecutive harvests. Maturity is indicated with a dash line. Figure S7. Berry weight, pH, sugar content, and titratable acidity of V. vinifera cv. Touriga Nacional, obtained during maturation, on the three parcels and three consecutive harvests. Technological maturity is indicated with a dash line. ** Overripe grapes. Table S1: Volatile components determined for Vitis vinifera L. cv. Arinto variety obtained from three vineyard parcels, at Bairrada Appellation, and from three years of harvest, at technological maturity. Table S2: Volatile components determined in the glycosidically linked fraction of mature grapes of Vitis vinifera L. cv. Arinto variety obtained from three vineyard parcels and three harvests under study, grouped by chemical classes. Table S3: Volatile components determined for Vitis vinifera L. cv. Bical variety obtained from three vineyard parcels, at Bairrada Appellation, and from three years of harvest, at technological maturity. Table S4: Volatile components determined in the glycosidically linked fraction of mature grapes of Vitis vinifera L. cv. Bical variety obtained from three vineyard parcels and three harvests under study, grouped by chemical classes. Table S5: Volatile components determined for Vitis vinifera L. cv. Sauvignon Blanc variety obtained from three vineyard parcels, at Bairrada Appellation, and from three years of harvest, at technological maturity. Table S6: Volatile components determined in the glycosidically linked fraction of mature grapes of Vitis vinifera L. cv. Sauvignon Blanc variety obtained from three vineyard parcels and three harvests under study, grouped by chemical classes. Table S7: Volatile components determined for Vitis vinifera L. cv. Baga variety obtained from three vineyard parcels, at Bairrada Appellation, and from three years of harvest, at technological maturity. Table S8: Volatile components determined in the glycosidically linked fraction of mature grapes of Vitis vinifera L. cv. Baga variety obtained from three vineyard parcels and three harvests under study, grouped by chemical classes. Table S9: Volatile components determined for Vitis vinifera L. cv. Castelão variety obtained from three vineyard parcels, at Bairrada Appellation, and from three years of harvest, at technological maturity. Table S10: Volatile components determined in the glycosidically linked fraction of mature grapes of Vitis vinifera L. cv. Castelão variety obtained from three vineyard parcels and three harvests under study, grouped by chemical classes. Table S11: Volatile components determined for Vitis vinifera L. cv. Touriga Nacional variety obtained from three vineyard parcels, at Bairrada Appellation, and from three years of harvest, at technological maturity. Table S12: Volatile components determined in the glycosidically linked fraction of mature grapes of Vitis vinifera L. cv. Touriga Nacional variety obtained from three vineyard parcels and three harvests under study, grouped by chemical classes.
Author Contributions
Conceptualization: S.P. and S.M.R.; methodology: S.P.; software, A.R.; validation: S.P., A.R. and S.M.R.; formal analysis: S.P.; investigation: S.P.; resources: S.M.R.; data curation: S.P.; writing—original draft preparation: S.P.; writing—review and editing: S.P., A.R., M.A.C. and S.M.R.; visualization: S.P. and S.M.R.; supervision: M.A.C. and S.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
Thanks are due to the University of Aveiro and FCT/MCTES, QREN, COMPETE for the financial support for the LAQV-REQUIMTE (UIDB/50006/2020) and CESAM (UIDP/50017/2020+UIDB/50017/2020) research units and CQ-VR at UTAD Vila Real (UIDP/00616/2020), through PT national funds, and when applicable, co-financed by FEDER, within the PT2020 Partnership Agreement and Compete 2020, and to the Portuguese NMR Network. Sílvia Petronilho thanks the PhD grant (SFRH/BDE/33805/2009) from FCT, Portugal, and Empresa Manuel dos Santos Campolargo, Herdeiros, and its FCT Post-doc grant (SFRH/BPD/117213/2016). This work is also supported by NORTE 2020, under the PT 2020 Partnership Agreement, through the ERDF and FSE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petronilho, S.; Barros, A.S.; Coimbra, M.A.; Rocha, S.M. Efficient Use of Non-renewable Natural Resources for Quality Wine through Sustainable Viticulture. In Agricultural Systems in the 21st Century; Nova Publishers: Hauppauge, NY, USA, 2013; pp. 195–230. [Google Scholar]
- Lamastra, L.; Fragkoulis, G.; Trevisan, M.; Capri, E. Enhancing the Ecosystem Services in Viticulture Farms: Approaches towards a Sustainable Management. In Environmental Management; Sarkar, S., Ed.; Sciyo InTech: Rijeka, Croatia, 2010; pp. 69–94. [Google Scholar]
- Coelho, E.; Coimbra, M.A.; Nogueira, J.M.F.; Rocha, S.M. Quantification approach for assessment of sparkling wine volatiles from different soils, ripening stages, and varieties by stir bar sorptive extraction with liquid desorption. Anal. Chim. Acta 2009, 635, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Suklje, K.; Carlin, S.; Antalick, G.; Blackman, J.; Deloire, A.; Vrhovsek, U.; Schmidtke, L. Regional Discrimination of Australian Shiraz Wine Volatome by Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry. J. Agricult. Food Chem. 2019, 67, 10273–10284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Kontoudakis, N.; Šuklje, K.; Antalick, G.; Blackman, J.W.; Rutledge, D.N.; Schmidtke, L.M.; Clark, A.C. Changes in Red Wine Composition during Bottle Aging: Impacts of Grape Variety, Vineyard Location, Maturity, and Oxygen Availability during Aging. J. Agric. Food Chem. 2020, 68, 13331–13343. [Google Scholar] [CrossRef]
- Anesi, A.; Stocchero, M.; Dal Santo, S.; Commisso, M.; Zenoni, S.; Ceoldo, S.; Tornielli, G.; Siebert, T.; Herderich, M.; Pezzotti, M.; et al. Towards a scientific interpretation of the terroir concept: Plasticity of the grape berry metabolome. BMC Plant Biol. 2015, 15, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.V.; Davis, R. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality—Rewiew. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Martinez, R.F.; Ascacibar, F.J.M.-d.-P.; Espinoza, A.V.P.; Lorza, R.L. Predictive modelling in grape berry weight during maturation process: Comparison of data mining, statistical and artificial intelligence techniques. Span. J. Agric. Res. 2011, 9, 1156–1167. [Google Scholar] [CrossRef]
- Marais, J.; Calitz, F.; Haasbroek, P.D. Relationship between microclimatic data, aroma component concentrations and wine quality parameters in the prediction of Sauvignon blanc wine quality. S. Afr. J. Enol. Vitic. 2001, 22, 22–26. [Google Scholar] [CrossRef]
- Santos, A.O.; Wample, R.L.; Sachidhanantham, S.; Kaye, O. Grape Quality Mapping for Vineyard Differential Harvesting. Braz. Arch. Biol. Technol. 2012, 55, 193–204. [Google Scholar] [CrossRef][Green Version]
- Santos, J.A.; Malheiro, A.C.; Karremann, M.K.; Pinto, J.G. Statistical modelling of grapevine yield in the Port Wine region under present and future climate conditions. Int. J. Biometeorol. 2011, 55, 119–131. [Google Scholar] [CrossRef]
- Niimi, J.; Tomic, O.; Naes, T.; Bastian, S.; Jeffery, D.; Nicholson, E.; Maffei, S.; Boss, P. Objective measures of grape quality: From Cabernet Sauvignon grape composition to wine sensory characteristics. Lwt Food Sci. Technol. 2020, 123, 109105–109114. [Google Scholar] [CrossRef]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schutz, B.; Bansbach, M.; Schafer, H.; Spraul, M. Targeted and Nontargeted Wine Analysis by H-1 NMR Spectroscopy Combined with Multivariate Statistical Analysis. Differentiation of Important Parameters: Grape Variety, Geographical Origin, Year of Vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Sun, D.; Gorecki, A.; Blaszczak, W.; Fornal, J.; Jelinski, T. Quantitative and predictive study of the evolution of wine quality parameters during high hydrostatic pressure processing. Innov. Food Sci. Emerg. 2013, 20, 81–90. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Rocha, S.; Legin, A.; Pereira, V.; Marques, J. Evaluation of the feasibility of the electronic tongue as a rapid analytical tool for wine age prediction and quantification of the organic acids and phenolic compounds. The case-study of Madeira wine. Anal. Chim. Acta 2010, 662, 82–89. [Google Scholar] [CrossRef]
- Firmani, P.; Vitale, R.; Ruckebusch, C.; Marini, F. ANOVA-Simultaneous Component analysis modelling of low-level-fused spectroscopic data: A food chemistry case-study. Anal. Chim. Acta 2020, 1125, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Marcheafave, G.G.; Tormena, C.D.; Mattos, L.E.; Liberatti, V.R.; Ferrari, A.B.S.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S.; Pauli, E.D. The main effects of elevated CO2 and soil-water deficiency on 1H NMR-based metabolic fingerprints of Coffea arabica beans by factorial and mixture design. Sci. Total Environ. 2020, 749, 142350–142360. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Hoefsloot, H.; van der Greef, J.; Timmerman, M.; Westerhuis, J.; Smilde, A. ASCA: Analysis of multivariate data obtained from an experimental design. J. Chemom. 2005, 19, 469–481. [Google Scholar] [CrossRef]
- Smilde, A.; Jansen, J.; Hoefsloot, H.; Lamers, R.; van der Greef, J.; Timmerman, M. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Meyners, M. Permutation tests: Are there differences in product liking? Food Qual. Pref. 2001, 12, 345–351. [Google Scholar] [CrossRef][Green Version]
- Vis, D.; Westerhuis, J.; Smilde, A.; van der Greef, J. Statistical validation of megavariate effects in ASCA. BMC Bioinform. 2007, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.; Hoefsloot, H.; Smit, S.; Vis, D.; Smilde, A.; van Velzen, E.; van Duijnhoven, J.; van Dorsten, F. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, S.; Delgadillo, I.; Coimbra, M. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. ‘Baga’ ripening. Anal. Chim. Acta 2006, 563, 204–214. [Google Scholar] [CrossRef]
- Perestrelo, R.; Petronilho, S.; Câmara, J.S.; Rocha, S.M. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction as a powerful tool for quantification of ethyl carbamate in fortified wines. The case study of Madeira wine. J. Chromatogr. A 2010, 1217, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Coutinho, P.; Barros, A.; Coimbra, M.A.; Delgadillo, I.; Cardoso, A.D. Aroma Potential of Two Bairrada White Grape Varieties: Maria Gomes and Bical. J. Agric. Food Chem. 2000, 48, 4802–4807. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Gonçalves Silva, D.; Rudnitskaya, A.; Almeida, A.; Rocha, S.M. Shedding light on Aspergillus niger volatile exometabolome. Sci. Rep. 2016, 6, 27441–27454. [Google Scholar] [CrossRef][Green Version]
- Rocha, S.M.; Freitas, R.; Cardoso, P.; Santos, M.; Martins, R.; Figueira, E. Exploring the potentialities of comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry to distinguish bivalve species: Comparison of two clam species (Venerupis decussata and Venerupis philippinarum). J. Chromatogr. A 2013, 1315, 152–161. [Google Scholar] [CrossRef]
- Mondello, L. HS-SPME-GCxGC-MS analysis of Yerba Mate (Ilex paraguariensis) in Shimadzu GC-GC application compendium of comprehensive 2D GC. Shimadzu Corp. 2012, 1, 1–29. [Google Scholar]
- Robinson, A.L.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Development of a sensitive non-targeted method for characterizing the wine volatile profile using HS-SPME/GC×GC-TOFMS. J. Chromatogr. A 2011, 1218, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Coelho, E.; Zrostlíková, J.; Delgadillo, I.; Coimbra, M.A. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry of monoterpenoids as a powerful tool for grape origin traceability. J. Chromatogr. A 2007, 1161, 292–299. [Google Scholar] [CrossRef]
- Jalali, H.T.; Petronilho, S.; Villaverde, J.J.; Coimbra, M.A.; Domingues, M.R.M.; Ebrahimian, Z.J.; Silvestre, A.J.D.; Rocha, S.M. Deeper insight into the monoterpenic composition of Ferula gummosa oleo-gum-resin from Iran. Ind. Crops Prod. 2012, 36, 500–507. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of Iranian geranium oil using gas chromatography-mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef]
- Kim, M.R.; Abd El-Aty, A.M.; Kim, I.S.; Shim, J.H. Determination of volatile flavor components in danggui cultivars by solvent free injection and hydrodistillation followed by gas chromatographic-mass spectrometric analysis. J. Chromatogr. A 2006, 1116, 259–264. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Differences in the fragrances of pollen and different floral parts of male and female flowers of Laurus nobilis. J. Agric. Food Chem. 2002, 50, 4647–4652. [Google Scholar] [CrossRef] [PubMed]
- Flach, A.; Dondon, R.C.; Singer, R.B.; Koehler, S.; Amaral, M.D.E.; Marsaioli, A.J. The chemistry of pollination in selected Brazilian maxillariinae orchids: Floral rewards and fragrance. J. Chem. Ecol. 2004, 30, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, S.; Rocha, S.M.; Ramírez-Chávez, E.; Molina-Torres, J.; Rios-Chavez, P. Assessment of the terpenic profile of Callistemon citrinus (Curtis) Skeels from Mexico. Ind. Crops Prod. 2013, 46, 369–379. [Google Scholar] [CrossRef]
- Radulović, N.; Dekić, M.; Stojanović-Radić, Z. Chemical composition and antimicrobial activity of the volatile oils of Geranium sanguineum L. and G. robertianum L. (Geraniaceae). Med. Chem. Res. 2012, 21, 601–615. [Google Scholar] [CrossRef]
- Mondello, L.; Costa, R. A new generation of GC capillary columns: SLB-5ms. Report 2006, 20, 17–19. [Google Scholar]
- Silva, I.; Rocha, S.M.; Coimbra, M.A.; Marriott, P.J. Headspace solid-phase microextraction combined with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry for the determination of volatile compounds from marine salt. J. Chromatogr. A 2010, 1217, 5511–5521. [Google Scholar] [CrossRef]
- Jalali, H.T.; Petronilho, S.; Villaverde, J.J.; Coimbra, M.A.; Domingues, M.R.M.; Ebrahimian, Z.J.; Silvestre, A.J.D.; Rocha, S.M. Assessment of the sesquiterpenic profile of Ferula gummosa oleo-gum-resin (galbanum) from Iran. Contributes to its valuation as a potential source of sesquiterpenic compounds. Ind. Crops Prod. 2013, 44, 185–191. [Google Scholar] [CrossRef]
- Petronilho, S.; Maraschin, M.; Delgadillo, I.; Coimbra, M.A.; Rocha, S.M. Sesquiterpenic composition of the inflorescences of Brazilian chamomile (Matricaria recutita L.): Impact of the agricultural practices. Ind. Crops Prod. 2011, 34, 1482–1490. [Google Scholar] [CrossRef]
- Robinson, C.J. A New Essential Oil—Agonis Fragrans; A Report for the Rural Industries Research and Development Corporation; RIRDC Publication: Barton, ACT, Australia, 2006. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Boulton, R. The general relationship between potassium, sodium and pH in grape juice and wine. Am. J. Enol. Vitic. 1980, 32, 182–186. [Google Scholar]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality. Food 2007, 1, 1–22. [Google Scholar]
- Pinu, F.; Tumanov, S.; Grose, C.; Raw, V.; Albright, A.; Stuart, L.; Villas-Boas, S.; Martin, D.; Harker, R.; Greven, M. Juice Index: An integrated Sauvignon Blanc grape and wine metabolomics database shows mainly seasonal differences. Metabolomics 2019, 15, 3–21. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Mateus, N.; Machado, J.; de Freitas, V. Development changes of anthocyanins in Vitis vinifera grapes grown in the Douro Valley and concentration in respective wines. J. Sci. Food Agric. 2002, 82, 1689–1695. [Google Scholar] [CrossRef]
- Mateus, N.; Marques, S.; Goncalves, A.; Machado, J.; De Freitas, V. Proanthocyanidin composition of red Vitis vinifera varieties from the Douro valley during ripening: Influence of cultivation altitude. Am. J. Enol. Viticult. 2001, 52, 115–121. [Google Scholar]
- López-Tamames, E.; Carro-Marinõ, N.; Gunata, Y.Z.; Sapis, C.; Baumes, R.; Bayonove, C. Potential Aroma in Several Varieties of Spanish Grapes. J. Agric. Food Chem. 1997, 45, 1729–1735. [Google Scholar] [CrossRef]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazarotto, M.; Zini, C.A. Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A 2012, 1226, 124–139. [Google Scholar] [CrossRef]
- Gonzalez-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The Chemistry of Wine and Stabilization and Treatments. In Handbook of Enology; Ribéreau-Gayon, P., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2006; pp. 205–230. [Google Scholar]
- Moreno-Arribas, M.V.; Polo, C. Wine Chemistry and Biochemistry; Springer Science+Business Media, LLC: New York, USA, 2009. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications, 4th ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Marais, I. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–60. [Google Scholar] [CrossRef]
- Pinho, P.G.; Falqué, E.; Castro, M.; Silva, H.O.; Machado, B.; Ferreira, A.C.S. Further Insights into the Floral Character of Touriga Nacional Wines. J. Food Sci. 2007, 72, S396–S401. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef]
- Bakker, J.; Clarke, J.R. Wine Flavour Chemistry, 2nd ed.; Wiley-Blackwell, Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2012. [Google Scholar]
- Goto-Yamamoto, N.; Mori, K.; Numata, M.; Koyama, K.; Kitayama, M. Effects of Temperature and Water Regimes on Flavonoid Contents and Composition in the Skin of Red-Wine Grapes. J. Int. Sci. Vigne Vin. 2009, 43, 75–80. [Google Scholar]
- Spayd, S.; Tarara, J.; Mee, D.; Ferguson, J. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Keller, M.; Smith, J.; Bondada, B. Ripening grape berries remain hydraulically connected to the shoot. J. Exp. Bot. 2006, 57, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Skinkis, P.; Bordelon, B.; Butz, E. Effects of Sunlight Exposure on Berry and Wine Monoterpenes and Sensory Characteristics of Traminette. Am. J. Enol. Vitic. 2010, 61, 147–156. [Google Scholar]
- Zhang, H.; Fan, P.; Liu, C.; Wu, B.; Li, S.; Liang, Z. Sunlight exclusion from Muscat grape alters volatile profiles during berry development. Food Chem. 2014, 164, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and UV radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J. Agr. Food Chem. 2006, 54, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Bondada, B.; Keller, M. Not All Shrivels Are Created Equal—Morpho-Anatomical and Compositional Characteristics Differ among Different Shrivel Types That Develop during Ripening of Grape (Vitis vinifera L.) Berries. Am. J. Plant Sci. 2012, 3, 879–898. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Seguin, G. The concept of terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).