Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria fruticosa, Achillea fragrantissima, and Phoenix dactylifera
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Screening of Pollen Extracts of Selected Species from Palestine
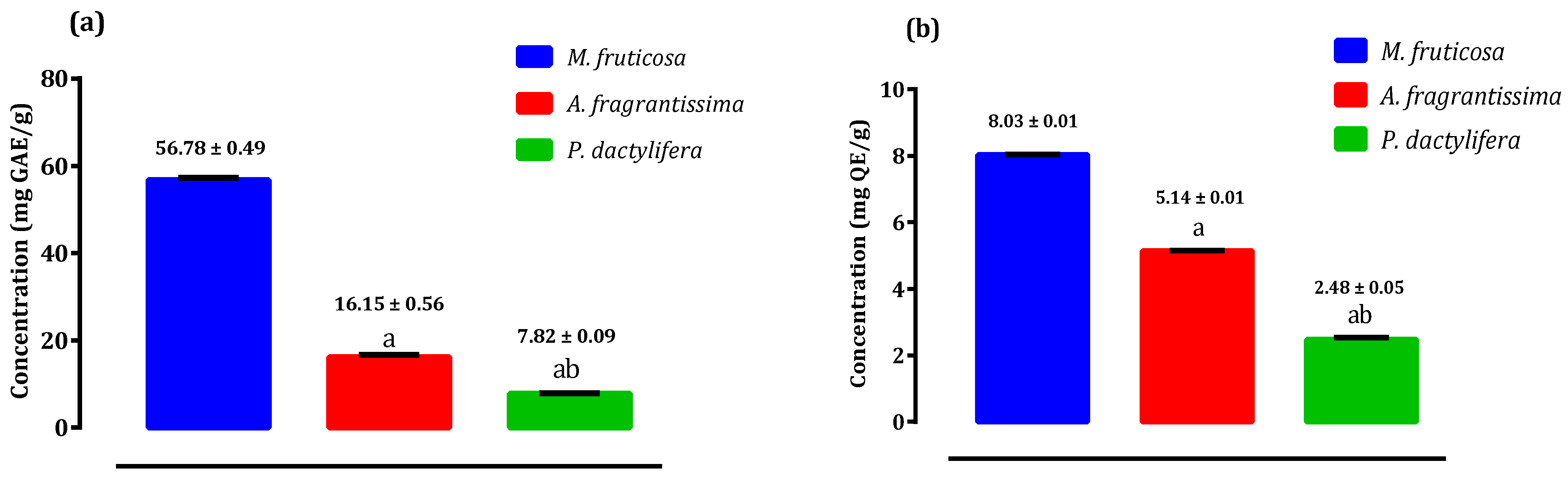
2.2. Antioxidant Potential of Pollen Extracts of Selected Species from Palestine
2.3. Antioxidant Activity of Pollen Extracts of Selected Species from Palestine
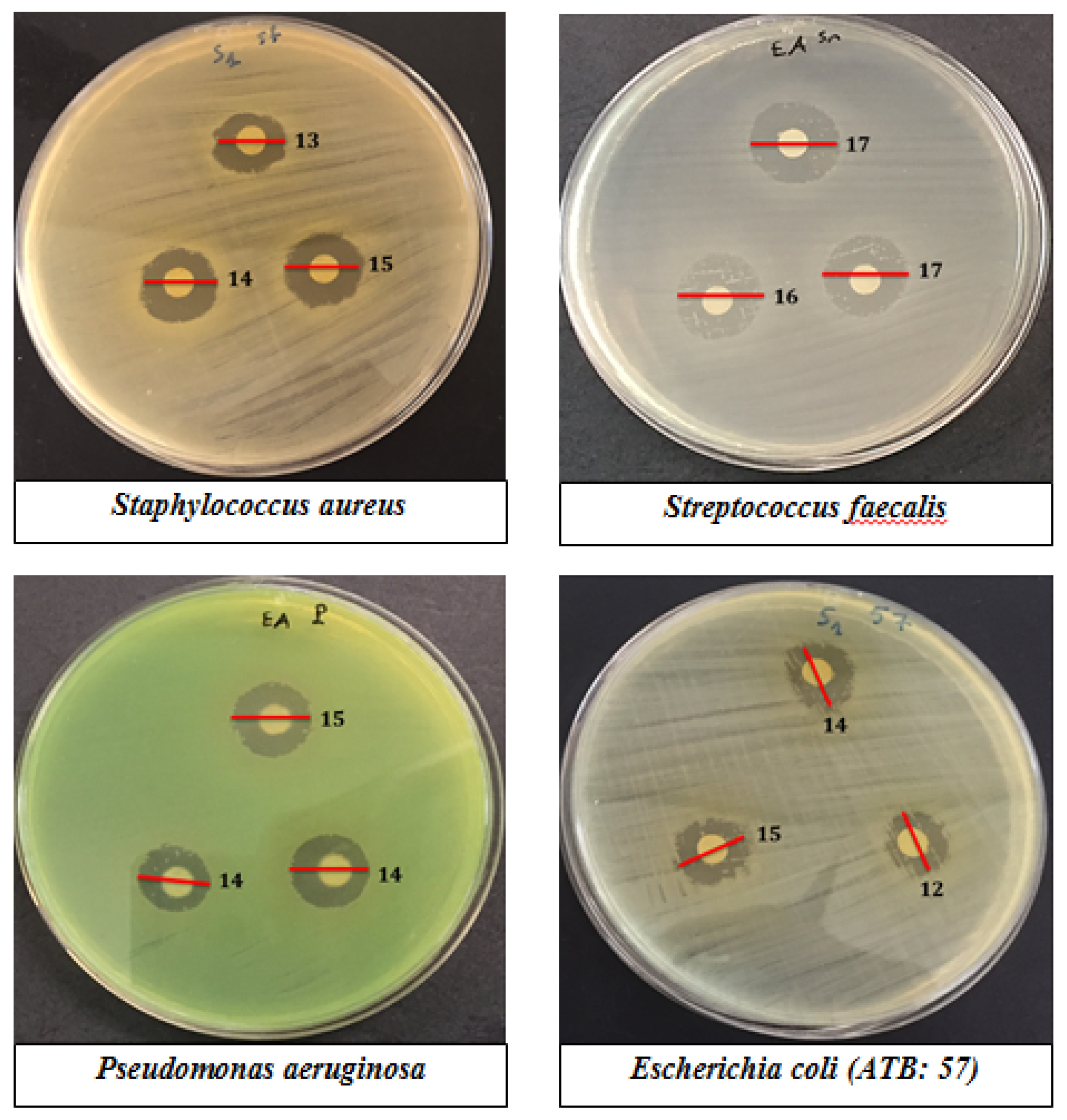
2.4. Antibacterial Activity of Pollen Extracts of Selected Species from Palestine
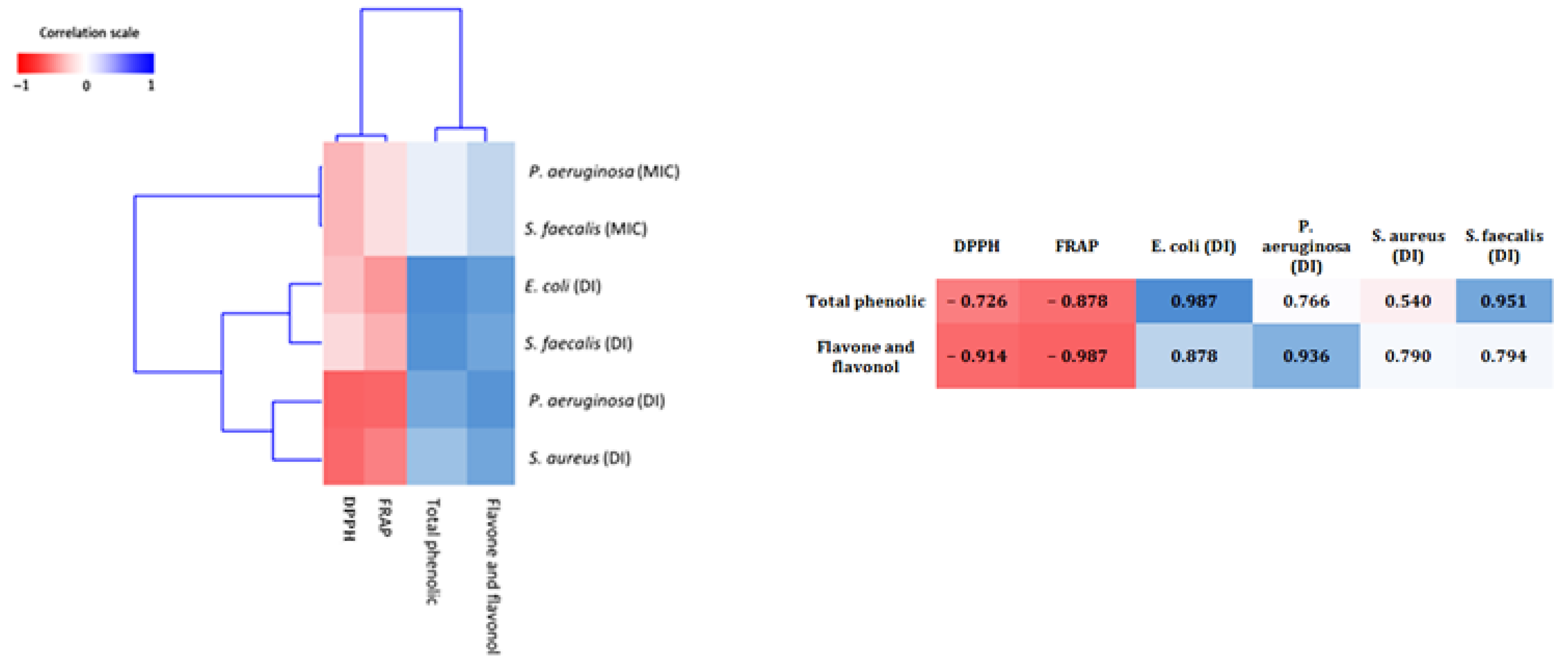
2.5. Correlations Analysis
3. Discussion
4. Materials and Methods
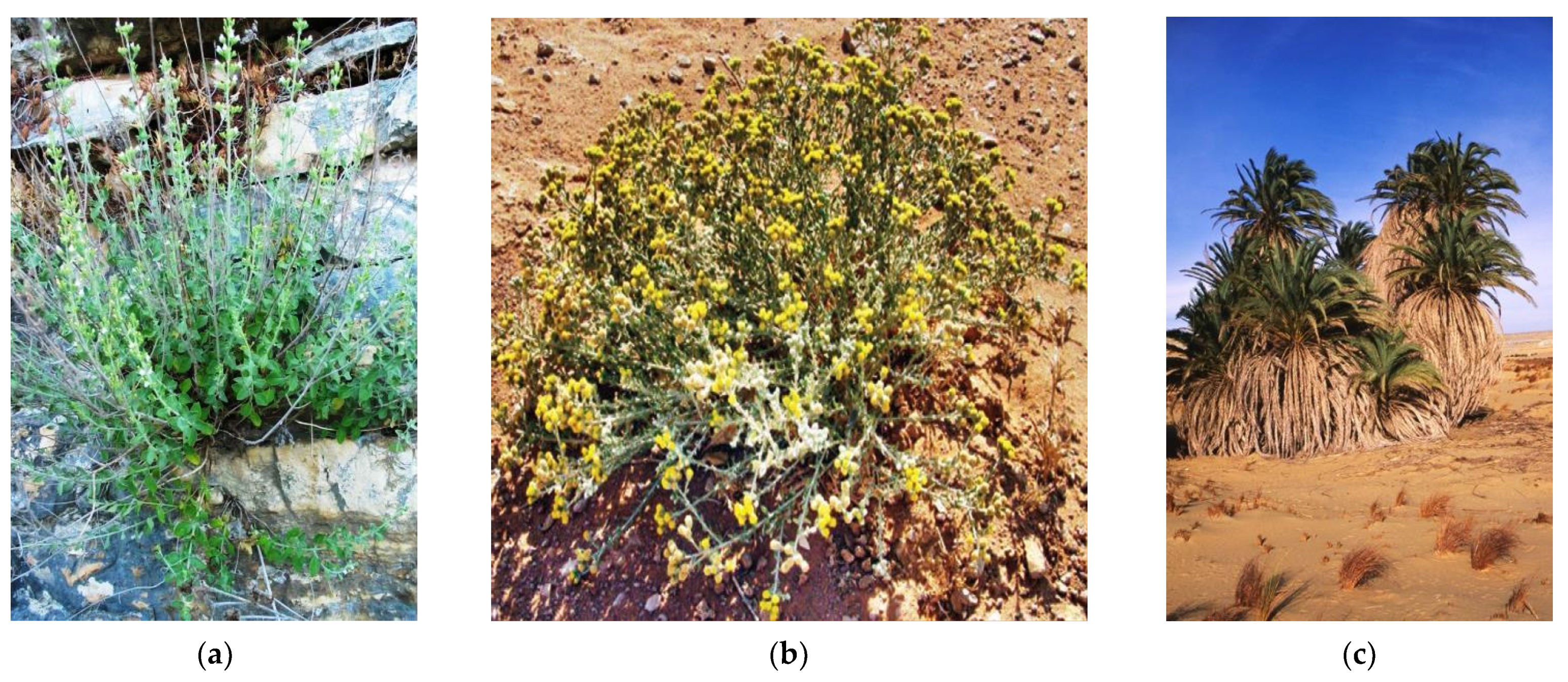
4.1. Species
4.2. Extracts Preparation
4.3. Phytochemical Screening of Pollen Extracts of Selected Species from Palestine
4.3.1. Test for Tannins
4.3.2. Test for Catechin Tannins
4.3.3. Test for Gallic Tannins
4.3.4. Test for Flavonoids
4.3.5. Test for Sterols (Shalkowski Test)
4.3.6. Test for Alkaloids
4.3.7. Test for Saponosides
4.3.8. Test for Oses and Holosides
4.3.9. Test for Mucilage
4.3.10. Test for Cardiac Glycosides
4.4. Antioxidant Potential of Pollen Extracts of Selected Species from Palestine
4.4.1. Total Phenolics Content Determination
4.4.2. Total Flavone and Flavonol Content
4.5. Antioxidant Activity of Pollen Extracts of Selected Species from Palestine
4.5.1. Scavenging 1,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Capacity
4.5.2. The Reducing Power Assay (FRAP)
4.6. Antibacterial Activity of Pollen Extracts of Selected Species from Palestine
4.6.1. Bacterial Strains and Inoculums Standardization
4.6.2. Agar Well Diffusion (AWD) Assay
4.6.3. Minimal Bactericidal Concentration (MBC) and Minimum Inhibitory Concentration (MIC)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, U.; Jacobo-Herrera, N.J.; Altemimi, A.B.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; El Moussaoui, A.; Cerruti, P.; Avella, M.; Grafov, A.; Bousta, D. Marketing and legal status of phytomedicines and food supplements in Morocco. J. Complement. Integr. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.; dos Santos, S.A.; Andrade, L.N.; Severino, P.; Carvalho, A.A. Natural Products as Treatment against Cancer: A Historical and Current Vision. Clin. Oncol. 2019, 4, 1562. [Google Scholar]
- Hall, W. Superbugs: An Arms Race against Bacteria; Harvard University Press: Cambridge, MA, USA, 2018; ISBN 0-674-98507-9. [Google Scholar]
- Yelin, I.; Kishony, R. Antibiotic Resistance. Cell 2018, 172, 1136–1136.e1. [Google Scholar] [CrossRef]
- Daraghmeh, J.; Imtara, H. In Vitro Evaluation of Palestinian Propolis as a Natural Product with Antioxidant Properties and Antimicrobial Activity against Multidrug-Resistant Clinical Isolates. Available online: https://www.hindawi.com/journals/jfq/2020/8861395/ (accessed on 15 February 2021).
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey Antibacterial Effect Boosting Using Origanum vulgare L. Essential Oil. Available online: https://www.hindawi.com/journals/ecam/2018/7842583/ (accessed on 15 February 2021).
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Cock, I.; Cheesman, M.; Ilanko, A.; Blonk, B. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Imtara, H.; Kmail, A.; Touzani, S.; Khader, M.; Hamarshi, H.; Saad, B.; Lyoussi, B. Chemical Analysis and Cytotoxic and Cytostatic Effects of Twelve Honey Samples Collected from Different Regions in Morocco and Palestine. Available online: https://www.hindawi.com/journals/ecam/2019/8768210/ (accessed on 16 February 2021).
- Thomas, C. Oxygen Radicals and the Disease Process; CRC Press: Boca Raton, FL, USA, 1998; ISBN 978-90-5702-226-5. [Google Scholar]
- Cardenas-Rodriguez, N.; Huerta-Gertrudis, B.; Rivera-Espinosa, L.; Montesinos-Correa, H.; Bandala, C.; Carmona-Aparicio, L.; Coballase-Urrutia, E. Role of Oxidative Stress in Refractory Epilepsy: Evidence in Patients and Experimental Models. Int. J. Mol. Sci. 2013, 14, 1455–1476. [Google Scholar] [CrossRef] [Green Version]
- Qusti, S.Y.; Abo-khatwa, A.N.; Lahwa, M.B. Screening of Antioxidant Activity and Phenolic Content of Selected Food Items Cited in the Holly Quran. EJBS 2010, 2, 40–51. [Google Scholar]
- Tabti, L.; Dib, M.E.A.; Gaouar, N.; Samira, B.; Tabti, B. Antioxidant and Antifungal Activity of Extracts of the Aerial Parts of Thymus capitatus (L.) Hoffmanns against Four Phytopathogenic Fungi of Citrus sinensis. Jundishapur J. Nat. Pharm. Prod. 2014, 9, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Dudai, N.; Larkov, O.; Ravid, U.; Putievsky, E.; Lewinsohn, E. Developmental Control of Monoterpene Content and Composition in Micromeria fruticosa (L.) Druce. Ann. Bot. 2001, 88, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Abu-Rabia, A. Ethno-Botanic Treatments for Paralysis (Falij) in the Middle East. Chin. Med. 2012, 3, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Yaniv, Z. Introduction: Medicinal Plants in Ancient Traditions. In Medicinal and Aromatic Plants of the Middle-East; Yaniv, Z., Dudai, N., Eds.; Medicinal and Aromatic Plants of the World; Springer: Dordrecht, The Netherlands, 2014; pp. 1–7. ISBN 978-94-017-9276-9. [Google Scholar]
- EBSCOhost|79348743|Constituents and Biological Activity of the Essential Oil and the Aqueous Extract of Micromeria fruticosa (L.) Druce subsp. serpyllifolia. Available online: https://web.b.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=1011601X&AN=79348743&h=Mqk0iip1Rdw2z4fdleqUtPNNcH69fqoWRpdkUJBtu1Imtg6ZI%2fumnnfM248q6Y%2fcSS7w9roD6asE3VDSfcSqlg%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d1011601X%26AN%3d79348743 (accessed on 18 December 2020).
- Gulluce, M.; Sökmen, M.; Sahin, F.; Sokmen, A.; Adiguzel, A.; Özer, H. Biological activities of the essential oil and methanolic extract of Micromeria fruticosa (L) Druce ssp serpyllifolia (Bieb) PH Davis plants from the eastern Anatolia region of Turkey. J. Sci. Food Agric. 2004, 84, 735–741. [Google Scholar] [CrossRef]
- Abu-Gharbieh, E.; Ahmed, N.G. Bioactive content, hepatoprotective and antioxidant activities of whole plant extract of Micromeria fruticosa (L) Druce ssp Serpyllifolia F Lamiaceae against Carbon tetrachloride-induced hepatotoxicity in mice. Trop. J. Pharm. Res. 2016, 15, 2099. [Google Scholar] [CrossRef] [Green Version]
- Al-Hamwi, M.; Aboul-Ela, M.; El-Lakany, A.; El Achi, N.; Ghanem, N.; El Hamaoui, B.; Bakkour, Y.; El Omar, F. Chemical Composition, Antimicrobial and Antioxidant Activities of the Ethanolic Extract of Micromeria fruticosa Growing in Lebanon. Int. J. Chem. Sci. 2015, 13, 325–335. [Google Scholar]
- Patocka, J. Achillea Fragrantissima: Pharmacology Review. Clin. Oncol. 2019, 4, 5. [Google Scholar]
- Hussain, M.I.; Farooq, M.; Syed, Q.A. Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.)—A review. Food Biosci. 2020, 34, 100509. [Google Scholar] [CrossRef]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabou, A.F.N.A.; Radwan, E. The current status of the date palm (Phoenix dactylifera) and its uses in the Gaza Strip, Palestine. Biodiversitas J. Biol. Divers. 2017, 18, 1047–1061. [Google Scholar] [CrossRef]
- Baliga, M.S.; Baliga, B.R.V.; Kandathil, S.M.; Bhat, H.P.; Vayalil, P.K. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res. Int. 2011, 44, 1812–1822. [Google Scholar] [CrossRef]
- Vayalil, P.K. Antioxidant and Antimutagenic Properties of Aqueous Extract of Date Fruit (Phoenix dactylifera L. Arecaceae). J. Agric. Food Chem. 2002, 50, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Saafi, E.B.; Louedi, M.; Elfeki, A.; Zakhama, A.; Najjar, M.F.; Hammami, M.; Achour, L. Protective effect of date palm fruit extract (Phoenix dactylifera L.) on dimethoate induced-oxidative stress in rat liver. Exp. Toxicol. Pathol. 2011, 63, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Ishurd, O.; Kennedy, J.F. The anti-cancer activity of polysaccharide prepared from Libyan dates (Phoenix dactylifera L.). Carbohydr. Polym. 2005, 59, 531–535. [Google Scholar] [CrossRef]
- Al-Qarawi, A.; Ali, B.; Al-Mougy, S.; Mousa, H. Gastrointestinal transit in mice treated with various extracts of date (Phoenix dactylifera L.). Food Chem. Toxicol. 2003, 41, 37–39. [Google Scholar] [CrossRef]
- Al-Ali, S.; Al-Judaibi, A. Biochemical and Molecular Effects of Phoenix dactylifera and Ziziphus spina-christi Extracts on Candida albicans. J. Biosci. Med. 2019, 7, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Abu-Reidah, I.M.; Arráez-Román, D.; Al-Nuri, M.; Warad, I.; Segura-Carretero, A. Untargeted metabolite profiling and phytochemical analysis of Micromeria fruticosa L. (Lamiaceae) leaves. Food Chem. 2019, 279, 128–143. [Google Scholar] [CrossRef]
- El-Ashmawy, I.M.; Al-Wabel, N.A.; Bayad, A.E. Achillea fragrantissima, rich in flavonoids and tannins, potentiates the activity of diminazine aceturate against Trypanosoma evansi in rats. Asian Pac. J. Trop. Med. 2016, 9, 228–234. [Google Scholar] [CrossRef] [Green Version]
- El-Shazly, A.M.; El-Shazly, A.M.; Hafez, S.S.; Wink, M. Comparative Study of the Essential Oils and Extracts of Achillea fragrantissima (Forssk.) Sch. Bip. and Achillea santolina L. (Asteraceae) from Egypt. Die Pharm. Int. J. Pharm. Sci. 2004, 59, 226–230. [Google Scholar]
- Iqbal, E.; Abu Salim, K.; Lim, L.B. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. Sci. 2015, 27, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Al-Samarai, A.H.; Al-Salihi, F.G.; Al-Samarai, R.R. Phytochemical Constituents and Nutrient Evaluation of Date Palm (Phoenix Dactylifera, L.) Pollen Grains. Tikrit J. Pure Sci. 2018, 21, 56–62. [Google Scholar]
- Farouk, A.; Metwaly, A.; Mohsen, M. Chemical Composition and Antioxidant Activity of Date Palm Pollen Grains (Phoenix dactylifera L. Palmae) Essential Oil for Siwe Cultivar Cultivated in Egypt. Middle East J. Appl. Sci. 2015, 5, 945–949. [Google Scholar]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Chemical Composition and Antioxidant Activity of Walnut Pollen Samples. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 361–365. [Google Scholar] [CrossRef]
- Bakour, M.; Campos, M.D.G.; Imtara, H.; Lyoussi, B. Antioxidant content and identification of phenolic/flavonoid compounds in the pollen of fourteen plants using HPLC-DAD. J. Apic. Res. 2019, 59, 35–41. [Google Scholar] [CrossRef]
- Kchaou, W.; Abbès, F.; Attia, H.; Besbes, S. In Vitro Antioxidant Activities of Three Selected Dates from Tunisia (Phoenix dactylifera L.). J. Chem. 2014, 2014, 367681. Available online: https://www.hindawi.com/journals/jchem/2014/367681/ (accessed on 18 December 2020). [CrossRef] [Green Version]
- El-Kholy, W.M.; Soliman, T.N.; Darwish, A.M.G. Evaluation of date palm pollen (Phoenix dactylifera L.) encapsulation, impact on the nutritional and functional properties of fortified yoghurt. PLoS ONE 2019, 14, e0222789. [Google Scholar] [CrossRef]
- Biglari, F.; Alkarkhi, A.F.; Easa, A.M. Cluster analysis of antioxidant compounds in dates (Phoenix dactylifera): Effect of long-term cold storage. Food Chem. 2009, 112, 998–1001. [Google Scholar] [CrossRef]
- Alasalvar, C.; Al-Farsi, M.; Quantick, P.C.; Shahidi, F.; Wiktorowicz, R. Effect of chill storage and modified atmosphere packaging (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots. Food Chem. 2005, 89, 69–76. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Liu, S.-C.; Lin, J.-T.; Wang, C.-K.; Chen, H.-Y.; Yang, D.-J. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009, 114, 577–581. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian Plants and Derived Products against Microbial Infections: A Review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [Green Version]
- Ndamane, Y.; Kambizi, L.; Songca, S.; Oluwafemi, O. Antibacterial Effectiveness of Tetradenia riparia Extract, a Plant Traditionally Used in the Eastern Cape Province to Treat Diseases of the Respiratory System. J. Med. Plant Res. 2013, 7, 2755–2760. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Salem, M.Z.; El-Hefny, M.; El-Kareem, M.S.A.; El-Shanhorey, N.A.; Mohamed, A.A.; Salem, A.Z. Antibacterial activity of the bioactive compounds identified in three woody plants against some pathogenic bacteria. Microb. Pathog. 2018, 121, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Flavonoids: An Overview|Journal of Nutritional Science|Cambridge Core. Available online: https://www.cambridge.org/core/journals/journal-of-nutritional-science/article/flavonoids-an-overview/C0E91D3851345CEF4746B10406908F52 (accessed on 18 December 2020).
- Omojate Godstime, C.; Enwa Felix, O.; Jewo Augustina, O.; Eze Christopher, O. Mechanisms of Antimicrobial Actions of Phytochemicals against Enteric Pathogens—A Review. J. Pharm. Chem. Biol. Sci. 2014, 2, 77–85. [Google Scholar]
- Dohou, R.; Yamni, K.; Tahrouch, S.; Hassani, L.I.; Badoc, A.; Gmira, N. Screening Phytochimique d’une Endémique Iberomarocaine, Thymelaea Lythroides. Bulletin-Société de Pharmacie de Bordeaux 2003, 142, 61–78. [Google Scholar]
- Bankole, A.E.; Adekunle, A.A.; Sowemimo, A.A.; Umebese, C.E.; Abiodun, O.; Gbotosho, G.O. Phytochemical screening and in vivo antimalarial activity of extracts from three medicinal plants used in malaria treatment in Nigeria. Parasitol. Res. 2016, 115, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Senhaji, O.; Faid, M.; Elyachioui, M.; Dehhaoui, M. Étude de l’activité antifongique de divers extraits de cannelle. J. Mycol. Méd. 2005, 15, 220–229. [Google Scholar] [CrossRef]
- N’Guessan, K.; Kadja, B.; Zirihi, G.; Traoré, D.; Ake-Assi, L. Screening phytochimique de quelques plantes médicinales ivoiriennes utilisées en pays Krobou (Agboville, Côte-d’Ivoire). Sci. Nat. 2009, 6. [Google Scholar] [CrossRef] [Green Version]
- Tamert, A.; Latreche, A.; Aouad, L. Criblage phytochimique et activité antimicrobienne des extraits de Thymus serpyllum et de Thymus vulgaris du mont de Tessala (Algérie occidentale). Phytothérapie 2017, 15, 384–394. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Physicochemical characterization and antioxidant activity of Palestinian honey samples. Food Sci. Nutr. 2018, 6, 2056–2065. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/fsn3.754 (accessed on 18 December 2020). [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci. Technol. 2014, 34, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Peterson, L.R.; Collins, S.M. Bactericidal testing for infectious disease therapy: The why and how to measure if we kill the bugs. Clin. Microbiol. Newsl. 2000, 22, 153–157. [Google Scholar] [CrossRef]

| Assay | Species | |||
|---|---|---|---|---|
| Micromeria fruticosa | Achilleafra grantissima | Phoenix dactylifera | ||
| Tannins | +++ | +++ | +++ | |
| Catechin tannins | ++ | +++ | − | |
| Gallic tannins | +++ | ++ | + | |
| Flavonoids | +++ (red = flavonols, flavanols) | +++ (orange-pink = flavones) | +++ (purple rose = flavonones) | |
| Sterol | +++ | +++ | ++ | |
| Alkaloids | Dragendorff test | +++ | ++ | + |
| Mayer test | − | − | − | |
| Saponosides | + | + | − | |
| Cardiac glycosides | +++ | +++ | + | |
| Oses and holosides | ++ | +++ | − | |
| Mucilage | − | − | − | |
| IC50 (mg/mL) | ||
|---|---|---|
| Species | DPPH (1,2-Diphenyl-1-Picrylhydrazyl) | FRAP |
| Micromeria fruticosa | 0.047 ± 0.003 | 0.039 ± 0.0003 |
| Achillea fragrantissima | 0.295 ± 0.002 a | 0.248 ± 0.007 a |
| Phoenix dactylifera | 1.820 ± 0.027 a, b | 0.585 ± 0.026 a, b |
| BHT (butylated hydroxytoluene) | 0.061 ± 0.0002 b, c | - |
| Ascorbic acid | - | 0.09 ± 0.0001 a, b, c |
| Bacterial Strains | Micromeria fruticosa | Achillea fragrantissima | Phoenix dactylifera |
|---|---|---|---|
| E. coli (ATB: 57) | 13.66 ± 1.5 | ND | ND |
| P. aeruginosa | 15.66 ± 1.5 | 15.33 ± 1.15 | 14 ± 1 |
| S. aureus | 14.33 ± 0.6 | 16.33 ± 0.6 | ND |
| S. faecalis | 16.33 ± 1.5 | 14.33 ± 1.15 | 14.66 ± 0.6 |
| Ethanol (70%) | ND | ND | ND |
| Species | Escherichia coli (ATB: 57) | Pseudomonas aeruginosa | Staphylococcus aureus | Streptococcus faecalis | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Micromeria fruticosa | 10 | >10 | 5 | >10 | 5 | 5 | 0.625 | 0.625 |
| Achillea fragrantissima | 10 | >10 | 10 | >10 | 5 | 10 | 1.25 | 1.25 |
| Phoenix dactylifera | 10 | >10 | 2.5 | 10 | 5 | 10 | 0.015 | 0.313 |
| Ethanol (70%) | − | − | − | − | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeq, O.; Mechchate, H.; Es-safi, I.; Bouhrim, M.; Jawhari, F.z.; Ouassou, H.; Kharchoufa, L.; N. AlZain, M.; M. Alzamel, N.; Mohamed Al kamaly, O.; et al. Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria fruticosa, Achillea fragrantissima, and Phoenix dactylifera. Plants 2021, 10, 676. https://doi.org/10.3390/plants10040676
Sadeq O, Mechchate H, Es-safi I, Bouhrim M, Jawhari Fz, Ouassou H, Kharchoufa L, N. AlZain M, M. Alzamel N, Mohamed Al kamaly O, et al. Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria fruticosa, Achillea fragrantissima, and Phoenix dactylifera. Plants. 2021; 10(4):676. https://doi.org/10.3390/plants10040676
Chicago/Turabian StyleSadeq, Omar, Hamza Mechchate, Imane Es-safi, Mohamed Bouhrim, Fatima zahra Jawhari, Hayat Ouassou, Loubna Kharchoufa, Mashail N. AlZain, Nurah M. Alzamel, Omkulthom Mohamed Al kamaly, and et al. 2021. "Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria fruticosa, Achillea fragrantissima, and Phoenix dactylifera" Plants 10, no. 4: 676. https://doi.org/10.3390/plants10040676
APA StyleSadeq, O., Mechchate, H., Es-safi, I., Bouhrim, M., Jawhari, F. z., Ouassou, H., Kharchoufa, L., N. AlZain, M., M. Alzamel, N., Mohamed Al kamaly, O., Bouyahya, A., Benoutman, A., & Imtara, H. (2021). Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria fruticosa, Achillea fragrantissima, and Phoenix dactylifera. Plants, 10(4), 676. https://doi.org/10.3390/plants10040676








