Therapeutic Potential of Lindera obtusiloba: Focus on Antioxidative and Pharmacological Properties
Abstract
1. Introduction
2. Genus Lindera and Antioxidant Properties of Its Plants
3. Ethnomedicinal Use of Lindera obtusiloba
4. Bioactive Compounds of Lindera obtusiloba
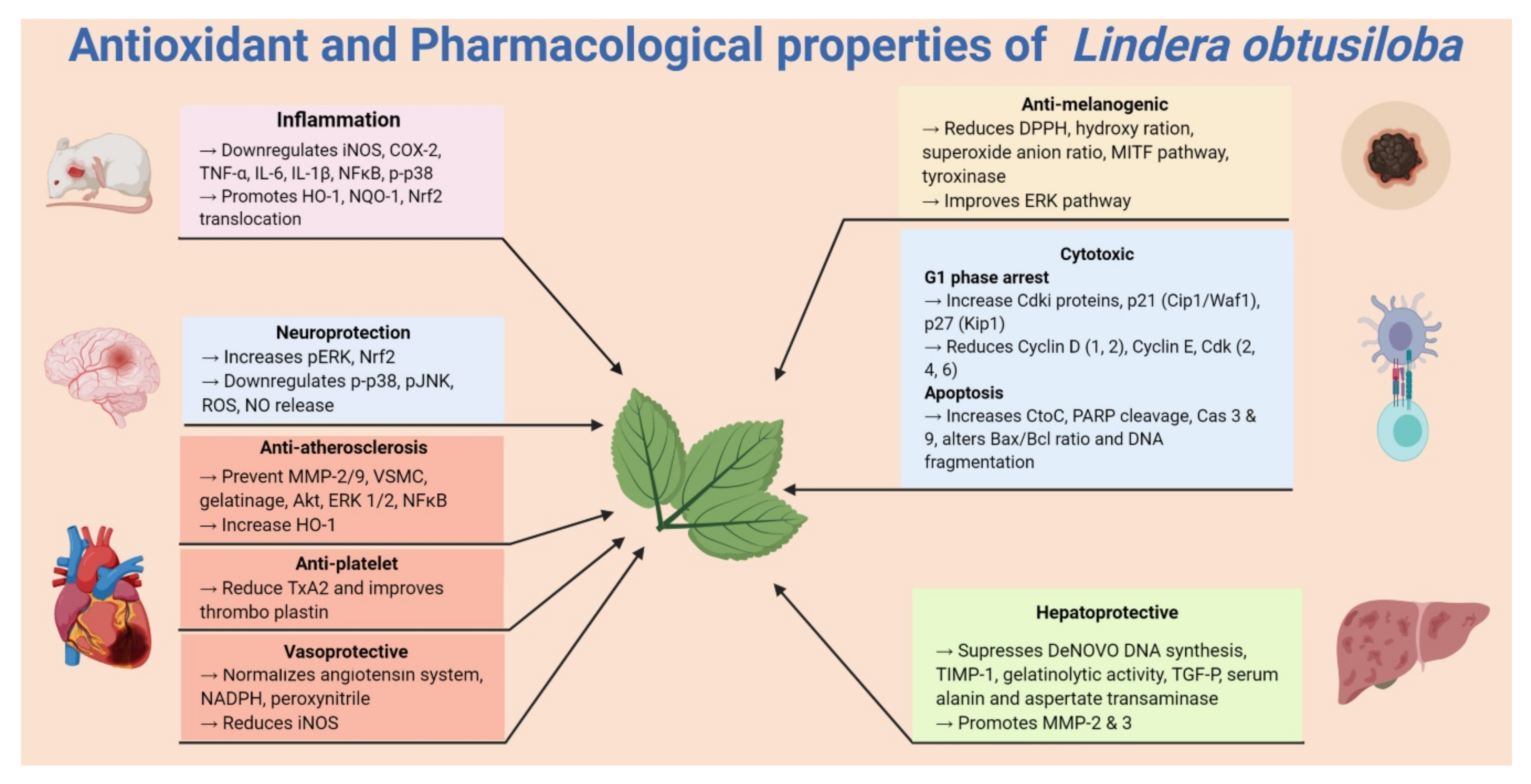
5. Pharmacological Properties of Lindera obtusiloba
5.1. Anti-Allergic and Anti-Inflammatory Activities
5.2. Antiplatelet Activity
5.3. Cytotoxic Activity
5.4. Hepatoprotective Activity
5.5. Vasoprotective and Antihypertensive Activity
5.6. Anti-Melanogenic Activities
5.7. Neuroprotective Activity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Tsui, H.-P. A study on the system of Lindera. Acta Phytotax. Sin. 1987, 25, 161–171. [Google Scholar]
- Cao, Y.; Xuan, B.; Peng, B.; Li, C.; Chai, X.; Tu, P. The genus Lindera: A source of structurally diverse molecules having pharmacological significance. Phytochem. Rev. 2016, 15, 869–906. [Google Scholar] [CrossRef]
- Han, Z.; Zheng, Y.; Chen, N.; Luan, L.; Zhou, C.; Gan, L.; Wu, Y. Simultaneous determination of four alkaloids in Lindera aggregata by ultra-high-pressure liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2008, 1212, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Chaofeng, Z.; Zhengtao, W. An advance in the study on the medicinal plant of Lindera. J. Shenyang Pharm. Univ. 2000, 17, 230–234. [Google Scholar]
- Han, H.; Xu, B.; Amin, A.; Li, H.; Yu, X.; Gong, M.; Zhang, L. Quercetin‑3‑O‑α‑L‑rhamnopyranoside derived from the leaves of Lindera aggregata (Sims) Kosterm. evokes the autophagy‑induced nuclear factor erythroid 2‑related factor 2 antioxidant pathway in human umbilical vein endothelial cells. Int. J. Mol. Med. 2019, 43, 461–474. [Google Scholar] [CrossRef]
- Noda, Y.; Mori, A. Antioxidant activities of Uyaku (Lindera strychnifolia) leaf extract: A natural extract used in traditional medicine. J. Clin. Biochem. Nutr. 2007, 41, 139–145. [Google Scholar] [CrossRef][Green Version]
- Joshi, S.C.; Verma, A.R.; Mathela, C.S. Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem. Toxicol. 2010, 48, 37–40. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. Transl. Integr. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, UK, 1985. [Google Scholar]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Element, A.R. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef] [PubMed]
- Ramana, K.V.; Reddy, A.; Majeti, N.V.; Singhal, S.S. Therapeutic potential of natural antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-W.; Ha, J.-H.; Shin, H.-G.; Jeong, S.-H.; Kim, J.-H.; Lee, J.; Park, J.-Y.; Kwon, H.-J.; Jung, K.; Lee, W.-S.; et al. Lindera obtusiloba Attenuates Oxidative Stress and Airway Inflammation in a Murine Model. of Ovalbumin-Challenged Asthma. Antioxidants 2020, 9, 563. [Google Scholar] [CrossRef]
- Freise, C.; Querfeld, U. The lignan (+)-episesamin interferes with TNF-α-induced activation of VSMC via diminished activation of NF-ĸB, ERK1/2 and AKT and decreased activity of gelatinases. Acta Physiol. 2015, 213, 642–652. [Google Scholar] [CrossRef]
- Hong, C.O.; Lee, H.A.; Rhee, C.H.; Choung, S.Y.; Lee, K.W. Separation of the antioxidant compound quercitrin from Lindera obtusiloba Blume and its antimelanogenic effect on B16F10 melanoma cells. Biosci. Biotechnol. Biochem. 2013, 77, 58–64. [Google Scholar] [CrossRef]
- Jung, S.-H.; Han, J.-H.; Park, H.-S.; Lee, J.-J.; Yang, S.-Y.; Kim, Y.H.; Heo, K.-S.; Myung, C.-S. Inhibition of collagen-induced platelet aggregation by the secobutanolide secolincomolide A from Lindera obtusiloba Blume. Front. Pharmacol. 2017, 8, 560–571. [Google Scholar] [CrossRef]
- Nanni, V.; Canuti, L.; Gismondi, A.; Canini, A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Nardi, G.M.; Januario, A.G.F.; Freire, C.G.; Megiolaro, F.; Schneider, K.; Perazzoli, M.R.A.; Raap Do Nascimento, S.; Gon, A.C.; Bolda Marino, L.N.; Wgner, G.; et al. Anti-inflammatory activity of berry fruits in mice model of inflammation is based on oxidative stress modulation. Pharmacogn. Res. 2016, 8, S42–S49. [Google Scholar]
- Wang, J.W.; Chen, X.Y.; Hu, P.Y.; Tan, M.M.; Tang, X.G.; Huang, M.C.; Lou, Z.H. Effects of Linderae radix extracts on a rat model of alcoholic liver injury. Exp. Ther. Med. 2016, 11, 2185–2192. [Google Scholar] [CrossRef]
- Xu, C.; Yang, B.; Zhu, W.; Li, X.; Tian, J.; Zhang, L. Characterisation of polyphenol constituents of Linderae aggregate leaves using HPLC fingerprint analysis and their antioxidant activities. Food Chem. 2015, 186, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-C.; Wu, Y.-H.; Hung, H.-Y.; Lam, S.-H.; Ma, G.-H.; Kuo, L.-M.; Hwang, T.-L.; Kuo, D.-H.; Wu, T.-S. Anti-inflammatory principles from Lindera aggregata. Bioorganic Med. Chem. Lett. 2020, 30, 127224. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. 7, 8-Dihydroxycoumarin (daphnetin) protects INS-1 pancreatic β-cells against streptozotocin-induced apoptosis. Phytomedicine 2017, 24, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.S.; Zheng, Y.L.; Mo, J.X.; Liu, X.; Li, X.H.; Zhou, C.X. Sesquiterpene lactones from the root tubers of Lindera aggregata. J. Nat. Prod. 2009, 72, 1497–1501. [Google Scholar] [CrossRef]
- Hyland, B.P.M. A revision of Lauraceae in Australia (excluding Cassytha). Aust. Syst. Bot. 1989, 2, 135–367. [Google Scholar] [CrossRef]
- Wofford, B.E. A new Lindera (Lauraceae) from North. America. J. Arnold Arbor. 1983, 64, 325–331. [Google Scholar] [CrossRef]
- Kim, J.A.; Jung, Y.S.; Kim, M.Y.; Yang, S.Y.; Lee, S.; Kim, Y.H. Protective effect of components isolated from Lindera erythrocarpa against oxidative stress-induced Apoptosis of H9c2 Cardiomyocytes. Phytother. Res. 2011, 25, 1612–1617. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Yuan, L.; Wu, Y.; Peng, X.; Kai, G.; Liu, Y. Aqueous extracts of Lindera aggregate (Sims) Kosterm leaves regulate serum/hepatic lipid and liver function in normal and hypercholesterolemic mice. J. Pharmacol. Sci. 2020, 143, 45–51. [Google Scholar] [CrossRef]
- Wang, N.; Minatoguchi, S.; Arai, M.; Uno, Y.; Hashimoto, K.; Chen, X.H.; Fujiwara, H. Lindera strychnifolia is Protective Against Post-ischemic Myocardial Dysfunction Through Scavenging Hydroxyl Radicals and Opening the Mitochondrial K ATP Channels in Isolated Rat Hearts. Am. J. Chin. Med. 2004, 32, 587–598. [Google Scholar] [CrossRef]
- Li, B.; Jeong, G.S.; Kang, D.G.; Lee, H.S.; Kim, Y.C. Cytoprotective effects of lindenenyl acetate isolated from Lindera strychnifolia on mouse hippocampal HT22 cells. Eur. J. Pharmacol. 2009, 614, 58–65. [Google Scholar] [CrossRef]
- Ichino, K.; Tanaka, H.; Ito, K.; Tanaka, T.; Mizuno, M. Two new dihydrochalcones from Lindera erythrocarpa. J. Nat. Prod. 1988, 51, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Yang, H.L.; Tsai, Y.C.; Hung, P.C.; Chang, S.H.; Lo, H.W.; Chou, C.W. Lucidone protects human skin keratinocytes against free radical-induced oxidative damage and inflammation through the up-regulation of HO-1/Nrf2 antioxidant genes and down-regulation of NF-κB signaling pathway. Food Chem. Toxicol. 2013, 59, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Wang, S.Y.; Chiu, C.C.; Tseng, C.K.; Lin, C.K.; Wang, H.C.; Lee, J.C. Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction. Antimicrob. Agents Chemother. 2013, 57, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.C.; Mathela, C.S. Antioxidant and antibacterial activities of the leaf essential oil and its constituents furanodienone and curzerenone from Lindera pulcherrima (Nees.) Benth. ex hook. f. Phcog. Res. 2012, 4, 80–84. [Google Scholar]
- Kim, Y.S.; Kim, E.K.; Dong, X.; Park, J.S.; Shin, W.B.; Kim, S.J.; Lim, B.O. Lindera glauca (Siebold et Zucc.) Blume Stem Extracts protect against tert-Butyl hydroperoxide-induced oxidative stress. J. Med. Food 2019, 22, 508–520. [Google Scholar] [CrossRef]
- Kim, Y.U.; Yun, J.M. Antioxidative and Antiproliferative Effects of Lindera glauca Blume on Human Colorectal Cancer Cells. J. Korean Soc. Food Sci. Nutr. 2015, 44, 635–640. [Google Scholar] [CrossRef]
- Huh, G.W.; Park, J.H.; Kang, J.H.; Jeong, T.S.; Kang, H.C.; Baek, N.I. Flavonoids from Lindera glauca Blume as low-density lipoprotein oxidation inhibitors. Nat. Prod. Res. 2014, 28, 831–834. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P.; Do, M.H.; Lee, T.H.; Kim, S.Y. Anti-neuroinflammatory and neuroprotective effects of the Lindera neesiana fruit in vitro. Phytomedicine 2016, 23, 872–881. [Google Scholar] [CrossRef]
- Adhikari-Devkota, A.; Dirar, A.I.; Kurizaki, A.; Tsushiro, K.; Devkota, H.P. Extraction and Isolation of Kaempferol Glycosides from the Leaves and Twigs of Lindera neesiana. Separations 2019, 6, 10. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, Y.; Wang, K. Antinociceptive and free radical scavenging activities of alkaloids isolated from Lindera angustifolia Chen. J. Ethnopharmacol. 2006, 106, 408–413. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Wang, L.; Chen, S. Pharmacokinetics and Tissue Distribution Study of Pinosylvin in Rats by Ultra-High.-Performance Liquid Chromatography Coupled with Linear Trap Quadrupole Orbitrap Mass Spectrometry. Evid. Based Complementary Altern. Med. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yang, J.; Chen, S.; Sun, X.; Zhao, P.; Xie, Z. Screening, and identification of the binding position, of xanthine oxidase inhibitors in the roots of Lindera reflexa Hemsl using ultrafiltration LC–MS combined with enzyme blocking. Biomed. Chromatogr. 2019, 33, e4577. [Google Scholar] [CrossRef] [PubMed]
- Song, M.C.; Nigussie, F.; Jeong, T.S.; Lee, C.Y.; Regassa, F.; Markos, T.; Baek, N.I. Phenolic Compounds from the Roots of Lindera f ruticosa. J. Nat. Prod. 2006, 69, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, M.; Hadi, H.A.; Mohamad, J.; Khalilzadeh, M.A.; Cheahd, S.-C.; Fadaeinasab, M. Flavonoids and Linderone from Lindera oxyphylla and their Bioactivities. Comb. Chem. High Throughput Screen. 2013, 16, 160–166. [Google Scholar] [PubMed]
- Kuroda, M.; Sakurai, K.; Mimaki, Y. Chemical constituents of the stems and twigs of Lindera umbellata. J. Nat. Med. 2011, 65, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, J.; Chen, Y.; Xu, C.; Chen, X.; Shao, T.; Pan, H. Linderae radix ethanol extract attenuates alcoholic liver injury via attenuating inflammation and regulating gut microbiota in rats. Braz. J. Med. Biol. Res. 2019, 52, e7628. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Sun, Z.; Yan, X.; Wang, H.; Xu, H.; Zhang, Y. Linderane protects pancreatic beta cells from streptozotocin (STZ)-induced oxidative damage. Life Sci. 2019, 233, 116732. [Google Scholar] [CrossRef]
- Koskela, A.; Reinisalo, M.; Hyttinen, J.M.; Kaarniranta, K.; Karjalainen, R.O. Pinosylvin-mediated protection against oxidative stress in human retinal pigment epithelial cells. Mol. Vis. 2014, 20, 760–769. [Google Scholar]
- Lee, J.O.; Auger, C.; Park, D.H.; Kang, M.; Oak, M.H.; Kim, K.R.; Schini-Kerth, V.B. An ethanolic extract of Lindera obtusiloba stems, YJP-14, improves endothelial dysfunction, metabolic parameters and physical performance in diabetic db/db mice. PLoS ONE 2013, 8, e65227. [Google Scholar] [CrossRef]
- Bang, C.Y.; Choung, S.Y. Antioxidant and whitening effects of Lindera obtusiloba BL. 70% EtOH extract. Planta Med. 2009, 75, PI27. [Google Scholar] [CrossRef]
- Lim, D.W.; Lee, M.S.; Her, S.; Cho, S.; Lee, C.H.; Kim, I.H.; Han, D. Antidepressant-like effects of Lindera obtusiloba extracts on the immobility behavior of rats in the forced swim test. Molecules 2016, 21, 277. [Google Scholar] [CrossRef] [PubMed]
- Yook, C.S. Medicinal Plants of Korea; Jinmyeong Publishing Co: Seoul, Korea, 1981; p. 392. [Google Scholar]
- Kim, J.H.; Lee, J.; Kang, S.; Moon, H.; Chung, K.H.; Kim, K.R. Antiplatelet and antithrombotic effects of the extract of lindera obtusiloba leaves. Biomol. Ther. 2016, 24, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Lee, J.O.; Kang, S.H.; Sohn, J.D.; Kim, J.H.; Lim, J.W.; Lee, S.W. Method for Preventing and Treating Thrombotic Disorders Using a Pharmaceutical Composition Comprising an Extract of Lindera Obtusiloba. US Patent 2014/0255529 A1, 11 September 2014. [Google Scholar]
- Freise, C.; Erben, U.; Neuman, U.; Kim, K.; Zeitz, M.; Somasundaram, R.; Ruehl, M. An active extract of Lindera obtusiloba inhibits adipogenesis via sustained Wnt signaling and exerts anti-inflammatory effects in the 3T3-L1 preadipocytes. J. Nutr. Biochem. 2010, 21, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Freise, C.; Ruehl, M.; Erben, U.; Neumann, U.; Seehofer, D.; Kim, K.Y.; Somasundaram, R. A hepatoprotective Lindera obtusiloba extract suppresses growth and attenuates insulin like growth factor-1 receptor signaling and NF-kappaB activity in human liver cancer cell lines. Bmc Complementary Altern. Med. 2011, 11, 39. [Google Scholar] [CrossRef]
- Facciola, S. Cornucopia: A Source Book of Edible Plants; Kampong Publications: Vista, CA, USA, 1990. [Google Scholar]
- Zekun, L.; Haixia, C. GC-MS analysis of essential oil from the bark of Lindera obtusiloba. Chem. Nat. Compd. 2012, 48, 696–697. [Google Scholar] [CrossRef]
- Choi, H.G.; Lee, H.D.; Kim, S.H.; Na, M.K.; Kim, J.A.; Lee, S.H. Phenolic glycosides from Lindera obtusiloba and their anti-allergic inflammatory activities. Nat. Prod. Commun. 2013, 8, 1934578X1300800212. [Google Scholar] [CrossRef]
- Hong, C.O.; Rhee, C.H.; Won, N.H.; Choi, H.D.; Lee, K.W. Protective effect of 70% ethanolic extract of Lindera obtusiloba Blume on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem. Toxicol. 2013, 53, 214–220. [Google Scholar] [CrossRef]
- Choi, H.G.; Choi, Y.H.; Kim, J.H.; Kim, H.H.; Kim, S.H.; Kim, J.A.; Lee, S.H. A new neolignan and lignans from the stems of Lindera obtusiloba Blume and their anti-allergic inflammatory effects. Arch. Pharmacal Res. 2014, 37, 467–472. [Google Scholar] [CrossRef]
- Kwon, H.C.; Baek, N.I.; Choi, S.U.; Lee, K.R. New cytotoxic butanolides from Lindera obtusiloba BLUME. Chem. Pharm. Bull. 2000, 48, 614–616. [Google Scholar] [CrossRef]
- Nii, H.; Furukawa, K.; Iwakiri, M.; Kubota, T. Constituents of essential oils of Lindera obtusiloba Blume and Parabenzoin trilobum (Sieb, et Zucc.) Nakai fruit. J. Agric. Chem. Soc. Jpn. 1983, 57, 663–666. [Google Scholar]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Ju, M.K.; Majumder, R.; Shukla, S.; Han, Y.K. Attenuation of inflammatory responses by (+)-syringaresinol via MAP-Kinase-mediated suppression of NF-κB signaling in vitro and in vivo. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung-Ok, L.E.E.; Chulyoung, K.I.M.; Seung-Woo, L.E.E.; Min-Ho, O.A.K. Antiplatelet and antithrombotic activities of Lindera obtusiloba extract in vitro and in vivo. Korean Soc. Appl. Pharmacol. 2010, 18, 205–210. [Google Scholar]
- Kwon, H.C.; Choi, S.U.; Lee, J.O.; Bae, K.H.; Zee, O.P.; Lee, K.R. Two new lignans from Lindera obtusiloba Blume. Arch. Pharmacal Res. 1999, 22, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Oh, S.R.; Ahn, K.S.; Kwon, O.K.; Lee, H.K. (–)-Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis. Int. Immunopharmacol. 2008, 8, 967–973. [Google Scholar] [CrossRef]
- Trowitzsch-Kienast, W.; Rühl, M.; Kim, K.Y.; Emmerling, F.; Erben, U.; Somasundaram, R.; Freise, C. Absolute Configuration of Antifi brotic (+)-Episesamin Isolated from Lindera obtusiloba BLUME. Z. Für Nat. C 2011, 66, 460–464. [Google Scholar] [CrossRef]
- Ruehl, M.; Erben, U.; Kim, K.; Freise, C.; Dagdelen, T.; Eisele, S.; Somasundaram, R. Extracts of Lindera obtusiloba induce antifibrotic effects in hepatic stellate cells via suppression of a TGF-β-mediated profibrotic gene expression pattern. J. Nutr. Biochem. 2009, 20, 597–606. [Google Scholar] [CrossRef]
- O'Driscoll, G.; Green, D.; Rankin, J.; Stanton, K.; Taylor, R. Improvement in endothelial function by angiotensin converting enzyme inhibition in insulin-dependent diabetes mellitus. J. Clin. Investig. 1997, 100, 678–684. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef]
- Antoniades, C.; Antonopoulos, A.S.; Bendall, J.K.; Channon, K.M. Targeting redox signaling in the vascular wall: From basic science to clinical practice. Curr. Pharm. Des. 2009, 15, 329–342. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, H.J.; Um, S.H.; Sohn, E.H.; Kim, B.O.; Moon, E.Y.; Pyo, S. Sulforaphane suppresses vascular adhesion molecule-1 expression in TNF-α-stimulated mouse vascular smooth muscle cells: Involvement of the MAPK, NF-κB and AP-1 signaling pathways. Vasc. Pharmacol. 2012, 56, 131–141. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, M.K.; Koo, K.A.; Kim, S.H.; Sung, S.H.; Lee, N.G.; Kim, Y.C. Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. J. Neurosci. Res. 2004, 76, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Kim, S.H.; Jeong, E.J.; Park, J.H.; Kim, S.H.; Kim, Y.C.; Sung, S.H. New secoisolariciresinol derivatives from Lindera obtusiloba stems and their neuroprotective activities. Planta Med. 2010, 76, 294–297. [Google Scholar] [CrossRef] [PubMed]
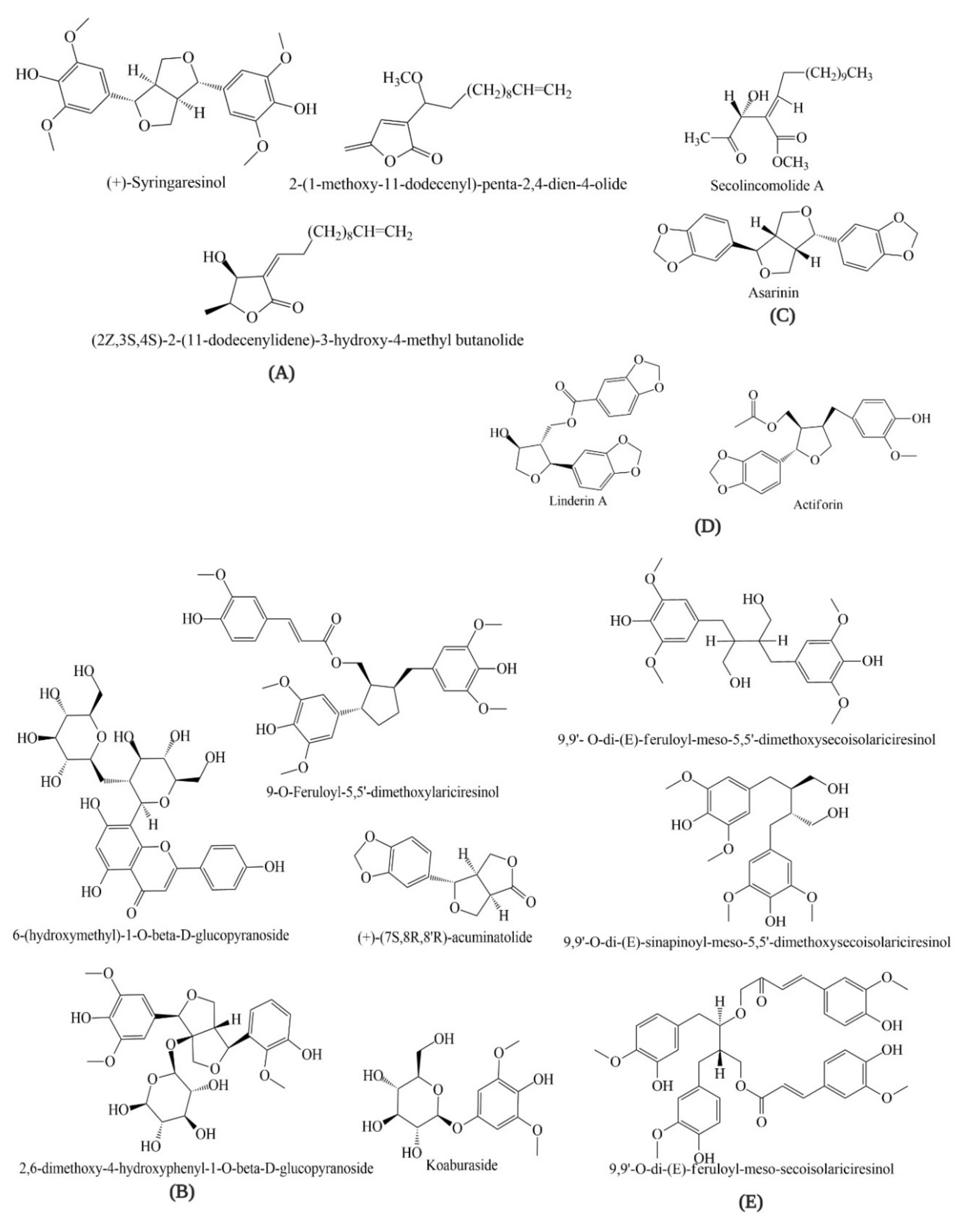
| Species | Extract/Compound | Plant Part | Model | Tested Concentrations | IC50 | Major Effects | Ref. |
|---|---|---|---|---|---|---|---|
| L. aggregata | Water and EtOH | root | alcoholic liver injury | 2 g/kg | - | decreased MDA and SOD levels | [21] |
| L. aggregata | 75% EtOH | root | 1, 2, and 4 g/kg | - | suppressed TLR4 overexpression and promoted the expression of occludin and claudin-1 in intestine tissue | [47] | |
| L. aggregata | EtOH | leaves | DPPH free radical scavenging assay | - | High Contents total phenols (May collection) 542.4 ± 12.9 μg/mL | Higher major compound (quercetin-3-O-α-l-rhamnoside) | [22] |
| L. aggregata | Quercetin-3-O-α-l-rhamnopyranoside | leaves | HUVEC cells | 62.5, 125, 250, and 500 µM | - | promoting Nrf2 and HO-1 | [5] |
| L. aggregata | Lindera aggredin C | whole plants | Human Neutrophils | - | 7.45 ± 0.74 µM | inhibition of superoxide anion generation | [23] |
| (+)-N-methyllaurotetanine | - | 8.36 ± 0.11 µM | |||||
| (+)-isoboldine | - | 5.81 ± 0.59 µM | |||||
| L. aggregata | linderane | root | INS-1 cells | 5, 10, and 20 µM | activation of Nrf2 pathway | [48] | |
| L. aggregata | Linderagalactone E, | root | HepG2 cells | - | 67.5 µM | hepatoprotective activity | [25] |
| linderane | - | 167.0 µM | |||||
| hydroxylindestenolide | - | 42.4 µM | |||||
| linderalactone | - | 98.0 µM | |||||
| L. aggregata | quercitrin | leaves | HCL mouse models | 0.3, 0.6, and 1.2 g/kg | - | combine Keap-1/Nrf2 system | [29] |
| L. aggregata | water | leaves | In vitro assay | - | 1.0 mg/mL (Hydroxyl radical) | scavenging activity of ROS and RNS, and inhibits lipid peroxidation | [6] |
| - | 0.01 mg/mL (Superoxide anion radical) | ||||||
| - | approximately 2 to 3 mg/mL (NO generation | ||||||
| - | 0.08 mg/mL (lipid peroxidation) | ||||||
| Lindera aggregata | Lindenenyl acetate | root | HT22 cells | 10, 20, 30, and 40 µM | - | increasing the activity of HO | [31] |
| Lindera aggregata | water | roots | Post-ischemic Myocardial rats | 0.75 and 1.5 g/kg | - | scavenging activities on DPPH radical | [30] |
| L. erythrocarpa | Lucidone | fruit | HaCaT cells | 0.5, 1, 5, and 10 µg/mL | - | increased expression HO-1 | [33] |
| L. erythrocarpa | Lucidone | fruit | Ava5 cells | 5, 10, 20, 30, 40, and 50µM | - | increased gene expression of HO-1 or Nrf2 | [34] |
| L. erythrocarpa | (−)-epicatechin | aerial parts | H9c2 cells | - | 1.7 µM | reduced the release of LDH | [28] |
| avicularin | - | 0.7 µM | |||||
| quercitrin | - | 22.3 µM | |||||
| L. pulcherrima | Extraction of oils | leaf | In vitro assay | - | 0.087 ± 0.03 mg/mL | DPPH radical scavenging activity | [7,35] |
| L. glauca | Water | Stem | In vitro assay | - | 11.920 ± 0.213 µg/mL (DPPH radical) | Free radical scavenging activity | [36] |
| - | 54.348 ± 2.124 (Alkyl radical) | ||||||
| - | 4.436 ± 0.141 (hydroxyl radical) | ||||||
| EtOH | - | 13.357 ± 0.312 µg/mL (DPPH) | |||||
| - | 56.714 ± 2.223 | ||||||
| - | 2.868 ± 0.124 | ||||||
| L. glauca | EtOH | Stem | In vitro assay | - | 30.5 ± 0.6 µg/mL | scavenging activities on DPPH radical | [37] |
| root | - | 29.4 ± 0.3 µg/mL | |||||
| L. neesiana | Water | fruit | N2a cells | 10, 50, and 100 µg/mL | - | increased Nrf2 secretion | [39] |
| L. neesiana | 60% EtOH | leaves and twigs | In vitro assay | - | 20.9 ± 1.04 µg/mL | DPPH radical scavenging activity | [40] |
| L. oxyphylla | Flavokawain B | bark | In vitro assay | - | 8.5 ± 0.004 μg/mL | DPPH radical scavenging activity, | [45] |
| L. reflexa | Pinosylvin | root | ARPE-19 cells | 5 µM | increase of HO-1 | [42,49] | |
| L. umbellata | (2S,3S)-2,3-bis[(4-hydroxy-3,5-dimethoxyphenyl)methyl]-1,4-butanediol 1,4-diferulate | Stem twig | In vitro assay | - | 22.5 ± 1.8 μg/mL | DPPH radical scavenging activity, | [46] |
| ssioriside | 21.5 ± 2.1 μg/mL | ||||||
| lyoniside | 26.3 ± 0.5 μg/mL | ||||||
| (+)-9′-O-(E)-feruloyl-5,5′-dimethoxylariciresinol | 23.6 ± 1.9 μg/mL | ||||||
| L. obtusiloba | MeOH extract | leaves | NCI-H292 cell | 25, 50, and 100 uM | - | increased the translocation of Nrf-2 into the nucleus with elevated HO-1 expression | [15] |
| L. obtusiloba | EtOH | stem | In vivo: type 2 diabetes mellitus mice model | 100 mg/kg | - | expression of the NADPH oxidase subunits NOX-1 and p47phox | [50] |
| L. obtusiloba | 70% EtOH extract | leaves | t-BHP rat model | 500 and 2000 mg/kg | - | decreased GSH level and oxidized NADPH | |
| In vitro assay | - | 249.5 ± 1.9 µg/mL | DPPH radical scavenging activity | ||||
| Quercitrin | - | 6.9 ± 0.4 | |||||
| Afzelin | - | 47.3 ± 2.4 | |||||
| L. obtusiloba | MeOH extract | leaves | In vitro assay | 4.21 ± 0.09 µg/mL | DPPH radical scavenging activity | ||
| Quercitrin | 107.5 ± 4.1 µM | ||||||
| Afzelin | 438.7 ± 14.2 µM | ||||||
| L. obtusiloba | 70% EtOH extract | leaves | In vitro assay | - | 243.14 µg/mL (DPPH) | DPPH, superoxide radical and hydroxyl radical scavenging activity | [51] |
| 35.47 µg/mL (enzymatic system of superoxide radical assay) | |||||||
| 1.21 mg/mL (Hydroxyl radical) | |||||||
| branch/stem mixed | 181.10 µg/mL (DPPH) | ||||||
| >100 µg/mL (enzymatic system of superoxide radical assay) |
| Compounds | Plant Part | Extraction Method | Study Model/Dose | Main Findings | Activity | Ref. |
|---|---|---|---|---|---|---|
| Anti-Allergic Inflammatory Activities | ||||||
| Isotachioside, Koaburaside, 2,6-dimethoxy-4-hydroxyphenyl-1-O-ß-d-glucopyranoside, 4,6-dihydroxy-2- methoxyphenyl-1-O-ß-d-glucopyranoside, Erigeside C, Salidroside, 6-hydroxyphenyl)-1-O-ß-d-glucopyranoside | Stem | Methanol | In vitro HMC-1 cells (10 µM) | Inhibited histamine release in mast cells. Hydroxyphenyl)-1-O-ß-d-glucopyranoside significantly inhibited in histamine release and IL-6 and TNF-α production in mast cells | Anti-allergic inflammatory activities | [60] |
| (+)-(7S,8R,80R)-acuminatolide, (+)-9′-0-O-trans-feruloyl-5,5–dimethoxylariciresinol | Stem | Methanol | In vitro HMC-1 cells (10 µM) | inhibited histamine release | Anti-allergic activity | |
| (+)-syringaresinol | Stem | Methanol | In vitro RAW 264.7 cells (25, 50, and 100 μM) | suppressed iNOS, COX-2, TNF-α, IL-1β, and IL-6 mRNA levels as well as COX-2 and NF-κB protein levels | Anti-inflammatory activity | [65] |
| In vivo Male ICR mice (30 mg/kg) | suppressed carrageenan-induced elevation of iNOS, COX-2, TNF-α, IL-1β, and IL-6 mRNA levels as well as COX-2 and NF-κB protein levels | Anti-inflammatory activity | ||||
| (+)-Episesamin | Twigs | 70% Ethanol | In vitro Hepatic stellate cells (10, 20, 50 µM) MOVAS-1 cell line (10 µM) | blocked cell proliferation and the profibrotic autocrine TGF-β expression HSC without significant cytotoxicity reduced TNF-α- and H2O2 -induced oxidative stress and in parallel induces anti-inflammatory haem oxygenase (HO)-1 expression | Antioxidant, Anti-inflammatory and other activities | [16] |
| Antiplatelet | ||||||
| Asarinin, Secoisolitsealiicolide B, Secolincomolide A | Stem | Methanol | In vivo Male white rabbits | inhibited of the GPVI and the COX-1-mediated metabolic pathways | Antiplatelet activity | [18] |
| Cytotoxicity | ||||||
| Linderin A, (+)-xanthoxyol | Stem | Methanol | In vitro HMC-1 cells (10 µM | inhibited histamine release and production of IL-6 and TNF-α. | Cytotoxicity and inflammatory activity | [62,67] |
| Pluviatilol | In vitro Tumour cells (3.40–19.27 µg/mL) | blocked cell proliferation of human tumour cell lines | Cytotoxicity | |||
| Actiforin | In vitro Tumour cells (3.40–19.27 µg/mL) HMC-1 cells (10 µM) | blocked cell proliferation of human tumour cell lines inhibited the histamine release and production of IL-6 and TNF-α. | Cytotoxicity and anti-inflammatory activity | |||
| 5,6-dihydroxymatairesinol, (+)-syringaresinol, (+)-9’-O-trans-feruloyl-5,5′-dimethoxylariciresinol, 2-(1-methoxy-11-dodecenyl)-penta-2,4-dien-4-olide, (2Z,3S,4S)-2-(11-dodecenylidene)-3-hydroxy-4-methyl butanolide, (2E,3R,4R)-2-(11-dodecenylidene)-3-hydroxy-4-methoxy-4-methyl butanolide, (−)-syringaresinol | Stem | Methanol | In vitro Tumour cells (3.40–19.27 µM) | blocked cell proliferation of human tumour cell lines | Cytotoxicity | [63] |
| Neuroprotective | ||||||
| 9,9′-O-di-(E)-feruloyl-meso-5,5′-dimethoxysecoisolariciresinol, 9,9′-O-di-(E)-sinapinoyl-meso-5,5′-dimethoxysecoisolariciresinol, 9,9′-O-di-(E)-feruloyl-meso-secoisolariciresinol | Stem | 80% Methanol | In vitro HT22 cells (1.0 and 10 µM) | protected from glutamate induced neurotoxicity in HT22 cells | Neuroprotective activity | [76] |
| Antiatherosclerosis | ||||||
| (+)-episesamin | Fruit | - | In vitro Human and murine VSMC | Diminished the activation of NF-ĸB, ERK1/2 and AKT and decreased activity of gelatinases inhibited the activation of Akt, NF-ĸB and MMP-2/-9 thus inhibiting TNF-α-induced proliferation of VSMC | Antiatherosclerosis Antioxidant activity | [16] |
| Hepatoprotective | ||||||
| (+)-episesamin | Twigs | 70% Ethanol | In vitro Hepatic stellate cells (10, 20, and 50 µM) | blocked the proliferation and the profibrotic autocrine TGF-β expression HSC without significant cytotoxicity | Hepatoprotective activity | [69,70] |
| Antimelanogenic | ||||||
| Quercetin-3-O-α-l-rhamnopyranoside, Kaempferol-3-O-α-l-rhamnoside | Leaves | Methanol | In vitro B16F10 melanoma cells (100 and 150 µM) | modulates of ERK and MITF expression | Antioxidant and antimelanogenic activity | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, M.E.; Azam, S.; Balakrishnan, R.; Akther, M.; Kim, I.-S. Therapeutic Potential of Lindera obtusiloba: Focus on Antioxidative and Pharmacological Properties. Plants 2020, 9, 1765. https://doi.org/10.3390/plants9121765
Haque ME, Azam S, Balakrishnan R, Akther M, Kim I-S. Therapeutic Potential of Lindera obtusiloba: Focus on Antioxidative and Pharmacological Properties. Plants. 2020; 9(12):1765. https://doi.org/10.3390/plants9121765
Chicago/Turabian StyleHaque, Md Ezazul, Shofiul Azam, Rengasamy Balakrishnan, Mahbuba Akther, and In-Su Kim. 2020. "Therapeutic Potential of Lindera obtusiloba: Focus on Antioxidative and Pharmacological Properties" Plants 9, no. 12: 1765. https://doi.org/10.3390/plants9121765
APA StyleHaque, M. E., Azam, S., Balakrishnan, R., Akther, M., & Kim, I.-S. (2020). Therapeutic Potential of Lindera obtusiloba: Focus on Antioxidative and Pharmacological Properties. Plants, 9(12), 1765. https://doi.org/10.3390/plants9121765







