Zinc in Dog Nutrition, Health and Disease: A Review
Abstract
Simple Summary
Abstract
1. Introduction
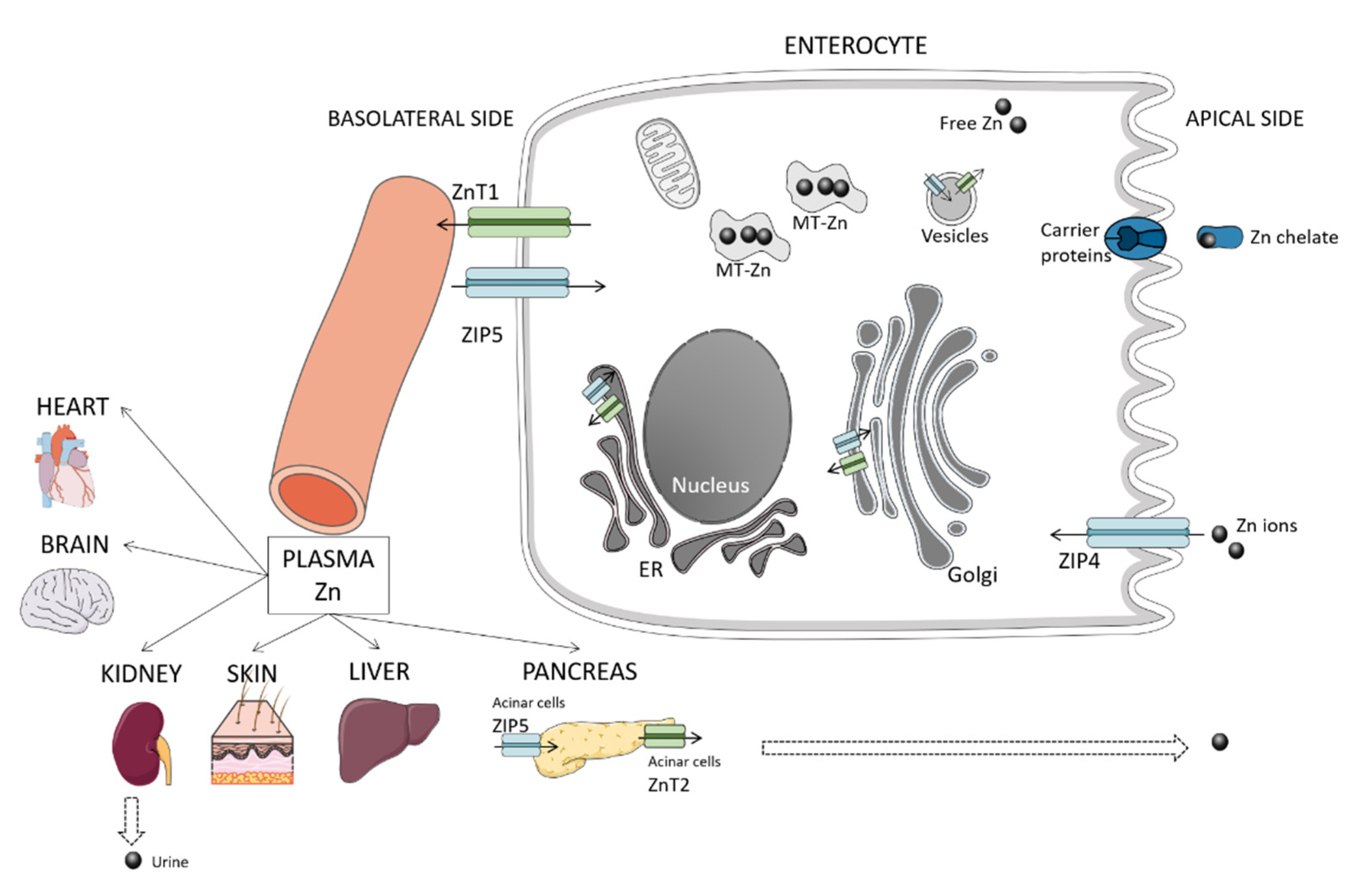
2. Overview of Zinc Homeostasis and Metabolism
2.1. Absorption
2.2. Excretion
3. Zinc Requirements and Biomarkers
3.1. Dietary Requirements for Healthy Individuals
3.2. Biomarkers of Zinc Status
3.2.1. Concentration of Zinc in Serum, Tissues, and Excretions
3.2.2. Zinc Dietary Intake
3.2.3. Metallothioneins
3.2.4. Zinc-Dependent Enzymes
3.2.5. Other Proteins
4. Zinc in Dog Foods
4.1. Legal Limits and Authorized Sources of Zinc for Animal Feed Supplementation
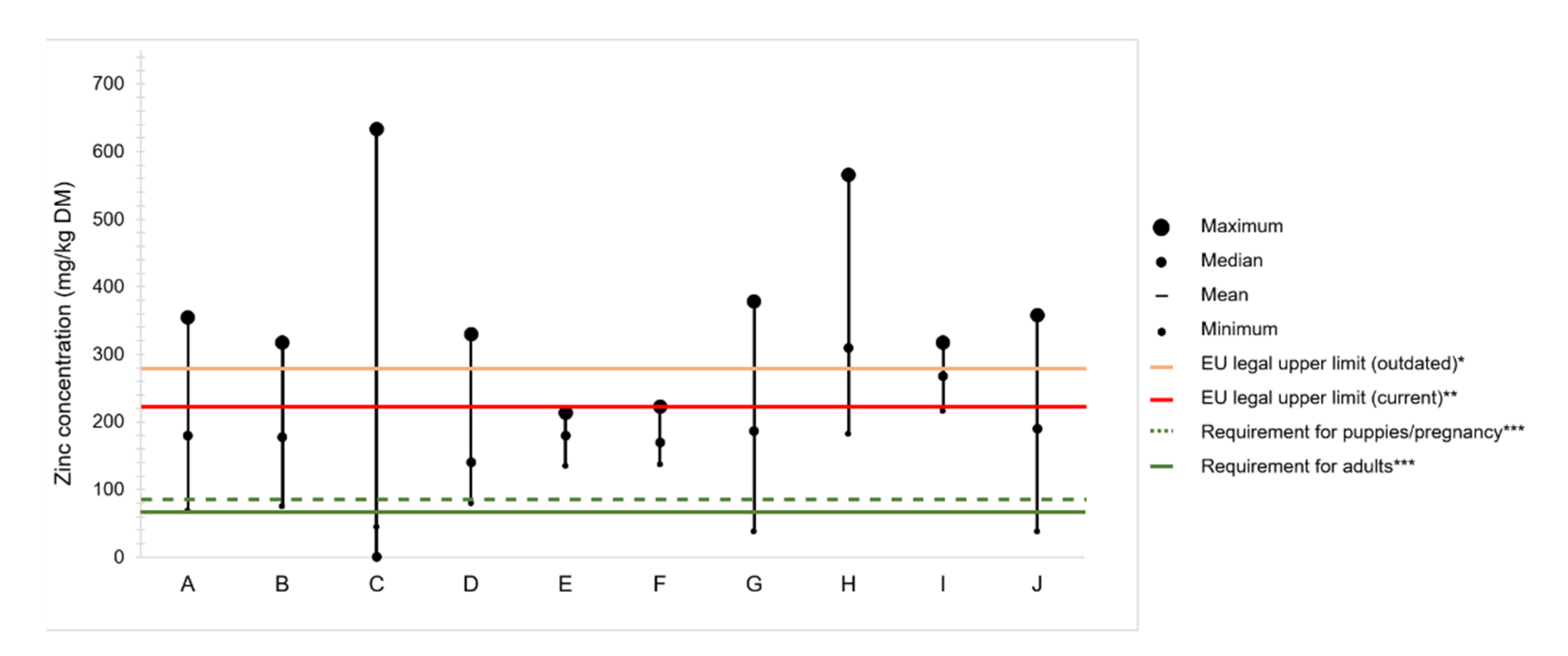
4.2. Zinc Content in Commercial Dog Foods
Variation of Zinc Content in Raw Ingredients
4.3. Bioavailability of Zinc Sources
Supplementation Strategies to Enhance Zinc Bioavailability
5. Role of Zinc Status in the Occurrence of Disease
5.1. Skin Disorders
5.1.1. Lethal Acrodermatitis
5.1.2. Canine Zinc-Responsive Dermatosis
5.1.3. Canine Atopic Dermatitis
5.1.4. Symmetrical Lupoid Onychomadesis
5.2. Neurological and Behavioral Disorders
5.3. Eye Disorders
5.4. Reproductive Disorders
5.5. Disorders Related to Natural Infections Caused by Pathogens
5.6. Hepatic and Renal Disorders
5.7. Cancer
5.8. Zinc Poisoning
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Raulin, J. Études Chimiques sur la Végétation; Masson & cie: Paris, France, 1905. [Google Scholar]
- Todd, W.R.; Elvehjem, C.A.; Hart, E.B. Zinc in the nutrition of the rat. Am. J. Physiol. Leg. Content 1933, 107, 146–156. [Google Scholar] [CrossRef]
- Prasad, A.S.; Halsted, J.A.; Nadimi, M. Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am. J. Med. 1961, 31, 532–546. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dogs; The National Academies Press: Washington, DC, USA, 1962. [Google Scholar] [CrossRef]
- Ozpinar, H.; Abas, I.; Bilal, T.; Demirel, G. Investigation of excretion and absorption of different zinc salts in puppies. Lab. Anim. 2001, 35, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, G.; Rompala, R.E. The influence of dietary sources of zinc, copper and manganese on canine reproductive performance and hair mineral content. J. Nutr. 1998, 128, 2603S–2605S. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.A.; Wiseman, J. A comparison of the bioavailability of three dietary zinc sources using four different physiologic parameters in dogs. J. Nutr. 1998, 128, 2809S–2811S. [Google Scholar] [CrossRef]
- Wedekind, K.J.; Lowry, S.R. Are organic zinc sources efficacious in puppies? J. Nutr. 1998, 128, 2593S–2595S. [Google Scholar] [CrossRef]
- Lowe, J.A.; Wiseman, J.; Cole, D.J. Zinc source influences zinc retention in hair and hair growth in the dog. J. Nutr. 1994, 124, 2575S–2576S. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.A.; Wiseman, J.; Cole, D.J. Absorption and retention of zinc when administered as an amino-acid chelate in the dog. J. Nutr. 1994, 124, 2572S–2574S. [Google Scholar] [CrossRef]
- Trevizan, L.; Fischer, M.M.; Rodenbusch, C.R.; Labres, R.V.; Kessler, A.d.M. Effects of diets containing organic and inorganic zinc sources on hair characteristics, zinc concentration in blood and hair, and the immune response of dogs. Acta Sci. Vet. 2013, 41, 1–7. [Google Scholar]
- Vester, B.M.; Karr-Lilienthal, L.K.; Tomlinson, D.J.; Swanson, K.S.; Fahey, G.C., Jr. Indicators of zinc status of weanling puppies are affected by zinc dietary concentration. Prof. Anim. Sci. 2007, 23, 448–453. [Google Scholar] [CrossRef]
- Pereira, A.M.; Guedes, M.; Matos, E.; Pinto, E.; Almeida, A.A.; Segundo, M.A.; Correia, A.; Vilanova, M.; Fonseca, A.J.M.; Cabrita, A.R.J. Effect of zinc source and exogenous enzymes supplementation on zinc status in dogs fed high phytate diets. Animals 2020, 10, 400. [Google Scholar] [CrossRef]
- White, S.D.; Bourdeau, P.; Rosychuk, R.A.; Cohen, B.; Bonenberger, T.; Fieseler, K.V.; Ihrke, P.; Chapman, P.L.; Schultheiss, P.; Zur, G.; et al. Zinc-responsive dermatosis in dogs: 41 cases and literature review. Vet. Dermatol. 2001, 12, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.A.; Ruedisueli, F.L.; Coe, S.L.; Watson, T.G.D. Effects of zinc and linoleic acid supplementation on the skin and coat quality of dogs receiving a complete and balanced diet. Vet. Dermatol. 2000, 11, 277–284. [Google Scholar] [CrossRef]
- Colombini, S. Canine zinc-responsive dermatosis. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 1373–1383. [Google Scholar] [CrossRef]
- Hara, T.; Takeda, T.A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Cousins, R.J. Gastrointestinal factors influencing zinc absorption and homeostasis. Int. J. Vitam. Nutr. Res. 2010, 80, 243–248. [Google Scholar] [CrossRef]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target Ther. 2017, 2, 17029. [Google Scholar] [CrossRef] [PubMed]
- Cousins, R.J. Chapter 72, Trace Element Absorption and Transport. In Physiology of the Gastrointestinal Tract, 5th ed.; Johnson, L.R., Ghishan, F.K., Kaunitz, J.D., Merchant, J.L., Said, H.M., Wood, J.D., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 1951–1961. [Google Scholar]
- Hardyman, J.E.; Tyson, J.; Jackson, K.A.; Aldridge, C.; Cockell, S.J.; Wakeling, L.A.; Valentine, R.A.; Ford, D. Zinc sensing by metal-responsive transcription factor 1 (MTF1) controls metallothionein and ZnT1 expression to buffer the sensitivity of the transcriptome response to zinc. Metallomics 2016, 8, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Naveh, Y.; Bentur, L.; Diamond, E. Site of zinc absorption in dog small intestine. J. Nutr. 1988, 118, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Ashmead, H. Amino Acid Chelation in Human and Animal Nutrition; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Nishito, Y.; Kambe, T. Absorption mechanisms of iron, copper, and zinc: An overview. J. Nutr. Sci. Vitaminol. 2018, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Lodemann, U.; Bondzio, A.; Gefeller, E.-M.; Vahjen, W.; Aschenbach, J.R.; Zentek, J.; Pieper, R. A High Amount of Dietary Zinc Changes the Expression of Zinc Transporters and Metallothionein in Jejunal Epithelial Cells in vitro and in vivo but Does Not Prevent Zinc Accumulation in Jejunal Tissue of Piglets. J. Nutr. 2013, 143, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, Y.Y.; Kirschke, C.P.; Gertz, E.R.; Lloyd, K.K. Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J. Biol. Chem. 2007, 282, 37053–37063. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.A.; Jackson, K.A.; Christie, G.R.; Mathers, J.C.; Taylor, P.M.; Ford, D. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J. Biol. Chem. 2007, 282, 14389–14393. [Google Scholar] [CrossRef]
- Pedraza-Chaverrí, J.; Torres-Rodríguez, G.A.; Cruz, C.; Mainero, A.; Tapia, E.; Ibarra-Rubio, M.E.; Silencio, J.L. Copper and zinc metabolism in aminonucleoside-induced nephrotic syndrome. Nephron 1994, 66, 87–92. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dogs; The National Academies Press: Washington, DC, USA, 1974. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dogs and Cats; National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Butterwick, R.F.; Erdman, J.W., Jr.; Hill, R.C.; Lewis, A.J.; Whittemore, C.T. Challenges in developing nutrient guidelines for companion animals. Br. J. Nutr. 2011, 106 (Suppl. 1), S24–S31. [Google Scholar] [CrossRef]
- FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs; FEDIAF: Bruxelles, Belgium, 2020. [Google Scholar]
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Baldwin, K.; Bartges, J.; Buffington, T.; Freeman, L.M.; Grabow, M.; Legred, J.; Ostwald, D. AAHA Nutritional assessment guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2010, 46, 285–296. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Talcott, P.A. Chapter 84, Zinc. In Small Animal Toxicology, 3rd ed.; Peterson, M.E., Talcott, P.A., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2013; pp. 847–851. [Google Scholar]
- Booles, D.; Burger, I.H.; Whyte, A.L.; Anderson, R.S.; Carlos, G.M.; Robinson, I.P. Effects of two levels of zinc intake on growth and trace element status in Labrador puppies. J. Nutr. 1991, 121, S79–S80. [Google Scholar] [CrossRef]
- Jamikorn, U.; Preedapattarapong, T. Comparative effects of zinc methionylglycinate and zinc sulfate on hair coat characteristics and zinc concentration in plasma, hair, and stool of dogs. Thai J. Vet. Med. 2008, 38, 9–16. [Google Scholar]
- Hambidge, M. Biomarkers of Trace Mineral Intake and Status. J. Nutr. 2003, 133, 948S–955S. [Google Scholar] [CrossRef]
- Krebs, N.F.; Miller, L.V.; Naake, V.L.; Lei, S.; Westcott, J.E.; Fennessey, P.V.; Michael Hambidge, K. The use of stable isotope techniques to assess zinc metabolism. J. Nutr. Biochem. 1995, 6, 292–301. [Google Scholar] [CrossRef]
- Victery, W.; Smith, J.M.; Vander, A.J. Renal tubular handling of zinc in the dog. Am. J. Physiol. Renal Physiol. 1981, 241, F532–F539. [Google Scholar] [CrossRef] [PubMed]
- Fieten, H.; Hooijer-Nouwens, B.D.; Biourge, V.C.; Leegwater, P.A.; Watson, A.L.; van den Ingh, T.S.; Rothuizen, J. Association of dietary copper and zinc levels with hepatic copper and zinc concentration in Labrador Retrievers. J. Vet. Intern. Med. 2012, 26, 1274–1280. [Google Scholar] [CrossRef]
- Passlack, N.; Mainzer, B.; Lahrssen-Wiederholt, M.; Schafft, H.; Palavinskas, R.; Breithaupt, A.; Zentek, J. Concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium, and lead in the liver and kidneys of dogs according to age, gender, and the occurrence of chronic kidney disease. J. Vet. Sci. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Zetzsche, A.; Schunter, N.; Zentek, J.; Pieper, R. Accumulation of copper in the kidney of pigs fed high dietary zinc is due to metallothionein expression with minor effects on genes involved in copper metabolism. J. Trace Elem. Med. Biol. 2016, 35, 1–6. [Google Scholar] [CrossRef]
- King, J.C.; Shames, D.M.; Lowe, N.M.; Woodhouse, L.R.; Sutherland, B.; Abrams, S.A.; Turnlund, J.R.; Jackson, M.J. Effect of acute zinc depletion on zinc homeostasis and plasma zinc kinetics in men. Am. J. Clin. Nutr. 2001, 74, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Blanchard, R.K.; Cousins, R.J. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc. Natl. Acad. Sci. USA 2006, 103, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, V.K.; Burnett, F.R.; Cousins, R.J. Metallothionein expression is increased in monocytes and erythrocytes of young men during zinc supplementation. J. Nutr. 1998, 128, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.K.; Hawksworth, G.M.; Woodhouse, L.R.; Sutherland, B.; King, J.C.; Beattie, J.H. Lymphocyte metallothionein mRNA responds to marginal zinc intake in human volunteers. Br. J. Nutr. 2000, 84, 747–756. [Google Scholar] [CrossRef]
- Sandoval, M.; Henry, P.R.; Luo, X.G.; Littell, R.C.; Miles, R.D.; Ammerman, C.B. Performance and tissue zinc and metallothionein accumulation in chicks fed a high dietary level of zinc. Poult. Sci. 1998, 77, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.X.; McDowell, L.R.; Cousins, R.J.; Martin, F.G.; Wilkinson, N.S.; Johnson, A.B.; Velasquez, J.B. Relative bioavailability of two organic and two inorganic zinc sources fed to sheep. J. Anim. Sci. 1995, 73, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Henry, P.R.; Davis, S.R.; Cousins, R.J.; Miles, R.D.; Littell, R.C.; Ammerman, C.B. Relative bioavailability of organic zinc sources based on tissue zinc and metallothionein in chicks fed conventional dietary zinc concentrations. Anim. Feed Sci. Technol. 2002, 101, 161–170. [Google Scholar] [CrossRef]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn superoxide dismutase: Not only a dismutase enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.A.; Cabelli, D.E. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim. Biophys. Acta 2010, 1804, 263–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Ward, T.L.; Ji, F.; Peng, C.; Zhu, L.; Gong, L.; Dong, B. Effects of zinc sources and levels of zinc amino acid complex on growth performance, hematological and biochemical parameters in weanling pigs. Asian-Australas. J. Anim. Sci. 2018, 31, 1267–1274. [Google Scholar] [CrossRef]
- Coleman, J.E. Zinc proteins: Enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 1992, 61, 897–946. [Google Scholar] [CrossRef]
- Nagalakshmi, D.; Dhanalakshmi, K.; Himabindu, D. Effect of dose and source of supplemental zinc on immune response and oxidative enzymes in lambs. Vet. Res. Commun. 2009, 33, 631–644. [Google Scholar] [CrossRef]
- Yalçinkaya, I.; Çinar, M.; Yildirim, E.; Erat, S.; Başalan, M.; Güngör, T. The effect of prebiotic and organic zinc alone and in combination in broiler diets on the performance and some blood parameters. Ital. J. Anim. Sci. 2016, 11. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Z.; Liu, L.; Luo, C.; Lu, G.; Li, Q.; Gao, X. Zinc regulates lipid metabolism and MMPs expression in lipid disturbance rabbits. Biol. Trace Elem. Res. 2015, 168, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, Ł.; Marek, A.; Grądzki, Z.; Laskowska, E.; Kwiecień, M. Effect of Zinc Sulfate and Zinc Glycine Chelate on Concentrations of Acute Phase Proteins in Chicken Serum and Liver Tissue. Biol. Trace Elem. Res. 2019, 187, 258–272. [Google Scholar] [CrossRef] [PubMed]
- EC. European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003-Appendix 4(II). (released 05.08.2020).
- EC. Regulation (EC) No 767/2009 of 13 July 2009 on the placing on the market and use of feed. 2009. [Google Scholar]
- EFSA. Scientific Opinion on the safety and efficacy of zinc compounds (E6) as feed additives for all animal species (zinc acetate, dihydrate; zinc chloride, anhydrous; zinc oxide; zinc sulphate, heptahydrate; zinc sulphate, monohydrate; zinc chelate of amino acids, hydrate; zinc chelate of glycine, hydrate), based on a dossier submitted by FEFANA asbl. EFSA J. 2015, 13, 46. [Google Scholar]
- EC. Regulation (EC) 2016/1095 of 6 July 2016 concerning the authorisation of Zinc acetate dihydrate, Zinc chloride anhydrous, Zinc oxide, Zinc sulphate heptahydrate, Zinc sulphate monohydrate, Zinc chelate of amino acids hydrate, Zinc chelate of protein hydrolysates, Zinc chelate of glycine hydrate (solid) and Zinc chelate of glycine hydrate (liquid) as feed additives for all animal species. 2016. [Google Scholar]
- EC. Regulation (EC) 2016/973 of 17 June 2016 concerning the authorisation of zinc bislysinate as a feed additive for all animal species. 2016. [Google Scholar]
- EC. Regulation (EC) No 335/2010 of 22 April 2010 concerning the authorisation of zinc chelate of hydroxy analogue of methionine as a feed additive for all animal species. 2010. [Google Scholar]
- EC. Regulation (EC) 2019/1125 of 5 June 2019 concerning the authorisation of zinc chelate of methionine sulfate as a feed additive for all animal species. 2019. [Google Scholar]
- Alomar, D.; Hodgkinson, S.; Abarzua, D.; Fuchslocher, R.; Alvarado, C.; Rosales, E. Nutritional evaluation of commercial dry dog foods by near infrared reflectance spectroscopy. J. Anim. Physiol. Anim. Nutr. 2006, 90, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, C.A.; Hodgkinson, S.M.; Alomar, D.; Boroschek, D. Evaluation of the chemical composition of dry dogfoods commercialized in Chile used for growing dogs. Arq. Bras. Med. Vet. Zootec. 2008, 60, 218–226. [Google Scholar] [CrossRef][Green Version]
- Elias, C.; De Nadai Fernandes, E.A.; Bacchi, M.A. Neutron activation analysis for assessing chemical composition of dry dog foods. J. Radioanal. Nucl. Chem. 2011, 291, 245–250. [Google Scholar] [CrossRef]
- Kelly, D.G.; White, S.D.; Weir, R.D. Elemental composition of dog foods using nitric acid and simulated gastric digestions. Food Chem. Toxicol. 2013, 55, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.; Alborough, R.; Jones, L.; Davis, C.; Williams, C.; Gardner, D.S. Mineral analysis of complete dog and cat foods in the UK and compliance with European guidelines. Sci. Rep. 2017, 7, 17107. [Google Scholar] [CrossRef]
- Costa, S.; Pereira, A.; Passos, E.; Alves, J.; Garcia, C.; Araujo, R. Evaluation of the chemical composition of dry feeds for dogs and cats. J. Braz. Chem. Soc. 2018. [Google Scholar] [CrossRef]
- Pereira, A.M.; Pinto, E.; Matos, E.; Castanheira, F.; Almeida, A.A.; Baptista, C.S.; Segundo, M.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Mineral composition of dry dog foods: Impact on nutrition and potential toxicity. J. Agric. Food Chem. 2018, 66, 7822–7830. [Google Scholar] [CrossRef] [PubMed]
- Goi, A.; Manuelian, C.L.; Curro, S.; Marchi, M. Prediction of mineral composition in commercial extruded dry dog food by near-infrared reflectance spectroscopy. Animals 2019, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.A.S.; Cave, N.J.; Adolphe, J.L.; Shoveller, A.K.; Verbrugghe, A. Plant-based (vegan) diets for pets: A survey of pet owner attitudes and feeding practices. PLoS ONE 2019, 14, e0210806. [Google Scholar] [CrossRef]
- Morelli, G.; Bastianello, S.; Catellani, P.; Ricci, R. Raw meat-based diets for dogs: Survey of owners’ motivations, attitudes and practices. BMC Vet. Res. 2019, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Zafalon, R.V.A.; Risolia, L.W.; Vendramini, T.H.A.; Ayres Rodrigues, R.B.; Pedrinelli, V.; Teixeira, F.A.; Rentas, M.F.; Perini, M.P.; Alvarenga, I.C.; Brunetto, M.A. Nutritional inadequacies in commercial vegan foods for dogs and cats. PLoS ONE 2020, 15, e0227046. [Google Scholar] [CrossRef] [PubMed]
- Pedrinelli, V.; Zafalon, R.V.A.; Rodrigues, R.B.A.; Perini, M.P.; Conti, R.M.C.; Vendramini, T.H.A.; de Carvalho Balieiro, J.C.; Brunetto, M.A. Concentrations of macronutrients, minerals and heavy metals in home-prepared diets for adult dogs and cats. Sci. Rep. 2019, 9, 13058. [Google Scholar] [CrossRef]
- Dillitzer, N.; Becker, N.; Kienzle, E. Intake of minerals, trace elements and vitamins in bone and raw food rations in adult dogs. Br. J. Nutr. 2011, 106 (Suppl. 1), S53–S56. [Google Scholar] [CrossRef] [PubMed]
- Ávila, D.V.; Souza, S.O.; Costa, S.S.; Araujo, R.G.; Garcia, C.A.; Alves, J.D.; Passos, E.A. Determination of Zn in Dry Feeds for Cats and Dogs by Energy-Dispersive X-ray Fluorescence Spectrometry. J. AOAC Int. 2016, 99, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A. The fascinating facets of plant selenium accumulation-biochemistry, physiology, evolution and ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- Marschner, H. Zinc Uptake from Soils. In Zinc in Soils and Plants; Robson, A.D., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 59–77. [Google Scholar] [CrossRef]
- Longnecker, N.E.; Robson, A.D. Distribution and transport of zinc in plants. In Zinc in Soils and Plants; Robson, A.D., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 79–91. [Google Scholar] [CrossRef]
- Henry, P.R.; Littell, R.C.; Ammerman, C.B. Effect of high dietary zinc concentration and length of zinc feeding on feed intake and tissue zinc concentration in sheep. Anim. Feed Sci. Technol. 1997, 66, 237–245. [Google Scholar] [CrossRef]
- Bellof, G.; Most, E.; Pallauf, J. Concentration of copper, iron, manganese and zinc in muscle, fat and bone tissue of lambs of the breed German Merino Landsheep in the course of the growing period and different feeding intensities. J. Anim. Physiol. Anim. Nutr. 2007, 91, 100–108. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Zhang, J.; Zhang, N.; Yang, X.; Qu, H.; Xi, L.; Han, J. Effects of dietary zinc levels on the growth performance, organ zinc content, and zinc retention in broiler chickens. Rev. Bras. Cienc. Avic. 2018, 20, 127–132. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J. Bioavailability of trace elements. Food Chem. 1992, 43, 213–217. [Google Scholar] [CrossRef]
- Cao, J.; Henry, P.R.; Guo, R.; Holwerda, R.A.; Toth, J.P.; Littell, R.C.; Miles, R.D.; Ammerman, C.B. Chemical characteristics and relative bioavailability of supplemental organic zinc sources for poultry and ruminants. J. Anim. Sci. 2000, 78, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid-base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef]
- Li, J.; Gong, C.; Wang, Z.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Xiao, F.; Cao, Y.; et al. Oyster-derived zinc-binding peptide modified by plastein reaction via zinc chelation promotes the intestinal absorption of zinc. Mar. Drugs 2019, 17, 341. [Google Scholar] [CrossRef]
- Shen, W.; Matsui, T. Intestinal absorption of small peptides: A review. Int. J. Food Sci. Technol. 2018, 54, 1942–1948. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Konkol, D.; Wojnarowski, K. The use of nanominerals in animal nutrition as a way to improve the composition and quality of animal products. J. Chem. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Center, S.A. Metabolic, antioxidant, nutraceutical, probiotic, and herbal therapies relating to the management of hepatobiliary disorders. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 67–172. [Google Scholar] [CrossRef] [PubMed]
- Affolter, V.K.; Moore, P.F. Histologie features of normal canine and feline skin. Clin. Dermatol. 1994, 12, 491–497. [Google Scholar] [CrossRef]
- Bin, B.H.; Hojyo, S.; Seo, J.; Hara, T.; Takagishi, T.; Mishima, K.; Fukada, T. The Role of the Slc39a Family of Zinc Transporters in Zinc Homeostasis in Skin. Nutrients 2018, 10, 219. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and skin disorders. Nutrients 2018, 10, 199. [Google Scholar] [CrossRef]
- Bin, B.-H.; Bhin, J.; Takaishi, M.; Toyoshima, K.-E.; Kawamata, S.; Ito, K.; Hara, T.; Watanabe, T.; Irié, T.; Takagishi, T.; et al. Requirement of zinc transporter ZIP10 for epidermal development: Implication of the ZIP10-p63 axis in epithelial homeostasis. Proc. Natl. Acad. Sci. USA 2017, 114, 12243–12248. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hasegawa, S.; Ban, S.; Yamada, T.; Date, Y.; Mizutani, H.; Nakata, S.; Tanaka, M.; Hirashima, N. ZIP2 protein, a zinc transporter, is associated with keratinocyte differentiation. J. Biol. Chem. 2014, 289, 21451–21462. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc in keratinocytes and langerhans cells: Relevance to the epidermal homeostasis. J. Immunol. Res. 2018, 2018, 5404093. [Google Scholar] [CrossRef]
- McEwan, N.A.; McNeil, P.E.; Thompson, H.; McCandlish, I.A. Diagnostic features, confirmation and disease progression in 28 cases of lethal acrodermatitis of bull terriers. J. Small Anim. Pract. 2000, 41, 501–507. [Google Scholar] [CrossRef]
- Jezyk, P.F.; Haskins, M.E.; MacKay-Smith, W.E.; Patterson, D.F. Lethal acrodermatitis in bull terriers. J. Am. Vet. Med. Assoc. 1986, 188, 833–839. [Google Scholar]
- Uchida, Y.; Moon-Fanelli, A.A.; Dodman, N.H.; Clegg, M.S.; Keen, C.L. Serum concentrations of zinc and copper in bull terriers with lethal acrodermatitis and tail-chasing behavior. Am. J. Vet. Res. 1997, 58, 808–810. [Google Scholar]
- Bauer, A.; Jagannathan, V.; Hogler, S.; Richter, B.; McEwan, N.A.; Thomas, A.; Cadieu, E.; Andre, C.; Hytonen, M.K.; Lohi, H.; et al. MKLN1 splicing defect in dogs with lethal acrodermatitis. PLoS Genet. 2018, 14, e1007264. [Google Scholar] [CrossRef]
- Lee, F.F.; Bradley, C.W., 2nd; Cain, C.L.; White, S.D.; Outerbridge, C.A.; Murphy, L.A.; Mauldin, E.A. Localized parakeratotic hyperkeratosis in sixteen Boston terrier dogs. Vet. Dermatol. 2016, 27, 384–e396. [Google Scholar] [CrossRef]
- Broek, A.H.M.v.d.; Thoday, K.L. Skin disease in dogs associated with zinc deficiency: A report of five cases. J. Small Anim. Pract. 1986, 27, 313–323. [Google Scholar] [CrossRef]
- van den Broek, A.H.; Stafford, W.L. Diagnostic value of zinc concentrations in serum, leucocytes and hair of dogs with zinc-responsive dermatosis. Res. Vet. Sci. 1988, 44, 41–44. [Google Scholar] [CrossRef]
- Beigh, S.A.; Soodan, J.S.; Nazki, S.; Khan, A.M. Oxidative stress, hematobiochemical parameters, trace elements xidative stress, hematobiochemical parameters, trace elements and vitamins in dogs with zinc responsive dermatosis itamins in dogs with zinc responsive dermatosis. Vet. Arhiv. 2014, 84, 591–600. [Google Scholar]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, M.; Bongiovanni, L.; Russo, A.; Capuccini, S.; Mechelli, L.; Ordeix, L.; Della Salda, L. Oxidative stress in the pathogenesis of canine zinc-responsive dermatosis. Vet. Dermatol. 2011, 22, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.; Qin, X.; Ran-Ressler, R.; Brenna, J.T.; Glahn, R.P.; Tako, E. Dietary zinc deficiency affects blood linoleic acid: Dihomo-gamma-linolenic acid (LA:DGLA) ratio; a sensitive physiological marker of zinc status in vivo (Gallus gallus). Nutrients 2014, 6, 1164–1180. [Google Scholar] [CrossRef]
- Khnykin, D.; Miner, J.H.; Jahnsen, F. Role of fatty acid transporters in epidermis: Implications for health and disease. Dermatoendocrinology 2011, 3, 53–61. [Google Scholar] [CrossRef]
- van den Broek, A.H.; Simpson, J.W. Fat absorption in dogs with demodicosis or zinc-responsive dermatosis. Res. Vet. Sci. 1992, 52, 117–119. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Palma, E.; Cordaro, M.; D’Amico, R.; Peritore, A.F.; Licata, P.; Crupi, R. Canine atopic dermatitis: Role of luteolin as new natural treatment. Vet. Med. Sci. 2020, 6, 926–932. [Google Scholar] [CrossRef]
- McFadden, R.A.; Heinrich, N.A.; Haarstad, A.C.; Tomlinson, D.J. A double-blinded, randomized, controlled, crossover evaluation of a zinc methionine supplement as an adjunctive treatment for canine atopic dermatitis. Vet. Dermatol. 2017, 28, 569-e138. [Google Scholar] [CrossRef]
- Tran, J.L.; Horvath, C.; Krammer, S.; Holler, U.; Zentek, J. Blood vitamin concentrations in privately owned dogs fed non-standardized commercial diets and after intake of diets with specified vitamin concentrations. J. Anim. Physiol. Anim. Nutr. 2007, 91, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hayashiya, S.; Tani, K.; Morimoto, M.; Hayashi, T.; Hayasaki, M.; Nomura, T.; Une, S.; Nakaichi, M.; Taura, Y. Expression of T helper 1 and T helper 2 cytokine mRNAs in freshly isolated peripheral blood mononuclear cells from dogs with atopic dermatitis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002, 49, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Olivry, T.; Moore, P.F.; Affolter, V.K.; Naydan, D.K. Langerhans cell hyperplasia and IgE expression in canine atopic dermatitis. Arch. Dermatol. Res. 1996, 288, 579. [Google Scholar] [CrossRef]
- Wilkie, J.S.; Yager, J.A.; Eyre, P.; Parker, W.M. Morphometric analyses of the skin of dogs with atopic dermatitis and correlations with cutaneous and plasma histamine and total serum IgE. Vet. Pathol. 1990, 27, 179–186. [Google Scholar] [CrossRef]
- Seo, H.M.; Kim, Y.H.; Lee, J.H.; Kim, J.S.; Park, Y.M.; Lee, J.Y. Serum zinc status and its association with allergic sensitization: The fifth korea national health and nutrition examination survey. Sci. Rep. 2017, 7, 12637. [Google Scholar] [CrossRef]
- Steimer, T.; Bauer, A.; Kienzle, E.; Mueller, R.S. Canine symmetrical lupoid onychomadesis in bearded collies. Vet. Dermatol. 2019, 30, 411-e124. [Google Scholar] [CrossRef]
- Auxilia, S.T.; Hill, P.B.; Thoday, K.L. Canine symmetrical lupoid onychodystrophy: A retrospective study with particular reference to management. J. Small Anim. Pract. 2001, 42, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Hague, D.W.; Foss, K.; de Godoy, M.C.; Selmic, L.E. Comparison of serum trace nutrient concentrations in epileptics compared to healthy dogs. Front. Vet. Sci. 2019, 6, 467. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Gower-Winter, S.D.; Levenson, C.W. Zinc in the central nervous system: From molecules to behavior. Biofactors 2012, 38, 186–193. [Google Scholar] [CrossRef]
- Juárez-Rebollar, D.; Rios, C.; Nava-Ruíz, C.; Méndez-Armenta, M. Metallothionein in brain disorders. Oxid. Med. Cell Longev. 2017, 2017, 5828056. [Google Scholar] [CrossRef]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of autophagy in oxidative stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef]
- Lee, S.J.; Koh, J.Y. Roles of zinc and metallothionein-3 in oxidative stress-induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytes. Mol. Brain 2010, 3, 30. [Google Scholar] [CrossRef]
- Hagmeyer, S.; Haderspeck, J.C.; Grabrucker, A.M. Behavioral impairments in animal models for zinc deficiency. Front. Behav. Neurosci. 2015, 8, 443. [Google Scholar] [CrossRef]
- Soltanian, A.; Khoshnegah, J.; Heidarpour, M. Comparison of serum trace elements and antioxidant levels in terrier dogs with or without behavior problems. Appl. Anim. Behav. Sci. 2016, 180, 87–92. [Google Scholar] [CrossRef]
- Rahimi Niyyat, M.; Azizzadeh, M.; Khoshnegah, J. Effect of supplementation with omega-3 fatty acids, magnesium, and zinc on canine behavioral disorders: Results of a pilot study. Top. Companion Anim. Med. 2018, 33, 150–155. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N. Recent advances in the understanding of the role of zinc in ocular tissues. Metallomics 2014, 6, 189–200. [Google Scholar] [CrossRef]
- Marquez, A.; Urbina, M.; Quintal, M.; Obregon, F.; Salazar, V.; Lim, L. Extracellular zinc chelator in vivo on system of taurine in retina: Transport, concentrations and localization of transporter. J. Clin. Exp. Ophthalmol. 2017, 8. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Meadows, N.J.; Keeling, P.W.N.; Mitchell, W.D.; Thompson, R.P.H. Rod mediated retinal dysfunction in cats with zinc depletion: Comparison with taurine depletion. Clin. Sci. 1986, 71, 559–564. [Google Scholar] [CrossRef]
- Rosolen, S.G.; Neveux, N.; Sahel, J.A.; Picaud, S.; Froger, N. Evaluation of the taurine concentrations in dog plasma and aqueous humour: A pilot study. Adv. Exp. Med. Biol. 2013, 775, 145–154. [Google Scholar]
- Madl, J.E.; McIlnay, T.R.; Powell, C.C.; Gionfriddo, J.R. Depletion of taurine and glutamate from damaged photoreceptors in the retinas of dogs with primary glaucoma. Am. J. Vet. Res. 2005, 66, 791–799. [Google Scholar] [CrossRef]
- Wen, G.Y.; Sturman, J.A.; Wisniewski, H.M.; MacDonald, A.; Niemann, W.H. Chemical and ultrastructural changes in tapetum of beagles with a hereditary abnormality. Investig. Ophthalmol. Vis. Sci. 1982, 23, 733–742. [Google Scholar]
- Figueroa, R.; Weiss, H.J.; Cecil Smith, J.; Hackley, B.M.; McBean, L.D.; Swassing, C.R.; Halsted, J.A. Effect of ethambutol on the ocular zinc concentration in dogs. Am. Rev. Respir. Dis. 1971, 104, 592–594. [Google Scholar] [PubMed]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar] [PubMed]
- Mogielnicka-Brzozowska, M.; Kowalska, N.; Fraser, L.; Kordan, W. Proteomic characterization of zinc-binding proteins of canine seminal plasma. Reprod. Domest. Anim. 2015, 50, 1017–1021. [Google Scholar] [CrossRef]
- Alonge, S.; Melandri, M.; Leoci, R.; Lacalandra, G.M.; Caira, M.; Aiudi, G.G. The Effect of Dietary Supplementation of Vitamin E, Selenium, Zinc, Folic Acid, and N-3 Polyunsaturated Fatty Acids on Sperm Motility and Membrane Properties in Dogs. Animals 2019, 9, 34. [Google Scholar] [CrossRef]
- Ciribe, F.; Panzarella, R.; Pisu, M.C.; Di Cerbo, A.; Guidetti, G.; Canello, S. Hypospermia Improvement in Dogs Fed on a Nutraceutical Diet. Sci. World J. 2018, 2018, 9520204. [Google Scholar] [CrossRef]
- Mogielnicka-Brzozowska, M.; Strzezek, R.; Wasilewska, K.; Kordan, W. Prostasomes of canine seminal plasma-zinc-binding ability and effects on motility characteristics and plasma membrane integrity of spermatozoa. Reprod. Domest. Anim. 2015, 50, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Leemaqz, S.Y.; Goh, Z.; McAninch, D.; Jankovic-Karasoulos, T.; Leghi, G.E.; Phillips, J.A.; Colafella, K.M.; Tran, C.; O’Leary, S.; et al. Zinc is a critical regulator of placental morphogenesis and maternal hemodynamics during pregnancy in mice. Sci. Rep. 2017, 7, 15137. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Patra, R.C.; Nandi, S.; Swarup, D. Oxidative stress indices in gastroenteritis in dogs with canine parvoviral infection. Res. Vet. Sci. 2009, 86, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Varshney, J.P.; Patra, R.C. Erythrocytic antioxidant defense, lipid peroxides level and blood iron, zinc and copper concentrations in dogs naturally infected with Babesia gibsoni. Res. Vet. Sci. 2008, 85, 120–124. [Google Scholar] [CrossRef]
- Seyrek, K.; Karagenç, T.; Paşa, S.; Kıral, F.; Atasoy, A. Serum zinc, iron and copper concentrations in dogs infected with Hepatozoon canis. Acta Vet. Brno 2009, 78, 471–475. [Google Scholar] [CrossRef][Green Version]
- Montgomery, M.L.; Sheline, G.E.; Chaikoff, I.L. The elimination of administered zinc in pancreatic juice, duodenal juice, and bile of the dog as measured by its radioactive isotope (Zn65). J. Exp. Med. 1943, 78, 151–159. [Google Scholar] [CrossRef]
- Gingerich, K.K.; Parnell, N.K.; Moore, G.E. Serum Magnesium and Zinc Concentrations in Dogs with Inflammatory Bowel Disease. In Proceedings of the 26th Annual Forum of the American College of Veterinary Internal Medicine, San Antonio, TX, USA, 4–7 June 2008. [Google Scholar]
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, K.P.; Mandal, A.; Paswan, R.K.; Sinha, P.; Das, P.; Ali, V.; Bimal, S.; Lal, C.S. Intracellular zinc flux causes reactive oxygen species mediated mitochondrial dysfunction leading to cell death in Leishmania donovani. PLoS ONE 2017, 12, e0178800. [Google Scholar] [CrossRef]
- Heidarpour, M.; Soltani, S.; Mohri, M.; Khoshnegah, J. Canine visceral leishmaniasis: Relationships between oxidative stress, liver and kidney variables, trace elements, and clinical status. Parasitol. Res. 2012, 111, 1491–1496. [Google Scholar] [CrossRef]
- López-Alonso, M.; Miranda, M.; García-Partida, P.; Mendez, A.; Castillo, C.; Benedito, J.L. Toxic and trace metal concentrations in liver and kidney of dogs. Biol. Trace Elem. Res. 2007, 116, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; Jones, P.G.; Biourge, V.; van den Ingh, T.S.; Mesu, S.J.; Bode, P.; Rothuizen, J. Dietary management of hepatic copper accumulation in Labrador Retrievers. J. Vet. Intern. Med. 2009, 23, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Cedeno, Y.; Lopez-Alonso, M.; Miranda, M. Hepatic concentrations of copper and other metals in dogs with and without chronic hepatitis. J. Small Anim. Pract. 2016, 57, 703–709. [Google Scholar] [CrossRef]
- Hunt, D.M.; Wake, S.A.; Mercer, J.F.; Danks, D.M. A study of the role of metallothionein in the inherited copper toxicosis of dogs. Biochem. J. 1986, 236, 409–415. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ogra, Y.; Machida, N.; Watanabe, I. Changes in copper, zinc and cadmium distributions in the liver of Formosan squirrels with characteristic high copper accumulation. Metallomics 2019, 11, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Hyun, C.; Filippich, L.J. Inherited canine copper toxicosis in Australian Bedlington Terriers. J. Vet. Sci. 2004, 5, 19–28. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Schall, W.; Yuzbasiyan-Gurkan, V.; Mullaney, T.P.; Pace, C.; Lindgren, J.; Thomas, M.; Padgett, G. Use of zinc acetate to treat copper toxicosis in dogs. J. Am. Vet. Med. Assoc. 1992, 201, 564–568. [Google Scholar]
- Damianaki, K.; Lourenco, J.M.; Braconnier, P.; Ghobril, J.P.; Devuyst, O.; Burnier, M.; Lenglet, S.; Augsburger, M.; Thomas, A.; Pruijm, M. Renal handling of zinc in chronic kidney disease patients and the role of circulating zinc levels in renal function decline. Nephrol. Dial. Transplant. 2020, 35, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.S.; Kim, H.W.; Lee, S.; Nam, K.H.; Yun, H.R.; Jhee, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Dietary zinc intake and incident chronic kidney disease. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Wang, M.-Q.; Hu, R.; Yang, Y.; Huang, Y.-S.; Xian, S.-X.; Lu, L. Effect of zinc supplementation on maintenance hemodialysis patients: A systematic review and meta-analysis of 15 randomized controlled trials. Biomed. Res. Int. 2017, 2017, 1024769. [Google Scholar] [CrossRef]
- Brodzki, A. Copper and zinc concentration in skin neoplastic tissues in dogs. Bull. Vet. Inst. Pulawy 2007, 51, 271–273. [Google Scholar]
- Harro, C.C.; Smedley, R.C.; Buchweitz, J.P.; Langlois, D.K. Hepatic copper and other trace mineral concentrations in dogs with hepatocellular carcinoma. J. Vet. Intern. Med. 2019, 33, 2193–2199. [Google Scholar] [CrossRef]
- Enginler, S.O.; Toydemir, T.S.F.; Ates, A.; Ozturk, B.; Erdogan, O.; Ozdemir, S.; Kirsan, I.; Or, M.E.; Arun, S.S.; Barutcu, U.B. Examination of Oxidative/Antioxidative Status and Trace Element Levels in Dogs with Mammary Tumors. Bulg. J. Agric. Sci. 2015, 5, 1086–1091. [Google Scholar]
- Wang, F.; Jiao, P.; Qi, M.; Frezza, M.; Dou, Q.P.; Yan, B. Turning tumor-promoting copper into an anti-cancer weapon via high-throughput chemistry. Curr. Med. Chem. 2010, 17, 2685–2698. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, K.J.; Ogilvie, G.K.; Fettman, M.J.; Lana, S.E.; Walton, J.A.; Hansen, R.A.; Richardson, K.L.; Hamar, D.W.; Bedwell, C.L.; Andrews, G.; et al. Serum zinc, chromium, and iron concentrations in dogs with lymphoma and osteosarcoma. J. Vet. Intern. Med. 2001, 15, 585–588. [Google Scholar] [CrossRef]
- Brodzki, A.; Brodzki, P.; Tatara, M.R.; Kostro, K. Total antioxidative capacity and zinc concentration in dogs suffering from perianal tumours. Bull. Vet. Inst. Pulawy 2015, 59, 417–423. [Google Scholar] [CrossRef]
- Dash, S.K.; Singh, C.; Singh, G. Mineral status in female dogs with malignant mammary gland tumors fed with different habitual diets. Explor. Anim. Med. Res. 2018, 8, 59–63. [Google Scholar]
- NRC. Mineral. Tolerance of Animals: Second Revised Edition; National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Gurnee, C.M.; Drobatz, K.J. Zinc intoxication in dogs: 19 cases (1991–2003). J. Am. Vet. Med. Assoc. 2007, 230, 1174–1179. [Google Scholar] [CrossRef]
- Blundell, R.; Adam, F. Haemolytic anaemia and acute pancreatitis associated with zinc toxicosis in a dog. Vet. Rec. Case Rep. 2013, 172, 17. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, K.; Chiapella, A.; Weisman, J.; Crofton, L.M.; Hillebrandt, J. Zinc Toxicosis in a Boxer Dog Secondary to Ingestion of Holiday Garland. J. Med. Toxicol. 2017, 13, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Gandini, G.; Bettini, G.; Pietra, M.; Mandrioli, L.; Carpenè, E. Clinical and pathological findings of acute zinc intoxication in a puppy. J. Small Anim. Pract. 2002, 43, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.M.; Loewen, M.E.; Blakley, B.R. Diagnosis and treatment of zinc poisoning in a dog. Vet. Hum. Toxicol. 2004, 46, 272–275. [Google Scholar] [PubMed]
- van der Merwe, D.; Tawde, S. Antacids in the initial management of metallic zinc ingestion in dogs. J. Vet. Pharmacol. Ther. 2009, 32, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Ibim, S.E.M.; Trotman, J.; Musey, P.I.; Semafuko, W.E.B. Depletion of essential elements by calcium disodium EDTA treatment in the dog. Toxicology 1992, 73, 229–237. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kang, M.H.; Park, H.M. Treatment of zinc toxicosis in a dog with chelation using d-penicillamine. J. Vet. Emerg. Crit. Care. 2016, 26, 825–830. [Google Scholar] [CrossRef]
- Siow, J.W. Zinc toxicosis in a dog secondary to prolonged zinc oxide ingestion. Open Vet. J. 2018, 8, 458–462. [Google Scholar] [CrossRef]
| Adult | Puppies | Gestation and Peak Lactation | |
|---|---|---|---|
| Recommended adequate Zn allowances 1 | |||
| mg Zn/1000 kcal ME | 15 | 18.5 | 24 |
| mg Zn/kg DM | 60 | 75 | 96 |
| Recommended minimum Zn allowances 2 | |||
| mg Zn/1000 kcal ME 3 | 18–20.8 | 25 | 25 |
| mg Zn/kg DM 3 | 72–83.4 | 100 | 100 |
| Source | Authorized Zinc Source | Regulation | Reference |
|---|---|---|---|
| Inorganic | Zinc acetate dihydrate | 2016/1095/EU | [67] |
| Zinc chloride anhydrous | |||
| Zinc oxide | |||
| Zinc sulfate monohydrate/heptahydrate | |||
| Zinc chloride hydroxide monohydrate | |||
| Organic | Zinc chelate of amino acids hydrate | ||
| Zinc chelate of glycine hydrate | |||
| Zinc chelate of protein hydrolysates | |||
| Zinc bislysinate | 2016/973/EU | [68] | |
| Zinc chelate of hydroxy analog of methionine | 2010/335/EU | [69] | |
| Zinc chelate of methionine sulfate | 2019/1125/EU | [70] |
| Design/Duration/Zn Restriction 1 | Subjects | Zn Forms | Level Zn 3 | Biomarker of Zinc Status | Reference |
|---|---|---|---|---|---|
| 2 wks length for 3 Zn sources; 3 wks washout between each Zn source/2 wks 2 | 6 male Beagles/ 12 wks | Zinc sulfate | 0 | Fecal, plasma, and urinary [Zn], apparent fecal absorption | [5] |
| Zinc acetate | 2 mg/kg BW | ||||
| Zinc oxide | 4 mg/kg BW | ||||
| Randomized block design/35 days/ 5.4 mg/kg feed | 42 puppies/11 wks | Zinc oxide Zinc propionate | 40 4 | Weight gain, plasma [Zn], declaws, teeth, and testes [Zn] | [8] |
| 1 meal test for each Zn form with 1 wk between them/2 wks/ 56 mg/kg feed | 4 adult Beagles | Zinc oxide Zinc amino acid chelate | 50 | Fecal, plasma and urinary [Zn], AUC | [10] |
| 6 × 4 randomized block design/25 days per treatment/no restriction | 4 adult Beagles | Zinc oxide 5 Zinc amino acid Chelate 5 Zinc polysaccharide 5,6 | 50 | Fecal [Zn], hair growth rate, hair [Zn] | [9] |
| Randomized block design/20 days/30 days/ 56 mg/kg feed | 27 adult Beagles | Zinc oxide | 50 | Hair growth, hair [Zn], serum AP, AUC5 | [7] |
| Zinc amino acid chelate | 75 | ||||
| Zinc polysaccharide | 100 | ||||
| Cross-over design/3 wks 2 wks/58.5 mg/kg DM | 4 female Beagles | Zinc sulfate Zinc methionylglycinate | ≈61.5 | Hair growth, hair [Zn], plasma and fecal [Zn], serum ALT, zinc absorption | [41] |
| 3 Latin Squares 4 × 4/4 diets, 4 periods of 5 wks/no restriction | 12 Beagles/ 1 year | Zinc sulfate 7 | 75 | Plasma, hair, and urinary [Zn], serum, ALT, SOD activity and CRP, CD4/CD8 ratio in peripheral blood, coat quality (brightness, softness, greasiness, and scale) and growth (trichogram), CTTAD, flatulence | [13] |
| Zinc proteinate 7 | |||||
| Randomized block design/28 d/50 mg/kg DM (7 days) | 30 Hound-cross Mongrel/ 8 wks | Zinc oxide | 50 8 | Weight gain, hair weight and length, plasma and hair [Zn], liver, cortisol, bone, and total AP, concentrations, blood MT gene expression | [12] |
| Zinc methionine | 100 8 | ||||
| Randomized block design/30 days/no restriction | 18 dogs many breeds/ 2—6 years | Zinc oxide Zinc HMTBa 9 | 40 | Coat quality (brightness, looseness, texture, greasiness), whole-blood and hair [Zn], antibody against sheep red blood cells | [11] |
| Randomized block design/reproduction (≈12 wks) 10/ no restriction | 34 female Beagles/> 1 year + newborn puppies | Zinc oxide | 53 | BW (dams) and weight gain (lactation from birth to 6 wks), litter size, hair [Zn], hair and root morphology by scanning electron microscopy | [6] |
| Zinc proteinate 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.M.; Maia, M.R.G.; Fonseca, A.J.M.; Cabrita, A.R.J. Zinc in Dog Nutrition, Health and Disease: A Review. Animals 2021, 11, 978. https://doi.org/10.3390/ani11040978
Pereira AM, Maia MRG, Fonseca AJM, Cabrita ARJ. Zinc in Dog Nutrition, Health and Disease: A Review. Animals. 2021; 11(4):978. https://doi.org/10.3390/ani11040978
Chicago/Turabian StylePereira, Ana Margarida, Margarida R. G. Maia, António José Mira Fonseca, and Ana Rita Jordão Cabrita. 2021. "Zinc in Dog Nutrition, Health and Disease: A Review" Animals 11, no. 4: 978. https://doi.org/10.3390/ani11040978
APA StylePereira, A. M., Maia, M. R. G., Fonseca, A. J. M., & Cabrita, A. R. J. (2021). Zinc in Dog Nutrition, Health and Disease: A Review. Animals, 11(4), 978. https://doi.org/10.3390/ani11040978







