Fucoxanthin Exerts Anti-Tumor Activity on Canine Mammary Tumor Cells via Tumor Cell Apoptosis Induction and Angiogenesis Inhibition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Reagents
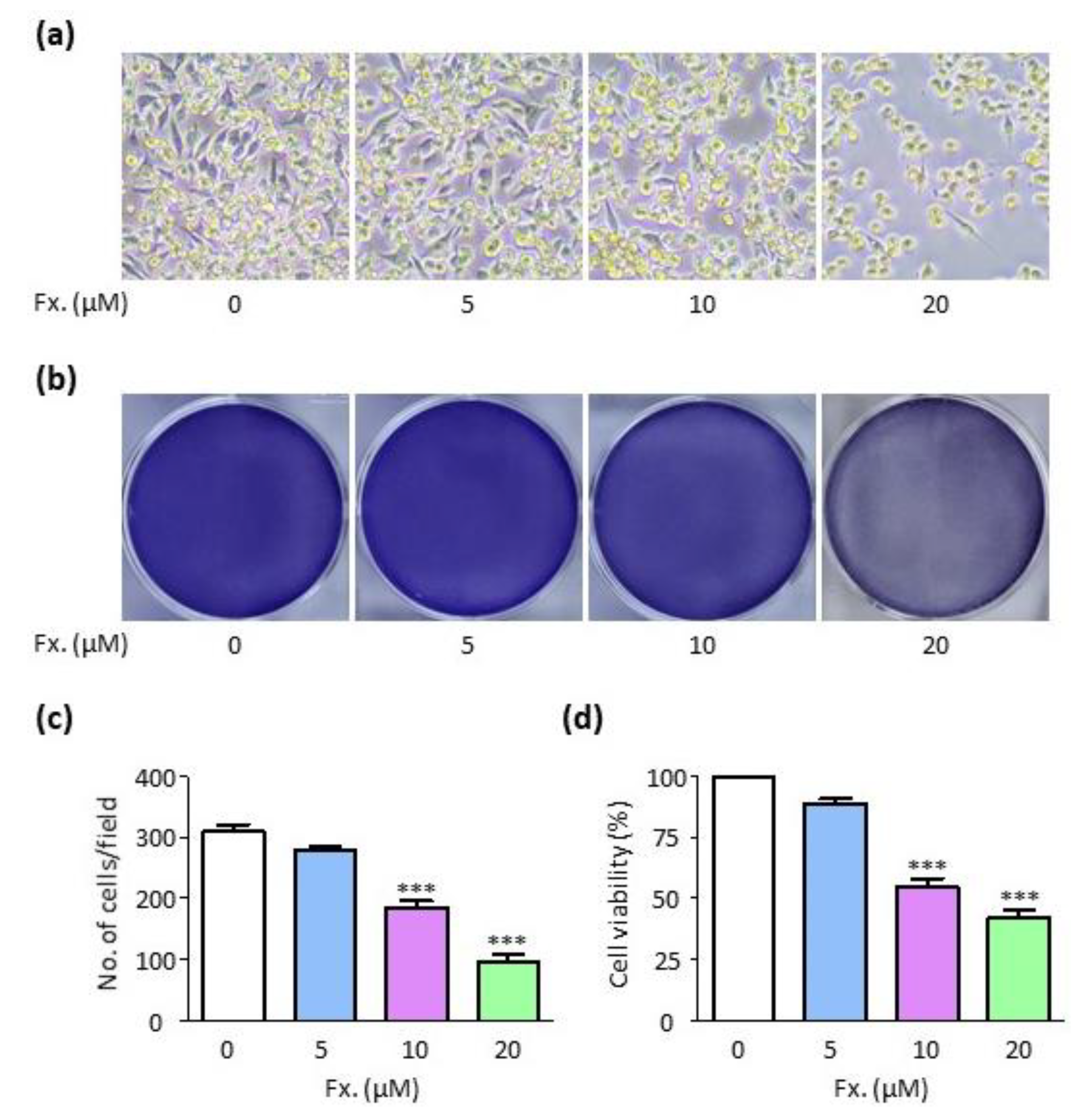
2.2. Cell Viability Assay
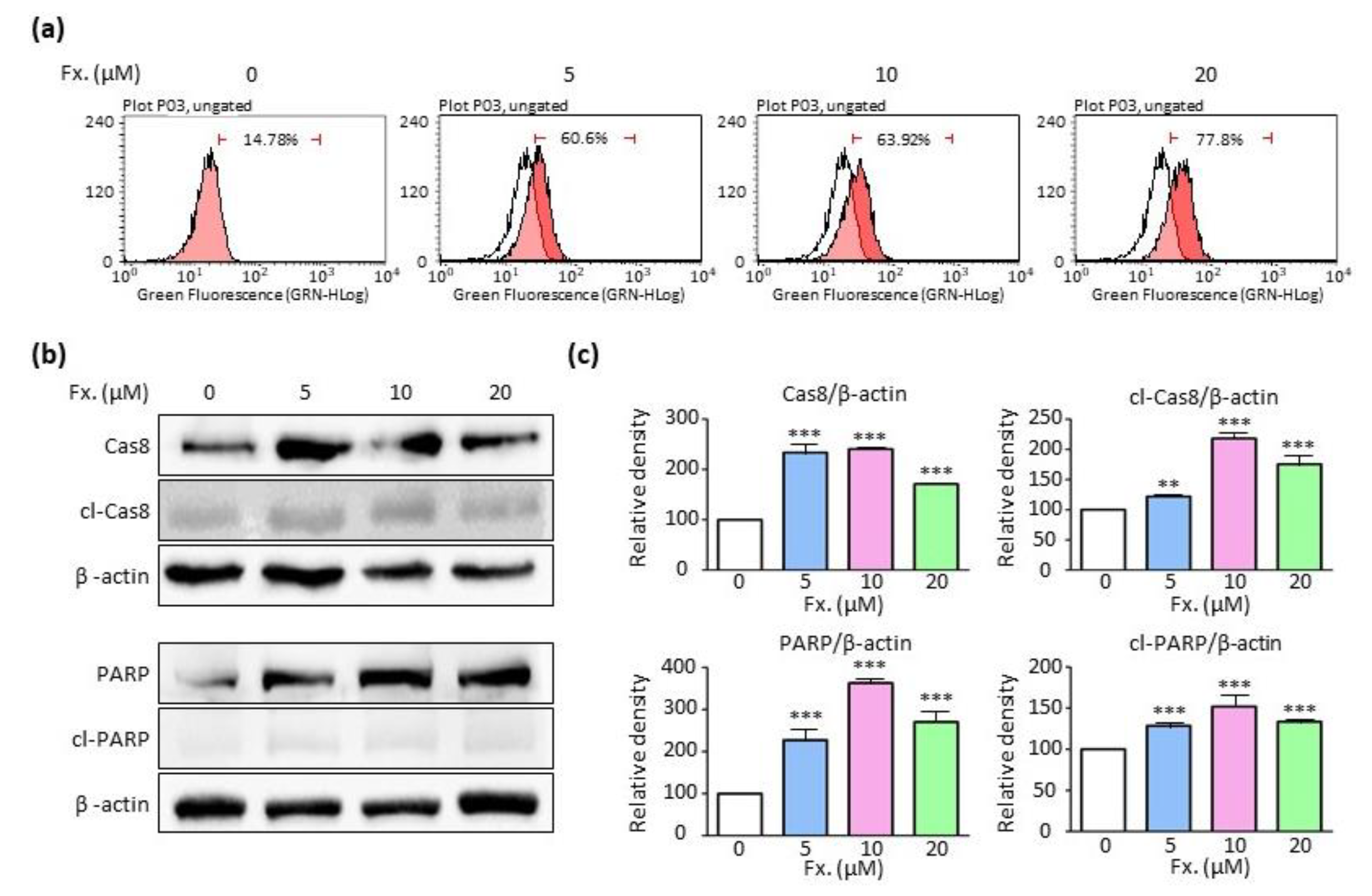
2.3. Annexin V Assay
2.4. Western Blotting
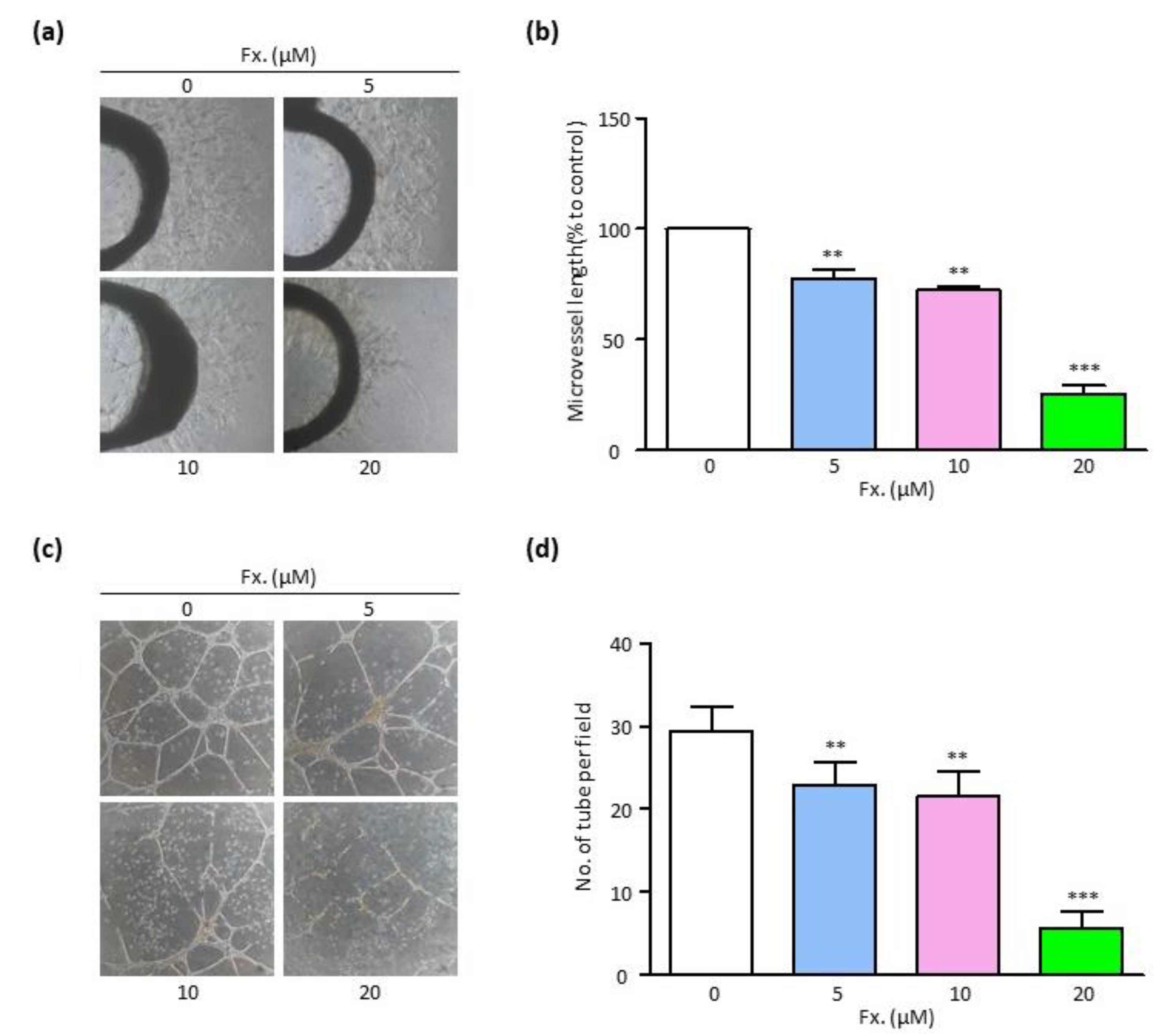
2.5. Rat Aortic Ring Assay
2.6. In Vitro Tube Formation Assay
2.7. In Vitro Migration Assay
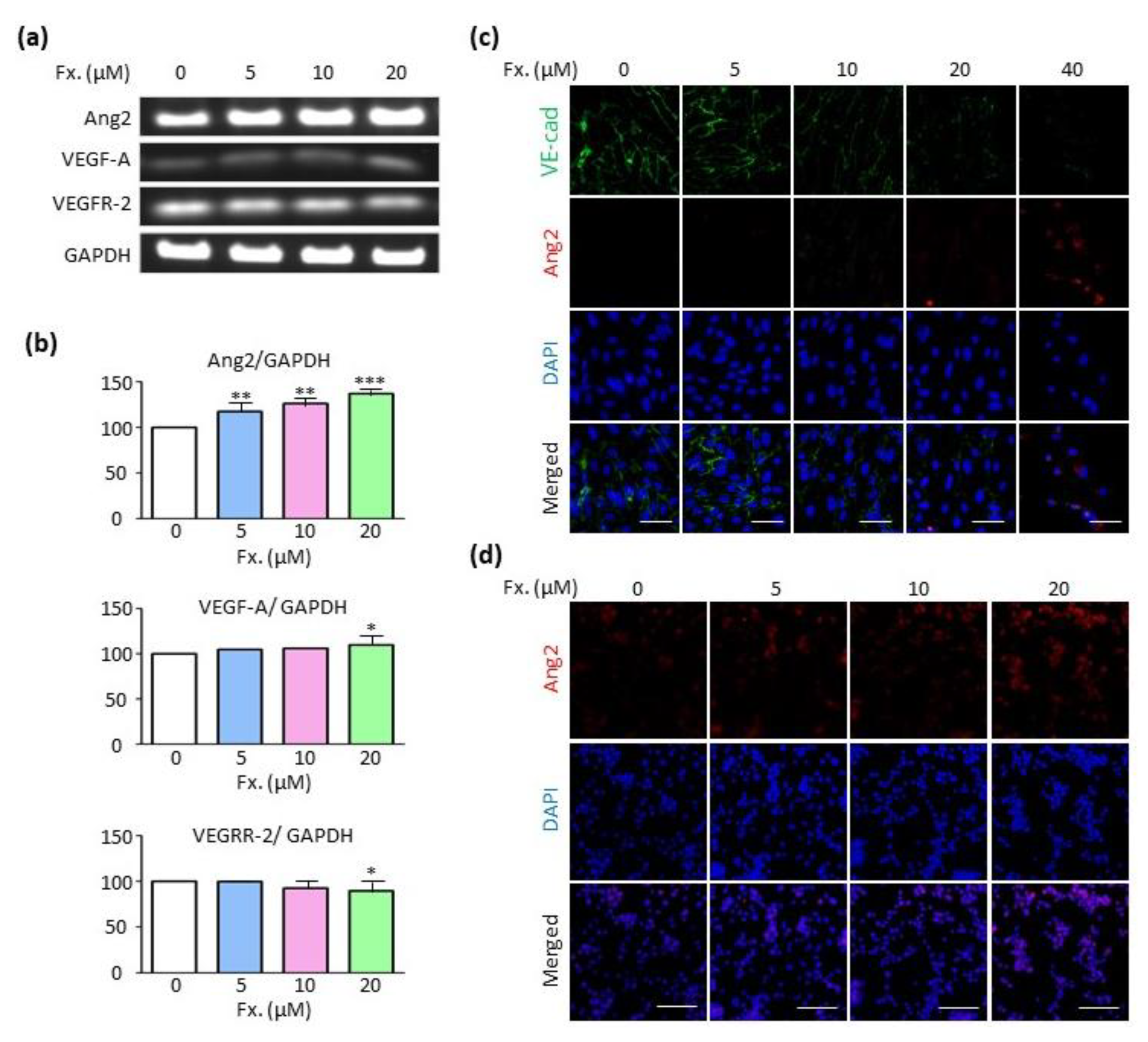
2.8. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.9. Immunocytochemistry
2.10. Statistical Analyses
3. Results
3.1. Fucoxanthin Reduces Cell Viability in CMT-U27 Cells
3.2. Fucoxanthin Causes Tumor Cell Death by Inducing Apoptosis
3.3. Fucoxanthin Suppresses Endothelial Cell Sprouting and Tube Formation
3.4. Fucoxanthin Inhibits the Cell Migration in CMT-U27 Cells and HUVECs
3.5. Fucoxanthin Regulates Ang2 Expression in the Absence of the Vascular Endothelial Growth Factor A (VEGF-A)/Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef]
- Moe, L. Population-based incidence of mammary tumours in some dog breeds. J. Reprod. Fertil. Suppl. 2001, 57, 439–443. [Google Scholar]
- Canadas-Sousa, A.; Santos, M.; Leal, B.; Medeiros, R.; Dias-Pereira, P. Estrogen receptors genotypes and canine mammary neoplasia. BMC Vet. Res. 2019, 15, 1–10. [Google Scholar] [CrossRef]
- Dall, G.V.; Hawthorne, S.; Seyed-Razavi, Y.; Vieusseux, J.; Wu, W.; Gustafsson, J.-A.; Byrne, D.; Murphy, L.; Risbridger, G.P.; Britt, K.L. Estrogen receptor subtypes dictate the proliferative nature of the mammary gland. J. Endocrinol. 2018, 237, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Sabattini, S.; Vascellari, M.; Marconato, L. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radiation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet. Comp. Oncol. 2018, 16, 497–504. [Google Scholar] [CrossRef]
- Bakirel, T.; Ustun Alkan, F.; Ustuner, O.; Çinar, S.; Anlas, C.; Bilge Sari, A. Response of cultured normal canine mammary epithelial cells to deracoxib—doxorubicin combination. Acta Vet. Hung. 2017, 65, 366–381. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Lafioniatis, S. Recent advances on canine mammary cancer chemotherapy: A review of studies from 2000 to date. Breast Cancer Res. 2016, 29, 43. [Google Scholar]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; De Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A Treasure from the Sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid. Based Complement Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Costa, E.; Abreu, M.; Gargiulo, D.; Rocha, E.; Ramos, A.A. Anticancer effects of seaweed compounds fucoxanthin and phloroglucinol, alone and in combination with 5-fluorouracil in colon cells. J. Toxicol. Environ. Health Part A 2017, 80, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Foo, S.C.; Yusoff, F.M.; Imam, M.U.; Foo, J.B.; Ismail, N.; Azmi, N.H.; Tor, Y.S.; Khong, N.M.; Ismail, M. Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways. Biotechnol. Rep. 2019, 21, e00296. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Afzal, S.; Elwakeel, A.; Sharma, D.; Radhakrishnan, N.; Dhanjal, J.K.; Sundar, D.; Kaul, S.C.; Wadhwa, R. Marine carotenoid fucoxanthin possesses anti-metastasis activity: Molecular evidence. Mar. Drugs 2019, 17, 338. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Asai, A.; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005, 220, 75–84. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Terasaki, M.; Nagao, A. Characterization of apoptosis induced by fucoxanthin in human promyelocytic leukemia cells. Biosci. Biotechnol. Biochem. 2005, 69, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Wu, K.; Yang, Y.; Yang, Y.; Wang, Y.; Li, J. Dandelion Polysaccharide Exerts Anti-Angiogenesis Effect on Hepatocellular Carcinoma by Regulating VEGF/HIF-1α Expression. Front. Pharmacol. 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Metibemu, D.S.; Akinloye, O.A.; Akamo, A.J.; Okoye, J.O.; Ojo, D.A.; Morifi, E.; Omotuyi, I.O. VEGFR-2 kinase domain inhibition as a scaffold for anti-angiogenesis: Validation of the anti-angiogenic effects of carotenoids from Spondias mombin in DMBA model of breast carcinoma in Wistar rats. Toxicol. Rep. 2021, 8, 489–498. [Google Scholar] [CrossRef]
- Zare, M.; Norouzi Roshan, Z.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention/treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 522–534. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Fromm, S.; Cunningham, C.; Dunne, M.; Veale, D.; Fearon, U.; Wade, S. Enhanced angiogenic function in response to fibroblasts from psoriatic arthritis synovium compared to rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Yin, H.; Chen, C.-Y.; Liu, Y.-W.; Tan, Y.-J.; Deng, Z.-L.; Yang, F.; Huang, F.-Y.; Wen, C.; Rao, S.-S.; Luo, M.-J. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics 2019, 9, 2678. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Agrawal, S. Brucine, an indole alkaloid from Strychnos nux-vomica attenuates VEGF-induced angiogenesis via inhibiting VEGFR2 signaling pathway in vitro and in vivo. Cancer Lett. 2013, 332, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Giannotta, M.; Trani, M.; Dejana, E. VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 2013, 26, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Ustün Alkan, F.; Ustüner, O.; Bakırel, T.; Cınar, S.; Erten, G.; Deniz, G. The effects of piroxicam and deracoxib on canine mammary tumour cell line. Sci. World J. 2012, 2012, 976740. [Google Scholar] [CrossRef] [PubMed]
- Masferrer, J.L.; Leahy, K.M.; Koki, A.T.; Zweifel, B.S.; Settle, S.L.; Woerner, B.M.; Edwards, D.A.; Flickinger, A.G.; Moore, R.J.; Seibert, K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000, 60, 1306–1311. [Google Scholar]
- Laird, A.D.; Vajkoczy, P.; Shawver, L.K.; Thurnher, A.; Liang, C.; Mohammadi, M.; Schlessinger, J.; Ullrich, A.; Hubbard, S.R.; Blake, R.A. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000, 60, 4152–4160. [Google Scholar] [PubMed]
- Bråkenhielm, E.; Veitonmäki, N.; Cao, R.; Kihara, S.; Matsuzawa, Y.; Zhivotovsky, B.; Funahashi, T.; Cao, Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA 2004, 101, 2476–2481. [Google Scholar] [CrossRef]
- Walker, N.; Harmon, B.; Gobe, G.; Kerr, J. Patterns of cell death. Methods Achiev. Exp. Pathol. 1988, 13, 18–54. [Google Scholar]
- Edinger, A.L.; Thompson, C.B. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004, 16, 663–669. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, C.; Neid, M. Cancer invasion and metastasis. Oncology 2005, 69, 14–16. [Google Scholar] [CrossRef]
- Jones, D.H.; Nakashima, T.; Sanchez, O.H.; Kozieradzki, I.; Komarova, S.V.; Sarosi, I.; Morony, S.; Rubin, E.; Sarao, R.; Hojilla, C.V. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006, 440, 692–696. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Dobrucki, L.W.; Tsutsumi, Y.; Kalinowski, L.; Dean, J.; Gavin, M.; Sen, S.; Mendizabal, M.; Sinusas, A.J.; Aikawa, R. Analysis of angiogenesis induced by local IGF-1 expression after myocardial infarction using microSPECT-CT imaging. J. Mol. Cell Cardiol. 2010, 48, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Ongusaha, P.P.; Kwak, J.C.; Zwible, A.J.; Macip, S.; Higashiyama, S.; Taniguchi, N.; Fang, L.; Lee, S.W. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res. 2004, 64, 5283–5290. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef]

| Gene | Primer Sequence | Size (bp) |
|---|---|---|
| Ang2 | 5′-GGATCTGGGGAGAGAGGAAC-3′ 5′-CTCTGCACCGAGTCATCGTA-3′ | 535 |
| VEGF-A | 5′- TGCAGATTATGCGGATCAAACC -3′ 5′- TGCATTCACATTTGTTGTGCTGTAG -3′ | 81 |
| VEGFR-2 | 5′-CCAGCAAAAGCAGGGAGTCTGT-3′ 5′-TGTCTGTGTCATCGGAGTGATATCC-3′ | 87 |
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ 5′-TCCACCACCCTGTTGCTGTA-3′ | 452 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.; Choi, J.; Park, J.-K.; Won, G.; Seol, J.-W. Fucoxanthin Exerts Anti-Tumor Activity on Canine Mammary Tumor Cells via Tumor Cell Apoptosis Induction and Angiogenesis Inhibition. Animals 2021, 11, 1512. https://doi.org/10.3390/ani11061512
Jang H, Choi J, Park J-K, Won G, Seol J-W. Fucoxanthin Exerts Anti-Tumor Activity on Canine Mammary Tumor Cells via Tumor Cell Apoptosis Induction and Angiogenesis Inhibition. Animals. 2021; 11(6):1512. https://doi.org/10.3390/ani11061512
Chicago/Turabian StyleJang, Hyuk, Jawun Choi, Jeong-Ki Park, Gayeon Won, and Jae-Won Seol. 2021. "Fucoxanthin Exerts Anti-Tumor Activity on Canine Mammary Tumor Cells via Tumor Cell Apoptosis Induction and Angiogenesis Inhibition" Animals 11, no. 6: 1512. https://doi.org/10.3390/ani11061512
APA StyleJang, H., Choi, J., Park, J.-K., Won, G., & Seol, J.-W. (2021). Fucoxanthin Exerts Anti-Tumor Activity on Canine Mammary Tumor Cells via Tumor Cell Apoptosis Induction and Angiogenesis Inhibition. Animals, 11(6), 1512. https://doi.org/10.3390/ani11061512






