In Vitro Screening of Sugarcane Cultivars (Saccharum spp. Hybrids) for Tolerance to Polyethylene Glycol-Induced Water Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material Selection and In Vitro Establishment
2.2. In Vitro Selection Pressure with PEG
2.3. Osmotic Potential Measurements of the Culture Medium
2.4. Total Protein Estimation
2.5. Proline Determination
2.6. Glycine-Betaine (GB) Determination
2.7. Experimental Design and Data Analysis
3. Results
3.1. PEG-Induced Osmotic Stress
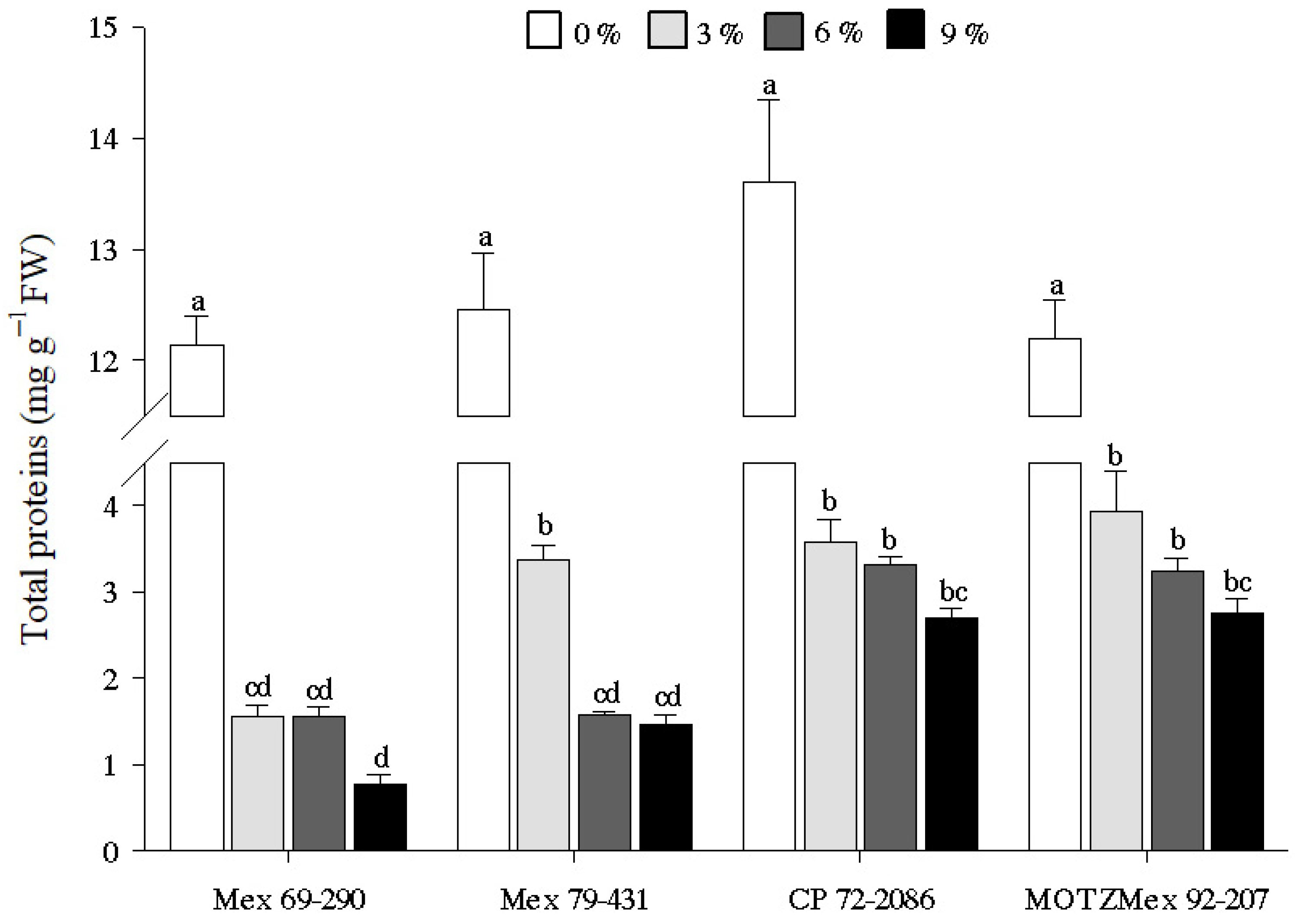
3.2. Total Protein Content
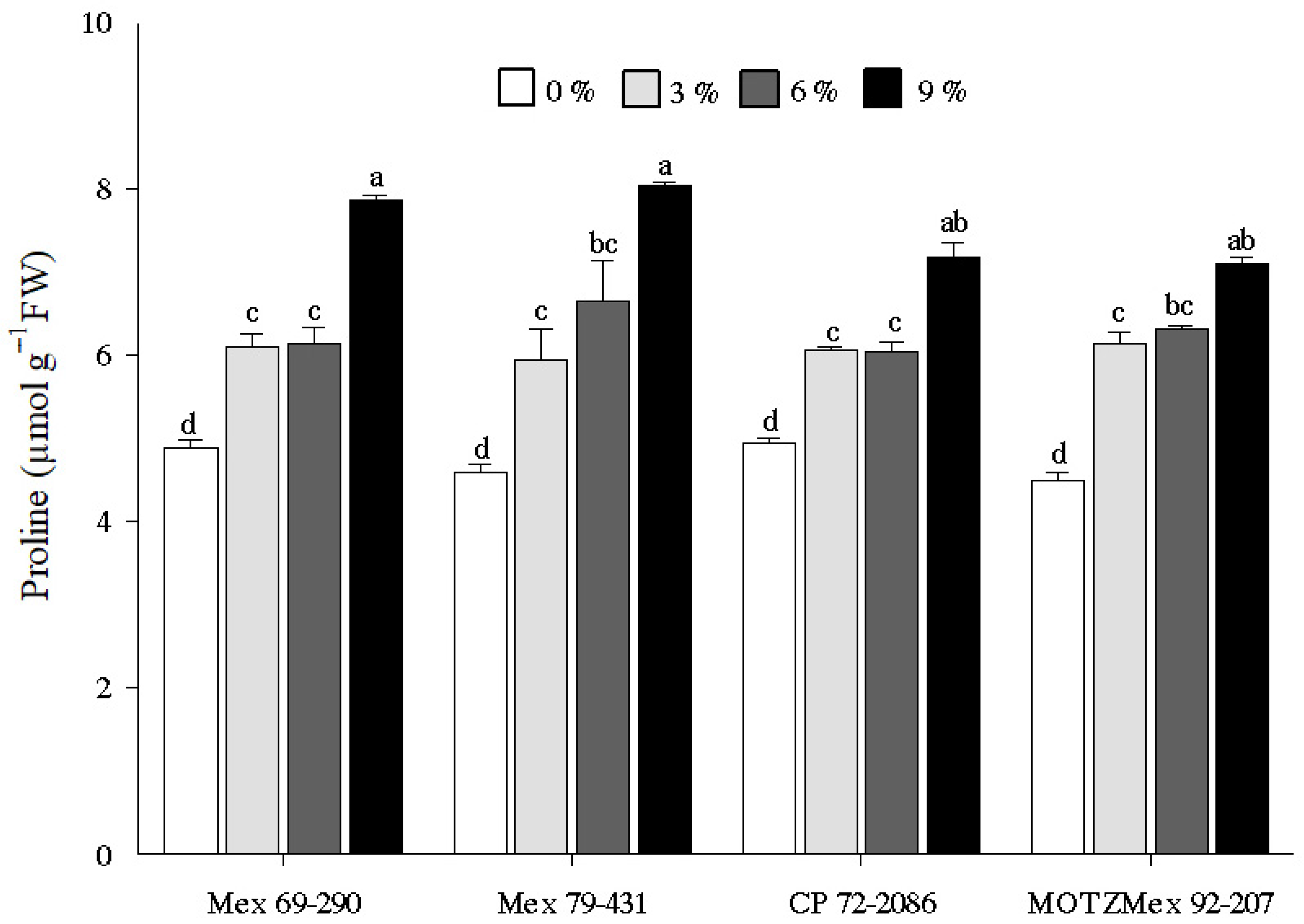
3.3. Proline Content
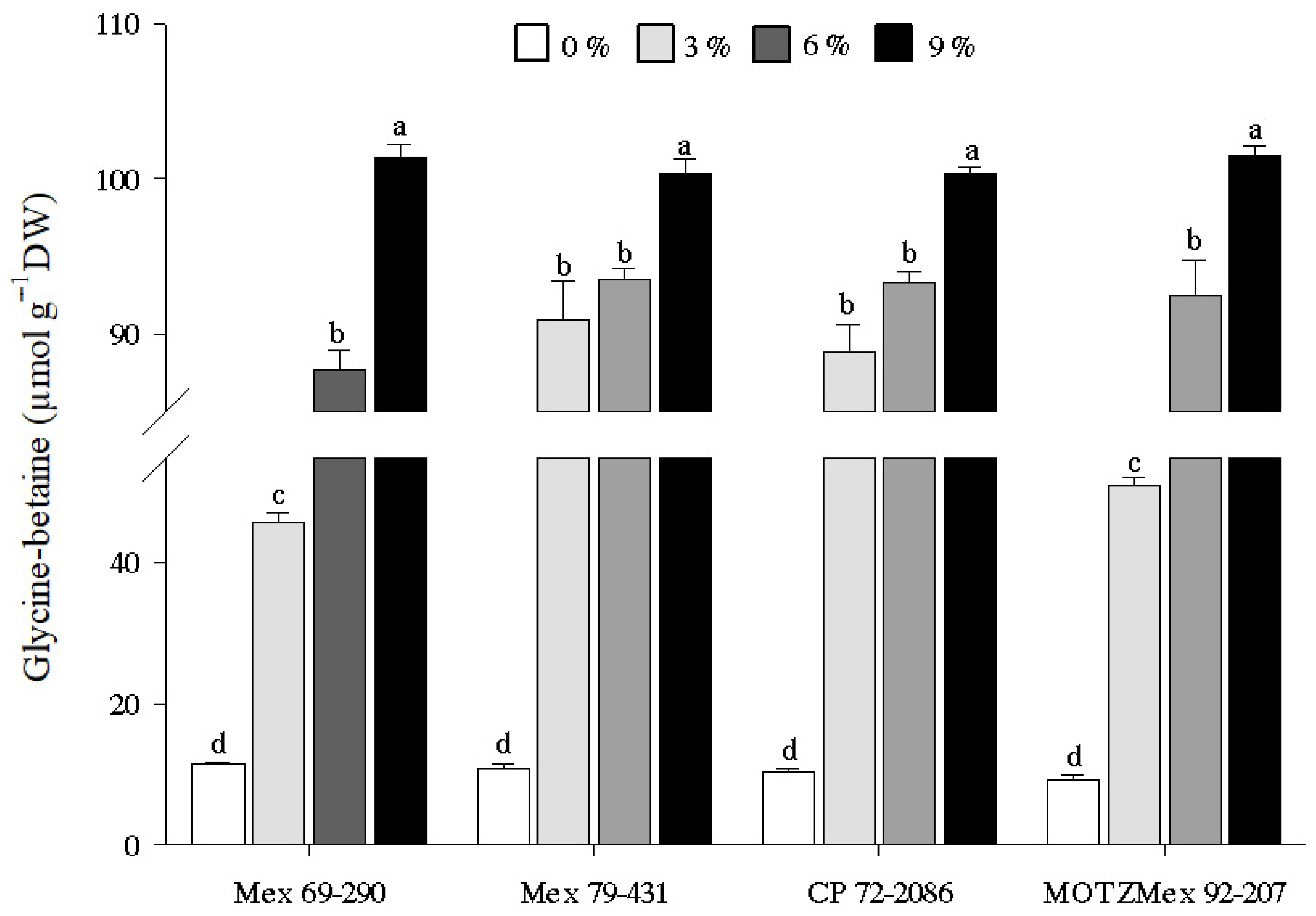
3.4. Glycine-Betaine Content
4. Discussion
4.1. Effect of PEG on In Vitro Development of Sugarcane Seedlings
4.2. Quantification of Total Protein Content
4.3. Proline Content Quantification
4.4. Quantification of Glycine-Betaine Content
4.5. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sentíes-Herrera, H.; Trejo-Téllez, L.; Gómez-Merino, F. The Mexican sugarcane production system: History, current status, and new trends. In Sugarcane: Production Systems, Uses and Economic Importance; Murphy, R., Ed.; Nova Science Publishers: New York, NY, USA, 2017; pp. 39–71. [Google Scholar]
- Mustafa, G.; Joyia, F.; Anwar, S.; Parvaiz, A.; Khan, M. Biotechnological interventions for the improvement of sugarcane crop and sugar production. In Sugarcane-Technology and Research; De Oliveira, A., Ed.; Intech Open: London, UK, 2018; pp. 113–138. [Google Scholar] [CrossRef] [Green Version]
- Sentiés-Herrera, H.E.; Gómez-Merino, F.C.; Valdez-Balero, A.; Silva-Rojas, H.V.; Trejo-Téllez, L.I. The agro-Industrial sugarcane system in Mexico: Current status, challenges and opportunities. J. Agric. Sci. 2014, 6, 26. [Google Scholar] [CrossRef]
- Gomathi, R.; Krishnapriya, V.; Arunkumar, R.; Govindaraj, P.; Ram, B. Physiological traits imparting drought stress tolerance to promising sugarcane (Saccharum spp.) clones. Plant Physiol. Rep. 2020, 25, 509–515. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R. Plant tissue culture environment as a switch-key of (epi) genetic changes. Pant Cell Tiss. Org. 2020, 140, 245–257. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.U.; Ali, G.; Dar, Z.A.; Maqbool, S.; Khulbe, R.K.; Bhat, A. Effect of Peg Induced Drought Stress on Maize (Zea mays L.) INBREDS. Plant Arch. 2019, 19, 1677–1681. [Google Scholar]
- Steuter, A.A.; Mozafar, A.; Goodin, J.R. Water potential of aqueous polyethylene glycol. Plant Physiol. 1981, 67, 64–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotech. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Naveed, M.S.; Manzoor, A.; Javed, A.; Tariq, M.A. In-vitro screening of different tomato genotypes against peg induced water stress. World J. Biol. Biotech. 2019, 4, 15–19. [Google Scholar] [CrossRef]
- Eliane, M.T.; Modeste, K.K.; André, S.B.; Edmond, K.K.; Mongomaké, K. Effect of water stress induced by polyethylene glycol 6000 on somatic embryogenesis in Cocoa (Theobroma cacao L.). Agric. Sci. 2019, 10, 1240–1254. [Google Scholar] [CrossRef] [Green Version]
- Raj, R.N.; Gokulakrishnan, J.; Prakash, M. Assessing drought tolerance using PEG-6000 and molecular screening by SSR markers in maize (Zea mays L.) hybrids. Maydica 2020, 64, 7. [Google Scholar]
- Koskosidis, A.; Ebrahim, K.; Mavromatis, A.; Pavli, O.; Vlachostergios, D.N. Effect of PEG-induced drought stress on germination of ten chickpea (Cicer arietinum L.) genotypes. Not. Bot. Horti. Agrobo. 2020, 48, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Singh, S.K.; Srivastav, M.; Prakash, J.; Lal, S.K.; Padaria, J.C.; Goswani, A.K.; Maurya, N.K. Poly ethylene glycol mediated in vitro screening and physico-biochemical changes induced in mango callus due to moisture stress. Pant Cell Tiss. Org. 2021, 1–18. [Google Scholar] [CrossRef]
- Joshi, R.; Shukla, A.; Sairam, R.K. In vitro screening of rice genotypes for drought tolerance using polyethylene glycol. Acta Physiol. Plant. 2011, 33, 2209. [Google Scholar] [CrossRef]
- Tsago, Y.; Andargie, M.; Takele, A. In vitro selection of sorghum (Sorghum bicolor L.) Moench) for polyethylene glycol (PEG) induced drought stress. Plant Sci. Today 2014, 1, 62–68. [Google Scholar] [CrossRef]
- Kaur, A.; Sarlach, R.S. Leaf area, relative water content and stay-green habit of iranian landraces (Triticum aestivum L.) under water stress in field conditions. Adv. Res. 2020, 21, 1–13. [Google Scholar] [CrossRef]
- Patade, V.Y.; Penna, S.; Kulkarni, U.G.; Bapat, V.A. Molecular profiling using RAPD technique of salt and drought tolerant regenerants of sugarcane. Sugar. Tech. 2006, 8, 63–68. [Google Scholar] [CrossRef]
- Hemaprabha, G.; Swapna, S.; Lavanya, D.L.; Sajitha, B.; Venkataramana, S. Evaluation of drought tolerance potential of elite genotypes and progenies of sugarcane (Saccharum sp. hybrids). Sugar. Tech. 2013, 15, 9–16. [Google Scholar] [CrossRef]
- Nikam, A.A.; Devarumath, R.M.; Shitole, M.G.; Ghole, V.S.; Tawar, P.N.; Suprasanna, P. Gamma radiation, in vitro selection for salt (NaCl) tolerance, and characterization of mutants in sugarcane (Saccharum officinarum L.). Vitro Cell. Dev. Biol. 2014, 50, 766–776. [Google Scholar] [CrossRef]
- De Oliveira, L.A.R.; Cardoso, M.N.; de Oliveira, A.C.A.; Machado, C.D.A.; Cardoso, B.T.; Muniz, A.D.S.; Ledo, A.D.S. Effects of in vitro drought stress on growth, proline accumulation and antioxidant defense in sugarcane. J. Agric. Sci. 2018, 10, 135–149. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; y Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Sahoo, M.R.; Dasgupta, M.; Kole, P.C.; Mukherjee, A. Photosynthetic, physiological and biochemical events associated with polyethylene glycol-mediated osmotic stress tolerance in taro (Colocasia esculenta L. Schott). Photosynthetica 2018, 56, 1069–1080. [Google Scholar] [CrossRef]
- Megha, B.R.; Mummigatti, U.V.; Chimmad, V.P.; Aladakatti, Y.R. Evaluation of hirsutum cotton genotypes for water stress using PEG-6000 by slanting glass plate technique. Int. J. Pure Appl. Biosci. 2017, 5, 740–750. [Google Scholar] [CrossRef]
- Kumar, A.P.; Reddy, N.N.; Lakshmi, J.N. PEG Induced screening for drought tolerance in tomato genotypes. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Leishman, M.R.; Westoby, M. The role of seed size in seedling establishment in dry soil conditions–experimental evidence from semi-arid species. J. Ecol. 1994, 82, 249–258. [Google Scholar] [CrossRef]
- Razzaq, H.; Nadeem Tahir, M.H.; Ahmad Sadaqat, H.; Sadia, B. Screening of sunflower (Helianthus annus L.) accessions under drought stress conditions, an experimental assay. J. Soil Sci. Plant Nutr. 2017, 17, 662–671. [Google Scholar] [CrossRef]
- Aazami, M.A.; Torabi, M.; Jalili, E. In vitro response of promising tomato genotypes for tolerance to osmotic stress. Afr. J. Biotechnol. 2010, 9, 4014–4017. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Tokarz, K.; Makowski, W.; Łukasiewicz, A. Different acclimatization mechanisms of two grass pea cultivars to osmotic stress in in vitro culture. Acta Physiol. Plant. 2017, 39, 96. [Google Scholar] [CrossRef] [Green Version]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Yu, Q.; Li, C.; Zhang, J.; Tian, Y.; Wang, H.; Zhang, Y.; Zhang, Z.; Xiang, Q.; Han, X.; Zhang, L. Genome-wide identification and expression analysis of the Dof gene family under drought stress in tea (Camellia sinensis). PeerJ 2020, 8. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. LEA proteins during water stress: Not just for plants anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, R.K.; Sonah, H.; Bélanger, R.R. Plant aquaporins: Genome-wide identification, transcriptomics, proteomics, and advanced analytical tools. Front. Plant Sci. 2016, 7, 1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, A.; Habibi, D.; Rohami, M.; Mafakheri, S. Effect of drought stress on antioxidant enzymes activity of some chickpea cultivars. Am Euras. J. Agric. Environ. Sci. 2011, 11, 782–785. [Google Scholar]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Bioch. 2017, 118, 427–437. [Google Scholar] [CrossRef]
- Harfouche, A.; Meilan, R.; Altman, A. Molecular and physiological responses to abiotic stress in forest trees and their relevance to tree improvement. Tree Physiol. 2014, 34, 1181–1198. [Google Scholar] [CrossRef] [Green Version]
- Thangella, P.A.; Pasumarti, S.N.; Pullakhandam, R.; Geereddy, B.R.; Daggu, M.R. Differential expression of leaf proteins in four cultivars of peanut (Arachis hypogaea L.) under water stress. 3 Biotech 2018, 8, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Celletti, S.; Cristofori, V.; Astolfi, S.; Ruggiero, B.; Rugini, E. Olive (Olea europaea L.) plants transgenic for tobacco osmotin gene are less sensitive to in vitro-induced drought stress. Acta Physiol. Plant. 2017, 39, 1–9. [Google Scholar] [CrossRef]
- Qayyum, A.; Razzaq, A.; Bibi, Y.; Khan, S.U.; Abbasi, K.S.; Sher, A.; Mehmood, A.; Ahmed, W.; Mahmood, I.; Manaf, A.; et al. Water stress effects on biochemical traits and antioxidant activities of wheat (Triticum aestivum L.) under In vitro conditions. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 283–290. [Google Scholar] [CrossRef]
- Mozafari, A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tiss. Org. 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Rao, S.; Jabeen, F.T.Z. In vitro selection and characterization of polyethylene glycol (PEG) tolerant callus lines and regeneration of plantlets from the selected callus lines in sugarcane (Saccharum officinarum L.). Physiol. Mol. Biol. Plants 2013, 19, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Hajihashemi, S.; Ehsanpour, A.A. Influence of exogenously applied paclobutrazol on some physiological traits and growth of Stevia rebaudiana under in vitro drought stress. Biologia 2013, 68, 414–420. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. Effect of silicon (Si) application on Phoenix dactylifera L. growth under drought stress induced by polyethylene glycol (PEG) in vitro. Am. J. Plant Sci. 2016, 7, 1711–1728. [Google Scholar] [CrossRef] [Green Version]
- Razavizadeh, R.; Komatsu, S. Changes in essential oil and physiological parameters of callus and seedlings of Carum copticum L. under in vitro drought stress. J. Food Meas. Charact. 2018, 12, 1581–1592. [Google Scholar] [CrossRef]
- Gao, H.; Xu, D.; Zhang, H.; Cheng, X.; Yang, Q. Effects of culture medium composition and PEG on hyperhydricity in Dendrobium officinale. In Vitro Cell. Dev. Biol. Plant 2020, 56, 143–149. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Murali, P.V.; Panneerselvam, R. Drought stress induced biochemical alterations in two varieties of Paspalum scrobiculatum L. Int. J. Curr. Sci. 2013, 7, 80–96. [Google Scholar]
- Huang, T.; Jander, G. Abscisic acid-regulated protein degradation causes osmotic stress-induced accumulation of branched-chain amino acids in Arabidopsis thaliana. Planta 2017, 246, 737–747. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Huang, B. Protein alterations in tall fescue in response to drought stress and abscisic acid. Crop. Sci. 2002, 42, 202–207. [Google Scholar] [CrossRef]
- Kishor, P.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Bioch. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Rai, M.K.; Kalia, R.K.; Singh, R.; Gangola, M.P.; Dhawan, A.K. Developing stress tolerant plants through in vitro selection—An overview of the recent progress. Environ. Exp. Bot. 2011, 71, 89–98. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Murmu, K.; Murmu, S.; Kundu, C.K.; Bera, P.S. Exogenous proline and glycine betaine in plants under stress tolerance. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 901–913. [Google Scholar] [CrossRef] [Green Version]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Chandra, P.; Wunnava, A.; Verma, P.; Chandra, A.; Sharma, R.K. Strategies to mitigate the adverse effect of drought stress on crop plants—influences of soil bacteria: A review. Pedosphere 2021, 31, 496–509. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, S.; Saxena, S. Biomass yield and steviol glycoside production in callus and suspension culture of Stevia rebaudiana treated with proline and polyethylene glycol. Appl. Biochem. Biotech. 2015, 176, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Ahmadi, A.; Hashemi, J.; Abbasi, A.; Tavarini, S.; Pompeiano, A.; Guglielminetti, L.; Angelini, L.G. Plant growth retardants (PGRs) affect growth and secondary metabolite biosynthesis in Stevia rebaudiana Bertoni under drought stress. S. Afr. J. Bot. 2019, 121, 394–401. [Google Scholar] [CrossRef]
- Obaid, O.H.; Reddy, S.K. In vitro Selection of Drought Stress Tolerance in Rice (Oryza sativa L.) var. Jarava. Plant Tissue Cult. Biotech. 2019, 29, 127–131. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Kumar, P.; Malik, P.S.; Kumar, M. Proline accumulation in the leaves of four potato cultivars in response to water stress. Plant Arch. 2020, 20, 3510–3514. [Google Scholar]
- Patade, V.Y.; Bhargava, S.; Suprasanna, P. Effects of NaCl and iso-osmotic PEG stress on growth, osmolytes accumulation and antioxidant defense in cultured sugarcane cells. Plant Cell Tiss. Org. 2012, 108, 279–286. [Google Scholar] [CrossRef]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arab. Book 2010, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Gorham, J. Betaines in higher plants-biosynthesis and role in stress metabolism. In Amino Acids and Their Derivatives in Higher Plants; Wallsgrove, R.M., Ed.; Cambridge University Press: New York, NY, USA, 1995; pp. 171–203. [Google Scholar]
- Umar, J.; Aliyu, A.; Shehu, K.; Abubakar, L. Influence of salt stress on proline and glycine betaine accumulation in tomato (Solanum lycopersicum L.). J. Hort. Plant 2018, 1, 19–25. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Nikam, T.D.; Penna, S. Biochemical, physiological and growth changes in response to salinity in callus cultures of Sesuvium portulacastrum L. Plant Cell Tiss. Org. 2010, 102, 17–25. [Google Scholar] [CrossRef]
- Helaly, M.N.; El-Hosieny, H.A.; El-Sarkassy, N.M.; Fuller, M.P. Growth, lipid peroxidation, organic solutes, and anti-oxidative enzyme content in drought-stressed date palm embryogenic callus suspension induced by polyethylene glycol. In Vitro Cell. Dev. Biol. Plant 2017, 53, 133–141. [Google Scholar] [CrossRef]
- Nasir, B.; Razzaq, H.; Tahir, M. Development of best screening method at seedling stage under drought stress for brassica Napus L. Big Data Agric. (BDA) 2019, 1, 11–14. [Google Scholar] [CrossRef]
- Karpe, A.; Nikam, A.A.; Chimote, K.P.; Kalwade, S.B.; Kawar, P.G.; Babu, H.; Devarumath, R.M.; Suprasanna, P. Differential responses to salinity stress of two varieties (CoC 671 and Co 86032) of sugarcane (Saccharum Officinarum L.). Afr. J. Biotechnol. 2012, 11, 9028–9035. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Ni, W. Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta 2020, 251, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Cao, F.; Richmond, M.E.A.; Qiu, C.; Wu, F. Foliar application of betaine improves water-deficit stress tolerance in barley (Hordeum vulgare L.). Plant Growth Regul. 2019, 89, 109–118. [Google Scholar] [CrossRef]
- Pei, L.; Li, H.; Zhou, Y.; Li, W.; Jiang, Y.; Li, H. Exogenous glycinebetaine application contributes to abiotic stress tolerance in maize. J. Plant Biol. 2020, 1–13. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. BBA Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masood, A.; Per, T.S.; Asgher, M.; Fatma, M.; Khan, M.I.R.; Rasheed, F.; Hussain, S.J.; Khan, N.A. Glycine betaine: Role in shifting plants toward adaptation under extreme environments. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 69–82. [Google Scholar]
- Sairam, R.K.; Saxena, D.C. Oxidative stress and antioxidants in wheat genotypes: Possible mechanism of water stress tolerance. J. Agron. Crop Sci. 2000, 184, 55–61. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behave 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Levitt, J. Responses of Plants to Environmental Stress, Volume 1: Chilling, Freezing, and High Temperature Stresses, 2nd ed.; Academic Press: New York, NY, USA, 1980; p. 698. [Google Scholar]
- Conadesuca. Décimo Segundo Reporte de Avance de Producción de la Zafra 2020/21, 2021. Available online: https://www.gob.mx/conadesuca/articulos/noveno-reporte-de-avance-de-produccion-de-la-zafra-2020-21?idiom=es (accessed on 10 January 2021).
- Stewart, G.R.; Lee, J.A. The role of proline accumulation in halophytes. Planta 1974, 120, 279–289. [Google Scholar] [CrossRef]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef] [Green Version]
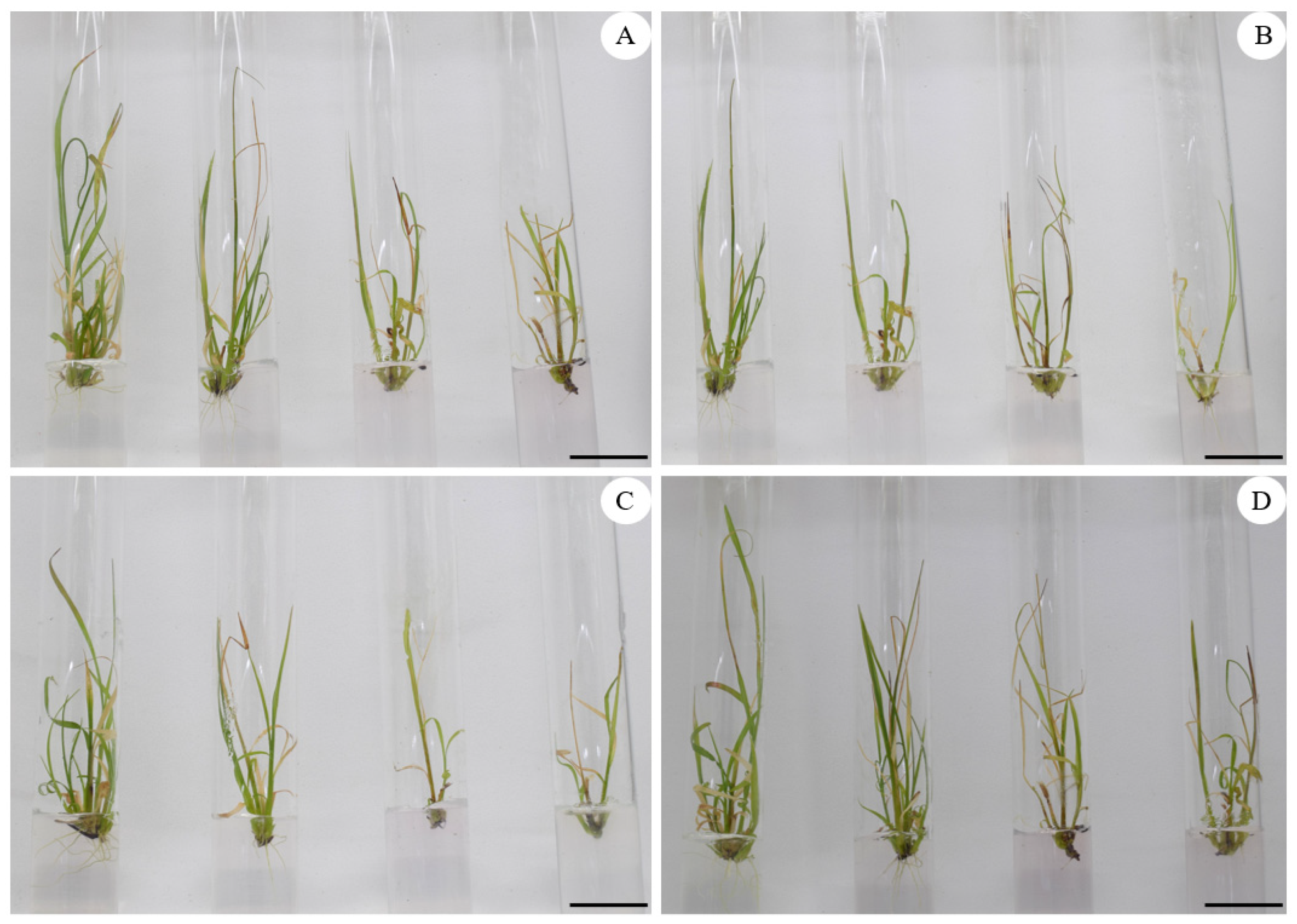
| Cultivar | PEG (%) | Number of Shoots per Explant | Shoot Length (cm) | Number of Leaves per Explant | Number of Roots per Explant | Root Length (cm) | Dry Matter (%) |
|---|---|---|---|---|---|---|---|
| Mex 69-290 | 0 | 8.16 ± 0.70 a | 8.02 ± 0.32 a | 3.24 ± 0.17 a | 9.50 ± 0.61 a | 3.10 ± 0.15 a | 7.57 ± 0.38 d |
| 3 | 7.33 ± 0.42 a | 8.17 ± 0.15 a | 3.33 ± 0.16 a | 9.33 ± 0.61 a | 3.16 ± 0.18 a | 10.27 ± 1.55 bcd | |
| 6 | 4.50 ± 0.22 bcd | 7.00 ± 0.28 abc | 2.61 ± 0.14 bcd | 2.66 ± 0.33 bc | 1.04 ± 0.15 d | 15.00 ± 0.67 a | |
| 9 | 3.66 ± 0.33 cde | 5.68 ± 0.23 e | 2.10 ± 0.16 cd | 1.83 ± 0.30 c | 1.12 ± 0.13 d | 15.10 ± 0.53 a | |
| Mex 79-431 | 0 | 7.16 ± 0.30 a | 7.36 ± 0.18 abc | 2.94 ± 0.18 ab | 8.00 ± 0.36 a | 3.20 ± 0.16 a | 7.29 ± 0.51 d |
| 3 | 4.66 ± 0.33 bcd | 6.76 ± 0.19 bcd | 2.86 ± 0.19 abc | 2.83 ± 0.30 bc | 2.20 ± 0.17 b | 8.70 ± 0.59 cd | |
| 6 | 3.50 ± 0.34 cde | 6.56 ± 0.20 bcde | 2.26 ± 0.11 bcd | 2.00 ± 0.36 c | 1.00 ± 0.16 d | 14.13 ± 0.25 ab | |
| 9 | 2.83 ± 0.30 de | 6.38 ± 0.33 cde | 2.15 ± 0.22 cd | 1.50 ± 0.22 c | 1.13 ± 0.17 d | 15.01 ± 0.37 a | |
| CP 72-2086 | 0 | 7.00 ± 0.36 a | 7.36 ± 0.15 abc | 3.26 ± 0.15 a | 7.83 ± 0.40 a | 3.03 ± 0.14 a | 7.51 ± 0.35 d |
| 3 | 4.80 ± 0.37 bc | 6.54 ± 0.24 bcd | 2.83 ± 0.20 abc | 4.20 ± 0.37 b | 2.04 ± 0.20 bc | 8.87 ± 0.23 cd | |
| 6 | 2.60 ± 0.24 e | 6.45 ± 0.63 cde | 2.72 ± 0.14 bcd | 2.40 ± 0.50 bc | 1.28 ± 0.14 cd | 14.18 ± 0.96 ab | |
| 9 | 2.60 ± 0.24 e | 5.95 ± 0.40 e | 2.16 ± 0.24 cd | 1.80 ± 0.37 c | 1.05 ± 0.13 d | 15.48 ± 1.17 a | |
| MOTZMex 92-207 | 0 | 7.00 ± 0.36 a | 8.23 ± 0.30 a | 3.03 ± 0.16 ab | 8.00 ± 0.36 a | 3.34 ± 0.10 a | 7.82 ± 0.64 d |
| 3 | 4.83 ± 0.30 bc | 7.65 ± 0.20 abc | 3.07 ± 0.13 ab | 4.50 ± 0.42 b | 2.17 ± 0.15 b | 12.53 ± 0.43 bc | |
| 6 | 3.20 ± 0.37 cde | 7.51 ± 0.44 abc | 2.27 ± 0.23 bcd | 3.20 ± 0.48 bc | 1.31 ± 0.12 cd | 13.56 ± 1.30 ab | |
| 9 | 2.20 ± 0.37 e | 6.42 ± 0.64 cde | 1.90 ± 0.27 d | 2.00 ± 0.44 c | 1.00 ± 0.13 d | 15.78 ± 1.16 a | |
| p-value | |||||||
| p (cultivar) | 0.000 | 0.001 | 0.301 | 0.000 | 0.064 | 0.046 | |
| p (PEG) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| p (cultivar × PEG) | 0.666 | 0.000 | 0.400 | 0.000 | 0.000 | 0.121 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Pérez, C.A.; Gómez-Merino, F.C.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. In Vitro Screening of Sugarcane Cultivars (Saccharum spp. Hybrids) for Tolerance to Polyethylene Glycol-Induced Water Stress. Agronomy 2021, 11, 598. https://doi.org/10.3390/agronomy11030598
Hernández-Pérez CA, Gómez-Merino FC, Spinoso-Castillo JL, Bello-Bello JJ. In Vitro Screening of Sugarcane Cultivars (Saccharum spp. Hybrids) for Tolerance to Polyethylene Glycol-Induced Water Stress. Agronomy. 2021; 11(3):598. https://doi.org/10.3390/agronomy11030598
Chicago/Turabian StyleHernández-Pérez, César A., Fernando Carlos Gómez-Merino, José L. Spinoso-Castillo, and Jericó J. Bello-Bello. 2021. "In Vitro Screening of Sugarcane Cultivars (Saccharum spp. Hybrids) for Tolerance to Polyethylene Glycol-Induced Water Stress" Agronomy 11, no. 3: 598. https://doi.org/10.3390/agronomy11030598








