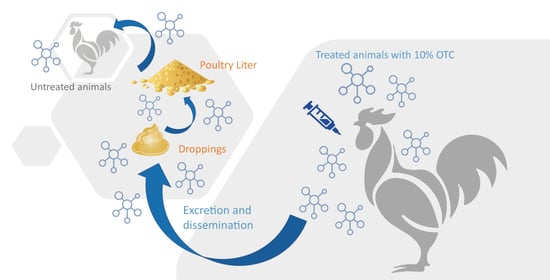
Evaluation of Antibiotic Dissemination into the Environment and Untreated Animals, by Analysis of Oxytetracycline in Poultry Droppings and Litter
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
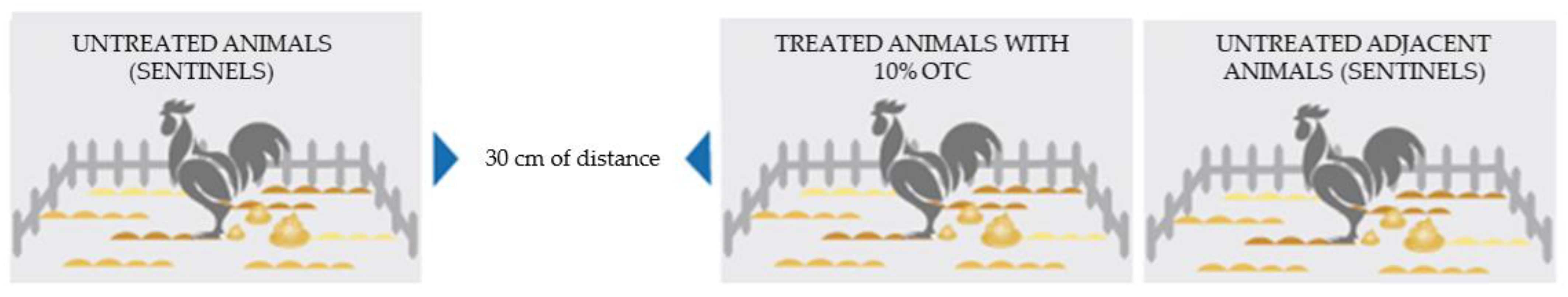
2.1. Experimental Animals
2.1.1. Animal Groups and Treatment
2.1.2. Sampling Collection
2.2. Chemical Analysis
2.2.1. Reagents, Solvents and Standards
2.2.2. Extraction Procedure
2.2.3. Instrumental Analysis
2.3. Validation of Analytical Methodology
3. Results
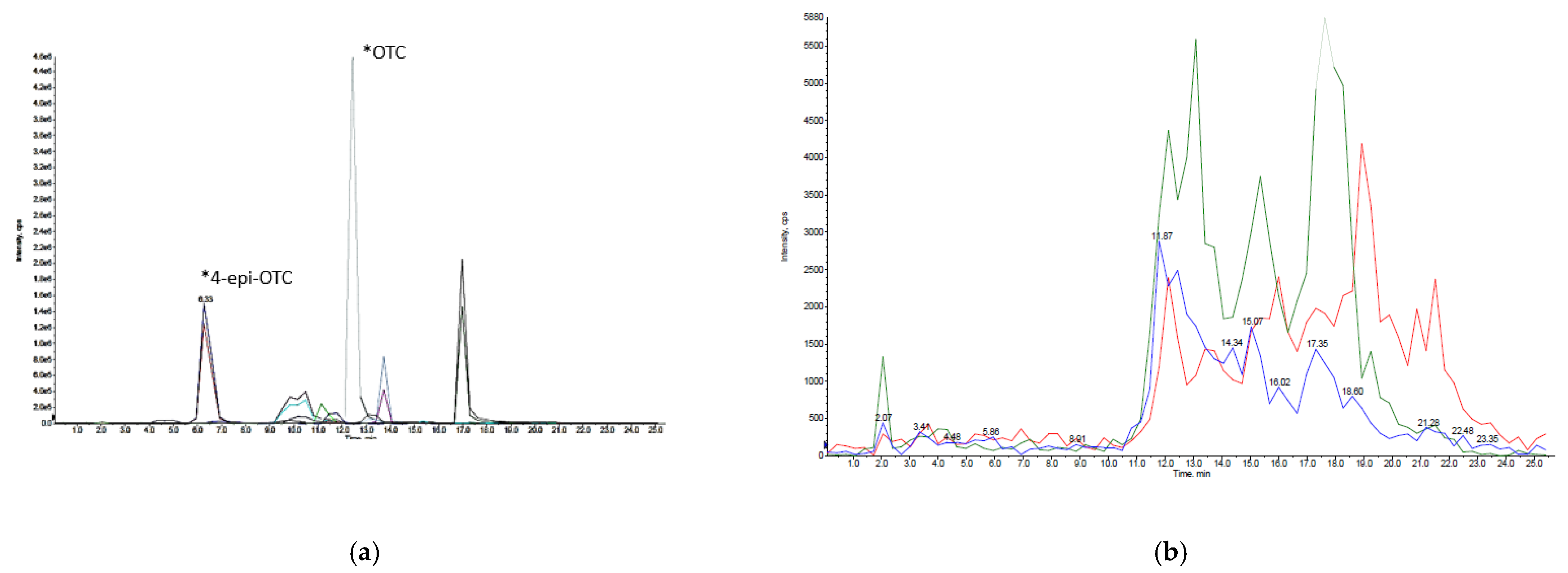
3.1. Optimization and Validation of Analytical Method
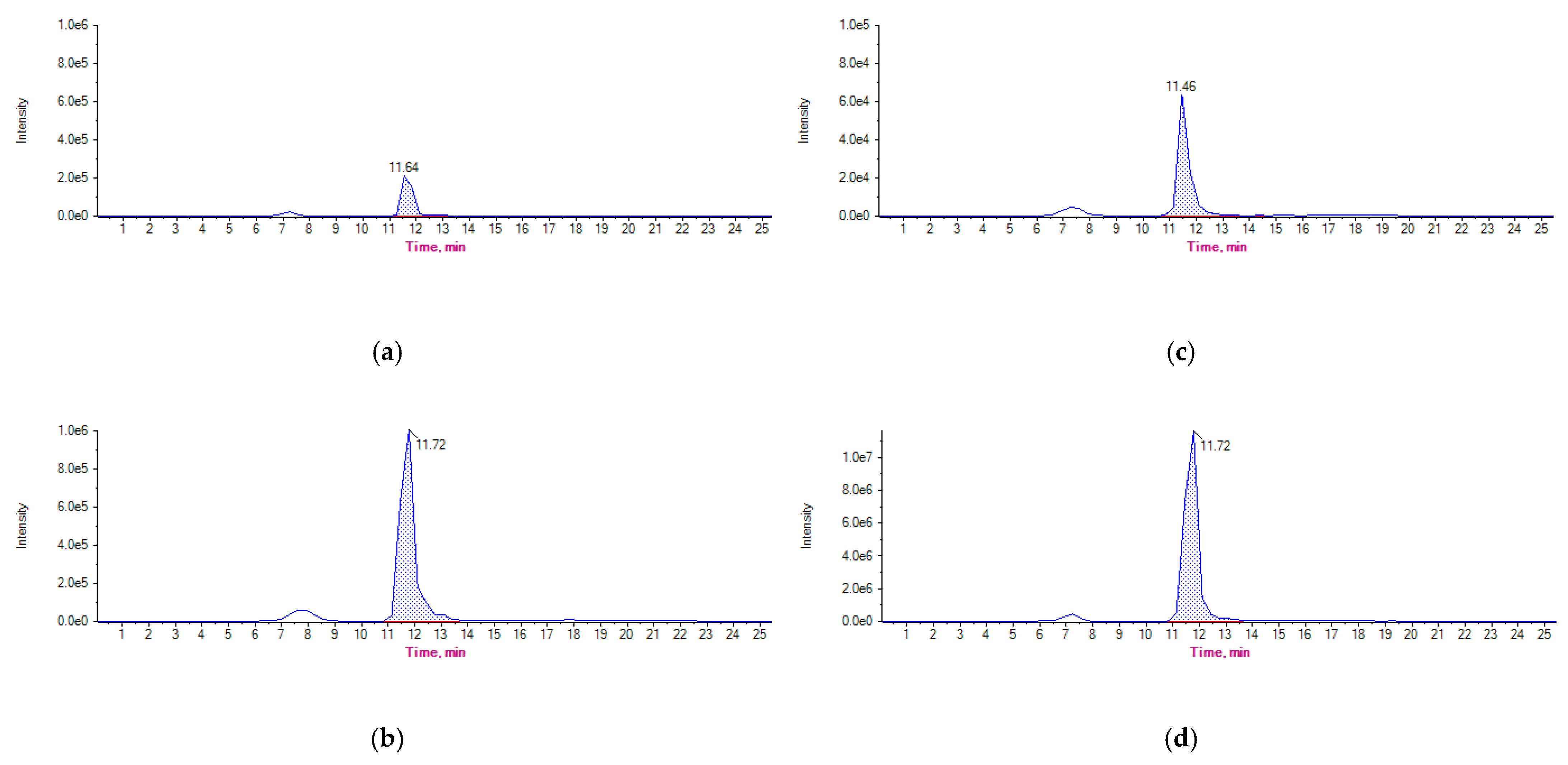
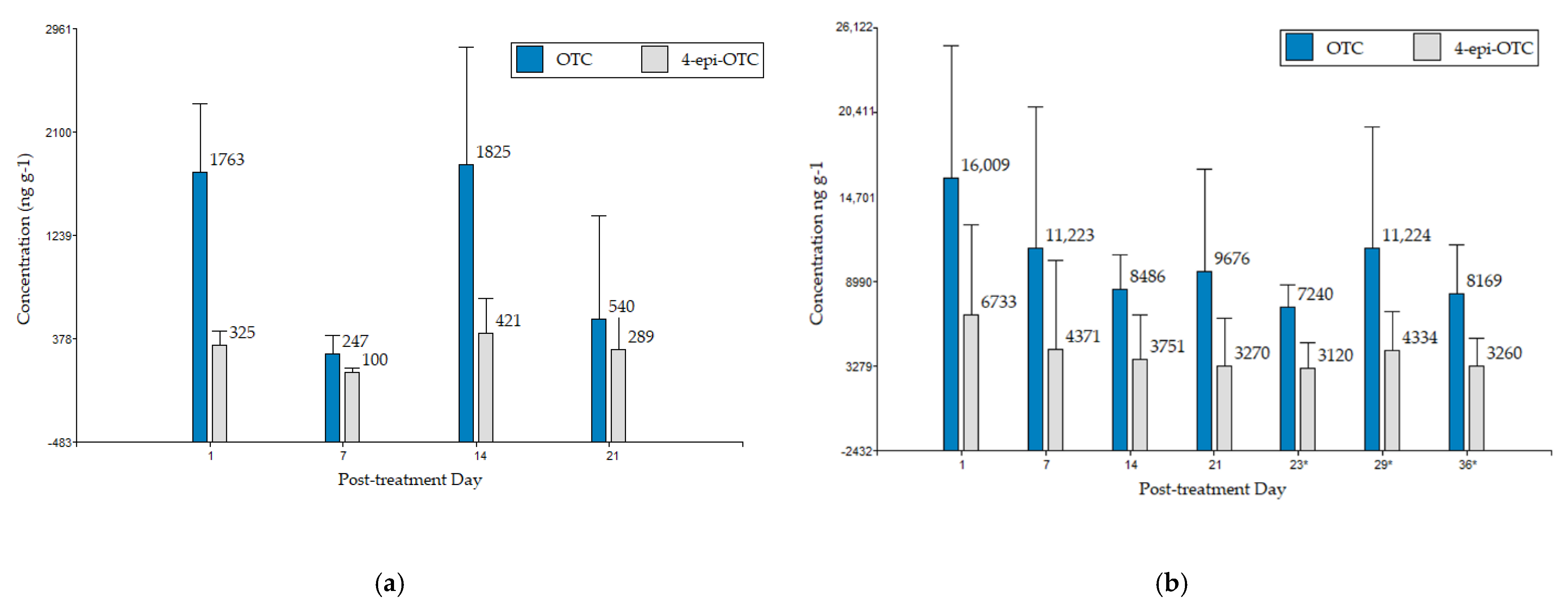
3.2. Detection and Quantification of OTC and 4-epi-OTC in Experimental Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daghrir, R.; Drogui, P. Tetracycline Antibiotics in the Environment: A Review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Granados-Chinchilla, F.; Rodríguez, C. Tetracyclines in Food and Feedingstuffs: From Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J. Anal. Methods Chem. 2017, 2017, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The Application of Antibiotics in Broiler Production and the Resulting Antibiotic Resistance in Escherichia Coli: A Global Overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health OIE Annual Report on Antimicrobial Agents Intended for Use in Animals 2018. Available online: https://www.oie.int/ (accessed on 25 October 2020).
- Sumano López, H.; Gutiérrez Olvera, L.; Leon Fraga, J. Farmacología Clínica en Aves Comerciales; de León Fraga, J., Ed.; McGraw-Hill Interamericana Editores, S.A. de C.V.: Mexico city, Mexico, 2010; ISBN 978-970-10-7077-2. [Google Scholar]
- Agyare, C.; Etsiapa Boamah, V.; Ngofi Zumbi, C.; Boateng Osei, F. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance. In Antimicrobial Resistance—A Global Threat; Kumar, Y., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-783-2. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, J.; Pokrant, E.; Krogh, M.; Briceño, C.; Hidalgo, H.; Maddaleno, A.; Araya-Jordán, C.; San Martín, B. Determination of Oxytetracycline and 4-Epi-Oxytetracycline Residues in Feathers and Edible Tissues of Broiler Chickens Using Liquid Chromatography Coupled with Tandem Mass Spectrometry. J. Food Prot. 2017, 80, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.; Pokrant, E.; Araya, D.; Briceño, C.; Hidalgo, H.; Maddaleno, A.; Araya-Jordán, C.; San Martin, B. Residue Depletion of Oxytetracycline (OTC) and 4-Epi-Oxytetracycline (4-Epi-OTC) in Broiler Chicken’s Claws by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). Food Addit. Contam. Part A 2017, 34, 494–500. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E. Sorption-Desorption Equilibrium and Diffusion of Tetracycline in Poultry Litter and Municipal Biosolids Soil Amendments. Chemosphere 2017, 188, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Sarker, Y.; Hasan, M.; Paul, T.; Rashid, S.; Alam, M.; Sikder, M. Screening of Antibiotic Residues in Chicken Meat in Bangladesh by Thin Layer Chromatography. J. Adv. Vet. Anim. Res. 2018, 5, 140. [Google Scholar] [CrossRef]
- Ziółkowski, H.; Grabowski, T.; Jasiecka, A.; Zuśka-Prot, M.; Barski, D.; Jaroszewski, J. Pharmacokinetics of Oxytetracycline in Broiler Chickens Following Different Routes of Administration. Vet. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, B.J.A.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A.M. The Analysis of Animal Faeces as a Tool to Monitor Antibiotic Usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef]
- Massé, D.; Saady, N.; Gilbert, Y. Potential of Biological Processes to Eliminate Antibiotics in Livestock Manure: An Overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef] [Green Version]
- Lykkeberg, A.K.; Halling-Sørensen, B.; Cornett, C.; Tjørnelund, J.; Honoré Hansen, S. Quantitative Analysis of Oxytetracycline and Its Impurities by LC-MS-MS. J. Pharm. Biomed. Anal. 2004, 34, 325–332. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Liang, J.; Zou, Y.; Wen, X.; Liao, X.; Wu, Y. Comparison of Oxytetracycline Degradation Behavior in Pig Manure with Different Antibiotic Addition Methods. Environ. Sci. Pollut. Res. 2015, 22, 18469–18476. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qi, W.; Feng, Y.; Liu, Y.; Ebrahim, S.; Long, J. Degradation Mechanisms of Oxytetracycline in the Environment. J. Integr. Agric. 2019, 18, 1953–1960. [Google Scholar] [CrossRef]
- EUROPEAN COMMISSION. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Off. J. Eur. Union 2010, 15, 1–72.
- Ghirardini, A.; Grillini, V.; Verlicchi, P. A Review of the Occurrence of Selected Micropollutants and Microorganisms in Different Raw and Treated Manure—Environmental Risk Due to Antibiotics after Application to Soil. Sci. Total Environ. 2020, 707, 136118. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of Veterinary Antibiotics in Manures from Feedlot Livestock in Eight Provinces of China. Sci.Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Li, W.; Lu, X.; Liu, B.; Wang, J. The Residues and Environmental Risks of Multiple Veterinary Antibiotics in Animal Faeces. Environ. Monit. Assess. 2013, 185, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Alavi, N.; Babaei, A.A.; Shirmardi, M.; Naimabadi, A.; Goudarzi, G. Assessment of Oxytetracycline and Tetracycline Antibiotics in Manure Samples in Different Cities of Khuzestan Province, Iran. Environ. Sci. Pollut. Res. 2015, 22, 17948–17954. [Google Scholar] [CrossRef]
- Mahmoud, M.A.M.; Abdel-Mohsein, H.S. Hysterical Tetracycline in Intensive Poultry Farms Accountable for Substantial Gene Resistance, Health and Ecological Risk in Egypt- Manure and Fish. Environ.l Pollu. 2019, 255, 113039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yates, S.R. Laboratory Study of Oxytetracycline Degradation Kinetics in Animal Manure and Soil. J. Agric. Food Chem. 2008, 56, 1683–1688. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Lahr, J.; Nibbeling, C.; Jansen, L.J.M.; Bongers, I.E.A.; Wipfler, E.L.; van de Schans, M.G.M. The Persistence of a Broad Range of Antibiotics during Calve, Pig and Broiler Manure Storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef]
- Kyakuwaire; Olupot; Amoding; Nkedi-Kizza; Basamba How Safe Is Chicken Litter for Land Application as an Organic Fertilizer? A Review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [CrossRef] [PubMed] [Green Version]
- Hartung, J.; Schulz, J. Risks Caused by Bio-Aerosols in Poultry Houses. In Poultry in the 21st Century: Avian Influenza and Beyond, 5–7 November 2007, Bangkok, Thailand; Food and Agriculture Organization: Rome, Italy, 2008. [Google Scholar]
- Stahl, J.; Zessel, K.; Schulz, J.; Finke, J.H.; Müller-Goymann, C.C.; Kietzmann, M. The Effect of Miscellaneous Oral Dosage Forms on the Environmental Pollution of Sulfonamides in Pig Holdings. BMC Vet. Res. 2016, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviagen Roos Nutrition Specifications 2019. Available online: https://tmea.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf (accessed on 25 November 2020).
- Ministerio De Salud Subsecretaría De Salud Pública Ley Núm. 20.380 Sobre Protección de Animales 2009. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1006858 (accessed on 16 December 2020).
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific PurposesText with EEA Relevance. 47. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 16 December 2020).
- Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of KillingText with EEA Relevance. 30. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:303:0001:0030:EN:PDF (accessed on 16 December 2020).
- Servicio Agricola y Ganadero (SAG) Protocolo de Tomas de Muestras de Suelos 2019. Available online: https://www.sag.gob.cl/sites/default/files/Protocolo%20toma%20muestras%20suelo.pdf (accessed on 25 November 2020).
- European Medicines Agency. ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology. 2006, p. 15. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 25 October 2020).
- European Commission 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Union 2002, L 221, 0008–0036.
- European Medicines Agency. VICH tpoic GL49: Studies to Evaluate the Metabolism and Residues Kinetics of Veterinary Drugs in Human Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies. 2015, p. 21. Available online: https://www.ema.europa.eu/en/vich-gl49-studies-evaluate-metabolism-residue-kinetics-veterinary-drugs-food-producing-animals (accessed on 25 October 2020).
- Grupo Infostat Infostat Version 2020; Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba: Argentina. Available online: http://www.infostat.com.ar (accessed on 16 December 2020).
- Jansen, L.J.M.; van de Schans, M.G.M.; de Boer, D.; Bongers, I.E.A.; Schmitt, H.; Hoeksma, P.; Berendsen, B.J.A. A New Extraction Procedure to Abate the Burden of Non-Extractable Antibiotic Residues in Manure. Chemosphere 2019, 224, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Odore, R.; De Marco, M.; Gasco, L.; Rotolo, L.; Meucci, V.; Palatucci, A.T.; Rubino, V.; Ruggiero, G.; Canello, S.; Guidetti, G.; et al. Cytotoxic Effects of Oxytetracycline Residues in the Bones of Broiler Chickens Following Therapeutic Oral Administration of a Water Formulation. Poult. Sci. 2015, 94, 1979–1985. [Google Scholar] [CrossRef]
- Mestorino, N.; Hernández, E.M.; Marchetti, L.; Errecalde, J.O. Pharmacokinetics and Tissue Residues of an Oxytetracycline/Diclofenac Combination in Cattle. Rev Sci Tech. 2007, 26, 679–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yévenes, K.; Pokrant, E.; Pérez, F.; Riquelme, R.; Avello, C.; Maddaleno, A.; Martín, B.S.; Cornejo, J. Assessment of Three Antimicrobial Residue Concentrations in Broiler Chicken Droppings as a Potential Risk Factor for Public Health and Environment. Int. J. Environ. Res. Public Health 2018, 16, 24. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, J.; Yevenes, K.; Avello, C.; Pokrant, E.; Maddaleno, A.; Martin, B.; Lapierre, L. Determination of Chlortetracycline Residues, Antimicrobial Activity and Presence of Resistance Genes in Droppings of Experimentally Treated Broiler Chickens. Molecules 2018, 23, 1264. [Google Scholar] [CrossRef] [Green Version]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [Green Version]
- Sarker, Y.A.; Rashid, Sm.Z.; Sachi, S.; Ferdous, J.; Das Chowdhury, B.L.; Tarannum, S.S.; Sikder, M.H. Exposure Pathways and Ecological Risk Assessment of Common Veterinary Antibiotics in the Environment through Poultry Litter in Bangladesh. J. Environ. Sci. Health B 2020, 55, 1061–1068. [Google Scholar] [CrossRef]
- Kasumba, J.; Appala, K.; Agga, G.E.; Loughrin, J.H.; Conte, E.D. Anaerobic Digestion of Livestock and Poultry Manures Spiked with Tetracycline Antibiotics. J. Environ. Sci. Health B 2020, 55, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Shields, S.J.; Garner, J.P.; Mench, J.A. Dustbathing by Broiler Chickens: A Comparison of Preference for Four Different Substrates. Appl. Anim. Behav. Sci. 2004, 87, 69–82. [Google Scholar] [CrossRef]
- Carballo, M.; Aguayo, S.; González, M.; Esperon, F.; Torre, A. de la Environmental Assessment of Tetracycline’s Residues Detected in Pig Slurry and Poultry Manure. JEP 2016, 07, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.D.; Bruland, G.L.; Agrawal, S.G.; Vasudevan, D. Factors Influencing the Sorption of Oxytetracycline to Soils. Environ. Toxicol. Chem. 2005, 24, 761–770. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Van Epps, A.; Blaney, L. Antibiotic Residues in Animal Waste: Occurrence and Degradation in Conventional Agricultural Waste Management Practices. Curr. Pollut. Rep. 2016, 2, 135–155. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Asai, T. Role of Antimicrobial Selective Pressure and Secondary Factors on Antimicrobial Resistance Prevalence in Escherichia Coli from Food-Producing Animals in Japan. J. Biomed. Biotechnol. 2010, 2010, 1–12. [Google Scholar] [CrossRef] [Green Version]

| Matrix | Analyte | Linearity (R2) 3 ± SD | Recovery 4 (%) | LD 5 (µg kg−1) | LC 6 (µg kg−1) |
|---|---|---|---|---|---|
| Droppings | OTC 1 | 0.995 ± 0.004 | 104.3 | 12.128 | 36.384 |
| 4-epi-OTC 2 | 0.997 ± 0.003 | 98.4 | 12.157 | 36.470 | |
| Litter | OTC | 0.993 ± 0.004 | 91.9 | 12.409 | 37.228 |
| 4-epi-OTC | 0.992 ± 0.007 | 96.0 | 10.783 | 32.349 |
| Days Post the End of Treatment (Days Post- Slaughter) | Average OTC+4-epi-OTC (µg kg−1) in Droppings | Average OTC+4-epi-OTC (µg kg−1) in Litter | ||||
|---|---|---|---|---|---|---|
| Group A 1 | Group B 2 | Group C 3 | Group A 1 | Group B 2 | Group C 3 | |
| 1 | 2087.41 | <LOD 4 | <LOD * | 22,741.68 | <LOD | <LOD |
| 7 | 347.63 | <LOD | <LOD * | 15,594.05 | <LOD | <LOD |
| 14 | 2244.66 | <LOD | N/D 5 | 12,236.68 | <LOD | <LOD |
| 21 | 733.00 | <LOD | N/D 5 | 12,946.13 | <LOD | <LOD ** |
| 23 (1) | - | - | - | 10,360.60 | <LOD | <LOD |
| 29 (7) | - | - | - | 15,557.62 | <LOD | <LOD |
| 36 (14) | - | - | - | 11,429.14 | <LOD | <LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokrant, E.; Yévenes, K.; Trincado, L.; Terraza, G.; Galarce, N.; Maddaleno, A.; Martín, B.S.; Lapierre, L.; Cornejo, J. Evaluation of Antibiotic Dissemination into the Environment and Untreated Animals, by Analysis of Oxytetracycline in Poultry Droppings and Litter. Animals 2021, 11, 853. https://doi.org/10.3390/ani11030853
Pokrant E, Yévenes K, Trincado L, Terraza G, Galarce N, Maddaleno A, Martín BS, Lapierre L, Cornejo J. Evaluation of Antibiotic Dissemination into the Environment and Untreated Animals, by Analysis of Oxytetracycline in Poultry Droppings and Litter. Animals. 2021; 11(3):853. https://doi.org/10.3390/ani11030853
Chicago/Turabian StylePokrant, Ekaterina, Karina Yévenes, Lina Trincado, Gigliola Terraza, Nicolás Galarce, Aldo Maddaleno, Betty San Martín, Lisette Lapierre, and Javiera Cornejo. 2021. "Evaluation of Antibiotic Dissemination into the Environment and Untreated Animals, by Analysis of Oxytetracycline in Poultry Droppings and Litter" Animals 11, no. 3: 853. https://doi.org/10.3390/ani11030853
APA StylePokrant, E., Yévenes, K., Trincado, L., Terraza, G., Galarce, N., Maddaleno, A., Martín, B. S., Lapierre, L., & Cornejo, J. (2021). Evaluation of Antibiotic Dissemination into the Environment and Untreated Animals, by Analysis of Oxytetracycline in Poultry Droppings and Litter. Animals, 11(3), 853. https://doi.org/10.3390/ani11030853