Detection of Antimicrobial Residues in Poultry Litter: Monitoring a Risk through a Selective and Sensitive HPLC–MS/MS Method
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
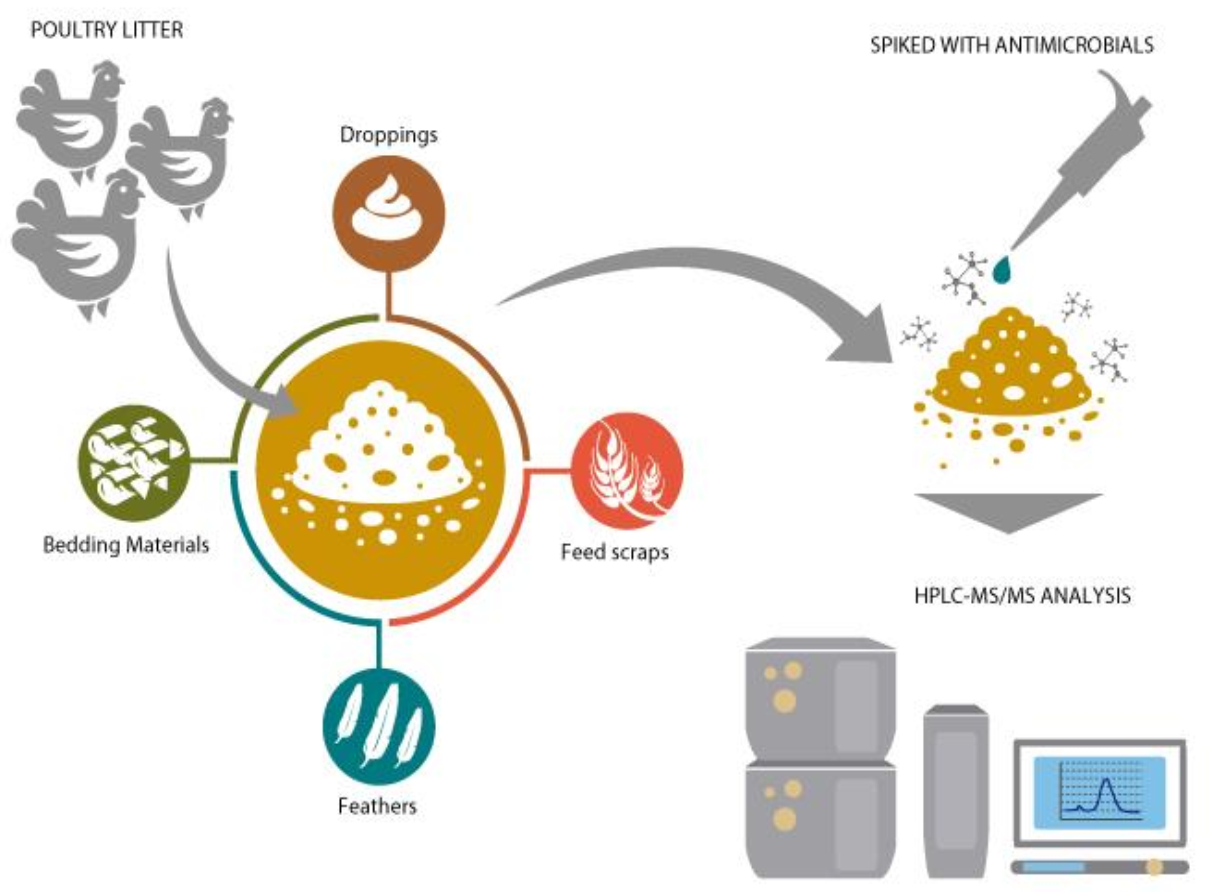
2.1. Sample Collection
2.2. Standard, Reagents, and Chemicals
2.3. Extraction of Antimicrobials
2.4. Instrument Analysis
2.5. Method Validation Procedure
2.6. Antimicrobial Monitoring in Commercial Flocks
3. Results
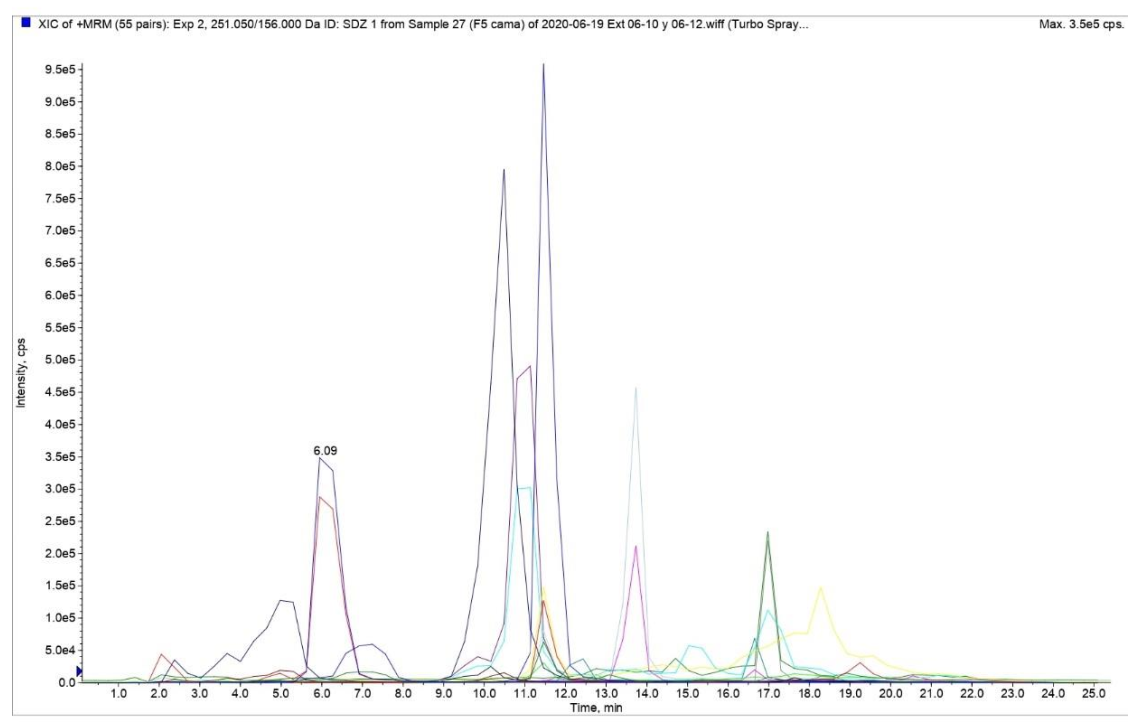
3.1. Implementation and Optimization of the Analytical Method
3.2. Validation of the Analytical Method
3.2.1. Selectivity and Specificity.
3.2.2. Detection Range
3.2.3. Linearity of Calibration Curves
3.2.4. Recovery and Precision
3.3. Analysis of Antimicrobial Concentrations in Commercial Flocks Litter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Ashworth, A.J.; Willett, C.; Cook, K.; Upadhyay, A.; Owens, P.R.; Ricke, S.C.; DeBruyn, J.M.; Moore, P.A., Jr. Review of Antibiotic Resistance, Ecology, Dissemination, and Mitigation in U.S. Broiler Poultry Systems. Front. Microbiol. 2019, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Food and Drug Administration Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; Food and Drug Administration, Department of Health and Human Services: Silver Spring, MD, USA, 2017; p. 67.
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.-L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in Poultry Manure and Their Associated Health Issues: A Systematic Review. J. Soils Sediments 2020, 20, 486–497. [Google Scholar] [CrossRef]
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The Application of Antibiotics in Broiler Production and the Resulting Antibiotic Resistance in Escherichia Coli: A Global Overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- SAG, Servicio Agrícola y Ganadero. Lista de Medicamentos Veterinarios Autorizados Por El SAG. Available online: https://www2.sag.gob.cl/pecuaria/medicamentos/medicamentos_list.asp (accessed on 6 May 2020).
- SAG, Servicio Agrícola y Ganadero. Declaración de Venta de Antimicrobianos. Available online: https://www.sag.gob.cl/ambitos-de-accion/declaracion-de-venta-de-antimicrobianos (accessed on 6 May 2020).
- Peng, P.; Wang, Y.; Liu, L.; Zou, Y.; Liao, X.; Liang, J.; Wu, Y. The Excretion and Environmental Effects of Amoxicillin, Ciprofloxacin, and Doxycycline Residues in Layer Chicken Manure. Poult. Sci. 2016, 95, 1033–1041. [Google Scholar] [CrossRef]
- Massé, D.; Saady, N.; Gilbert, Y. Potential of Biological Processes to Eliminate Antibiotics in Livestock Manure: An Overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef]
- Mehdi, Y.; Létourneau-Montminy, M.-P.; Gaucher, M.-L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of Antibiotics in Broiler Production: Global Impacts and Alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Herath, E.M.; Palansooriya, A.G.K.N.; Dandeniya, W.S.; Jinadasa, R.N. An Assessment of Antibiotic Resistant Bacteria in Poultry Litter and Agricultural Soils in Kandy District, Sri Lanka. Trop. Agric. Res. 2016, 27, 389. [Google Scholar] [CrossRef]
- Santos Dalólio, F.; da Silva, J.N.; Carneiro de Oliveira, A.C.; de Ferreira Tinôco, I.F.; Christiam Barbosa, R.; de Resende, M.O.; Teixeira Albino, L.F.; Teixeira Coelho, S. Poultry Litter as Biomass Energy: A Review and Future Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 941–949. [Google Scholar] [CrossRef]
- Cornejo, J.; Pokrant, E.; Krogh, M.; Briceño, C.; Hidalgo, H.; Maddaleno, A.; Araya-Jordán, C.; San Martín, B. Determination of Oxytetracycline and 4-Epi-Oxytetracycline Residues in Feathers and Edible Tissues of Broiler Chickens Using Liquid Chromatography Coupled with Tandem Mass Spectrometry. J. Food Prot. 2017, 80, 619–625. [Google Scholar] [CrossRef]
- Pokrant, E.; Medina, F.; Maddaleno, A.; San Martín, B.; Cornejo, J. Determination of Sulfachloropyridazine Residue Levels in Feathers from Broiler Chickens after Oral Administration Using Liquid Chromatography Coupled to Tandem Mass Spectrometry. PLoS ONE 2018, 13, e0200206. [Google Scholar] [CrossRef]
- Furtula, V.; Huang, L.; Chambers, P.A. Determination of Veterinary Pharmaceuticals in Poultry Litter and Soil by Methanol Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Environ. Sci. Health B 2009, 44, 717–723. [Google Scholar] [CrossRef]
- Leal, R.M.P.; Figueira, R.F.; Tornisielo, V.L.; Regitano, J.B. Occurrence and Sorption of Fluoroquinolones in Poultry Litters and Soils from São Paulo State, Brazil. Sci. Total Environ. 2012, 432, 344–349. [Google Scholar] [CrossRef]
- Van Epps, A.; Blaney, L. Antibiotic Residues in Animal Waste: Occurrence and Degradation in Conventional Agricultural Waste Management Practices. Curr. Pollut. Rep. 2016, 2, 135–155. [Google Scholar] [CrossRef]
- Codex Alimentarius Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Foods CX/MRL 2-2018. 2018. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXM%2B2%252FMRL2e.pdf (accessed on 24 November 2020).
- Marble, S.C.; Sibley, J.L.; Gilliam, C.H.; Torbert, H.A. Application of Composted Poultry Litter as a Fertilizer for Landscape Bedding Plants. HortScience 2011, 46, 1367–1372. [Google Scholar] [CrossRef]
- Tewolde, H.; Sistani, K.R.; Adeli, A. Fall- and Spring-Applied Poultry Litter Effectiveness as Corn Fertilizer in the Mid-Southern United States. Agron. J. 2013, 105, 1743–1748. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Ateenyi Basamba, T. How Safe Is Chicken Litter for Land Application as an Organic Fertilizer? A Review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Huang, Y.; Wu, L.; Liu, X.; Luo, Y. Residues and Risks of Veterinary Antibiotics in Protected Vegetable Soils Following Application of Different Manures. Chemosphere 2016, 152, 229–237. [Google Scholar] [CrossRef]
- Wei, R.; Ge, F.; Zhang, L.; Hou, X.; Cao, Y.; Gong, L.; Chen, M.; Wang, R.; Bao, E. Occurrence of 13 Veterinary Drugs in Animal Manure-Amended Soils in Eastern China. Chemosphere 2016, 144, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wan, W.; Mao, D.; Wang, C.; Mu, Q.; Qin, S.; Luo, Y. Occurrence and Distribution of Sulfonamides, Tetracyclines, Quinolones, Macrolides, and Nitrofurans in Livestock Manure and Amended Soils of Northern China. Environ. Sci. Pollut. Re.s 2015, 22, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics Impact Plant Traits, Even at Small Concentrations. Aob Plants 2017, 9. [Google Scholar] [CrossRef]
- Jafari Ozumchelouei, E.; Hamidian, A.H.; Zhang, Y.; Yang, M. Physicochemical Properties of Antibiotics: A Review with an Emphasis on Detection in the Aquatic Environment. Water Environ. Res. 2020, 92, 177–188. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Baidoo, S.K.; Chander, Y.; Rosen, C.J. Antibiotic Uptake by Plants from Soil Fertilized with Animal Manure. J. Environ. Qual. 2005, 34, 2082–2085. [Google Scholar] [CrossRef]
- American College of Allergy, Asthma and Immunology (ACAAI). Allergic Reaction to Antibiotic Residues in Foods? You May Have to Watch What Your Fruits and Veggies Eat. 2014. Available online: https://acaai.org/news/you-may-have-watch-what-your-fruits-and-veggies-eat (accessed on 12 August 2020).
- Blaser, M.J. Antibiotic Use and Its Consequences for the Normal Microbiome. Science 2016, 352, 544–545. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Hu, X.-G.; Yi, L.; Zhou, Q.-X.; Xu, L. Determination of Thirteen Antibiotics Residues in Manure by Solid Phase Extraction and High Performance Liquid Chromatography. Chin. J. Anal. Chem. 2008, 36, 1162–1166. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, W.-J.; Liu, Y.-W.; Xue, J.-M.; Zhang, S.-Q.; Li, Z.-J. A Simple, Sensitive, and Reliable Method for the Simultaneous Determination of Multiple Antibiotics in Vegetables through SPE-HPLC-MS/MS. Molecules 2018, 23, 1953. [Google Scholar] [CrossRef]
- Rashid, A.; Mazhar, S.H.; Zeng, Q.; Kiki, C.; Yu, C.-P.; Sun, Q. Simultaneous Analysis of Multiclass Antibiotic Residues in Complex Environmental Matrices by Liquid Chromatography with Tandem Quadrupole Mass Spectrometry. J. Chromatogr. B 2020, 1145, 122103. [Google Scholar] [CrossRef]
- US Department of Agriculture (USDA). Poultry Industry Manual. 2013. Available online: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/documents_manuals/poultry_ind_manual.pdf (accessed on 9 August 2020).
- Congreso Nacional de la República de Chile Ley N°20.380 Sobre la Protección de los Animales. 2017. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1006858 (accessed on 28 November 2020).
- European Parliament. European Parliament and the Council of the European Union Directive 2010/63/EU of 22 September 2010 of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2018, 276, 33–79. [Google Scholar]
- European Parliament and the Council of the European Union COUNCIL REGULATION (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. 2009. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:303:0001:0030:EN:PDF (accessed on 10 October 2020).
- European Commission 2002/657/EC: Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. 2002. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:221:0008:0036:EN:PDF (accessed on 17 October 2020).
- GL49: Studies to Evaluate the Metabolism and Residues Kinetics of Veterinary Drugs in Human Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/vich-gl49-studies-evaluate-metabolism-residue-kinetics-veterinary-drugs-food-producing-animals_en.pdf (accessed on 13 December 2020).
- Berendsen, B.J.A.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A.M. The Analysis of Animal Faeces as a Tool to Monitor Antibiotic Usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-Based Fertilizers: A Practical Approach towards Circular Economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Organizacion de las Naciones Unidas para la Alimentacion y la Agricultura Producción y Productos Avícolas. Available online: http://www.fao.org/poultry-production-products/production/es/ (accessed on 8 January 2021).
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.J.; Panneerselvam, P. Uses and Management of Poultry Litter. World’s Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Escario, M.; Miguel, E.; Pérez, R.A. Persistence and Availability of Veterinary Antibiotics in Soil and Soil-Manure Systems. Sci. Total Environ. 2018, 643, 1562–1570. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Lahr, J.; Nibbeling, C.; Jansen, L.J.M.; Bongers, I.E.A.; Wipfler, E.L.; van de Schans, M.G.M. The Persistence of a Broad Range of Antibiotics during Calve, Pig and Broiler Manure Storage. Chemosphere 2018, 204, 267–276. [Google Scholar] [CrossRef]
- Cornejo, J.; Yevenes, K.; Avello, C.; Pokrant, E.; Maddaleno, A.; Martin, B.; Lapierre, L. Determination of Chlortetracycline Residues, Antimicrobial Activity and Presence of Resistance Genes in Droppings of Experimentally Treated Broiler Chickens. Molecules 2018, 23, 1264. [Google Scholar] [CrossRef]
- Yévenes, K.; Pokrant, E.; Pérez, F.; Riquelme, R.; Avello, C.; Maddaleno, A.; Martín, B.S.; Cornejo, J. Assessment of Three Antimicrobial Residue Concentrations in Broiler Chicken Droppings as a Potential Risk Factor for Public Health and Environment. Int. J. Environ. Res. Public Health 2018, 16, 24. [Google Scholar] [CrossRef]
- Manikandan, M.; Chun, S.; Kazibwe, Z.; Gopal, J.; Singh, U.B.; Oh, J.-W. Phenomenal Bombardment of Antibiotic in Poultry: Contemplating the Environmental Repercussions. Int. J. Environ. Res. Public Health 2020, 17, 5053. [Google Scholar] [CrossRef]
- Organizacion Mundial de Sanidad Animal. Informe Anual de La OIE Sobre El Uso de Agentes Antimicrobianos En Los Animales: Comprendiendo Mejor La Situacion Mundial; OIE: Paris, France, 2016. [Google Scholar]
- World Organisation for Animal Health OIE. Annual Report on Antimicrobial Agents Intended for Use in Animals; OIE: Paris, France, 2018. [Google Scholar]
- Paranhos, A.G.O.; Pereira, A.R.; da Fonseca, I.C.; Sanson, A.L.; Afonso, R.J.C.F.; Aquino, S.F. Analysis of Tylosin in Poultry Litter by HPLC-UV and HPLC-MS/MS after LTPE. Int. J. Environ. Anal. Chem. 2020, 1–18. [Google Scholar] [CrossRef]
| Total Time (min) | Flow Rate(µL/min) | A (%) | B (%) |
|---|---|---|---|
| 0.00 | 200 | 85.0 | 15.0 |
| 5.00 | 200 | 85.0 | 15.0 |
| 5.10 | 200 | 60.0 | 40.0 |
| 10.00 | 200 | 60.0 | 40.0 |
| 10.10 | 200 | 10.0 | 90.0 |
| 15.00 | 200 | 10.0 | 90.0 |
| 16.00 | 200 | 85.0 | 15.0 |
| 25.00 | 200 | 85.0 | 15.0 |
| Parameters | Analytical Conditions |
|---|---|
| Ionization | Electrospray ionization (ESI) |
| Temperature | 550 °C |
| Curtain gas | 30 psi |
| Collision gas | 10 psi |
| Ion spray voltage | 4500 V |
| Ion source gas 1 | 60 psi |
| Ion source gas 2 | 80 psi |
| Analyte | Linearity (R2) 10 | R2 SD 11 | R2 RSD% 12 | LOD 13 (µg kg−1) | LOQ 14 (µg kg−1) |
|---|---|---|---|---|---|
| TC 1 | 0.985 | 0.004 | 0.373 | 10.711 | 32.133 |
| 4-epi-TC 2 | 0.977 | 0.001 | 0.070 | 8.951 | 26.852 |
| OTC 3 | 0.979 | 0.003 | 0.347 | 11.487 | 34.460 |
| 4-epi-OTC 4 | 0.977 | 0.002 | 0.179 | 9.379 | 28.138 |
| EFX 5 | 0.986 | 0.003 | 0.331 | 13.626 | 40.879 |
| CFX 6 | 0.980 | 0.011 | 1.127 | 20.861 | 62.582 |
| FLU 7 | 0.980 | 0.005 | 0.555 | 11.729 | 35.188 |
| SCP 8 | 0.982 | 0.006 | 0.561 | 9.191 | 27.574 |
| SDZ 9 | 0.977 | 0.011 | 1.171 | 11.705 | 35.116 |
| Analyte | Work Concentration (µg kg−1) | Repeatability RSD 10 (%) | Reproducibility RSD (%) | Average Recovery (%) |
|---|---|---|---|---|
| TC 1 | 25 | 1.78 | 5.17 | 94.6 |
| 50 | 1.69 | 4.64 | 105.4 | |
| 75 | 0.58 | 1.66 | 98.2 | |
| 4-epi-TC 2 | 25 | 2.51 | 9.85 | 91.7 |
| 50 | 2.82 | 8.34 | 108.3 | |
| 75 | 0.87 | 3.10 | 97.2 | |
| OTC 3 | 25 | 3.94 | 6.38 | 92.4 |
| 50 | 3.72 | 5.48 | 107.6 | |
| 75 | 1.29 | 2.01 | 97.5 | |
| 4-epi-OTC 4 | 25 | 5.05 | 7.13 | 95.8 |
| 50 | 4.65 | 6.57 | 104.2 | |
| 75 | 1.64 | 2.31 | 98.6 | |
| EFX 5 | 25 | 4.96 | 10.64 | 95.4 |
| 50 | 5.61 | 9.71 | 104.6 | |
| 75 | 1.72 | 3.44 | 98.5 | |
| CFX 6 | 25 | 7.58 | 9.60 | 95.8 |
| 50 | 8.23 | 8.83 | 104.2 | |
| 75 | 2.60 | 3.11 | 98.6 | |
| FLU 7 | 25 | 3.68 | 6.91 | 93.7 |
| 50 | 3.62 | 6.09 | 106.3 | |
| 75 | 1.22 | 2.20 | 97.9 | |
| SCP 8 | 25 | 3.31 | 4.91 | 93.0 |
| 50 | 3.29 | 4.27 | 107.0 | |
| 75 | 1.10 | 1.56 | 97.7 | |
| SDZ 9 | 25 | 1.59 | 10.01 | 95.6 |
| 50 | 1.92 | 9.17 | 104.4 | |
| 75 | 0.56 | 3.24 | 98.5 |
| Poultry Litter Sample | TC 1 + 4-epi-TC 2 (µg kg−1) | OTC 3 + 4-epi-OTC 4 (µg kg−1) | EFX 5 + CFX 6 (µg kg−1) | FLU 7 (µg kg−1) | SCP 8 (µg kg−1) | SDZ 9 (µg kg−1) |
|---|---|---|---|---|---|---|
| Farm 1 | nd | nd | 20.73 ± 10 10 | nd | nd | nd |
| Farm 2 | nd | nd | nd | nd | nd | nd |
| Farm 3 | nd | nd | nd | nd | 179.99 ± 83 | nd |
| Farm 4 | nd | nd | 24,307.11 ± 10 | nd | nd | nd |
| Farm 5 | nd | nd | nd | nd | nd | nd |
| Farm 6 | nd | 66.43 ± 53 | nd | nd | nd | nd |
| Farm 7 | 322.10 ± 229 | 10,364.41 ± 6791 | nd | nd | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yévenes, K.; Pokrant, E.; Trincado, L.; Lapierre, L.; Galarce, N.; Martín, B.S.; Maddaleno, A.; Hidalgo, H.; Cornejo, J. Detection of Antimicrobial Residues in Poultry Litter: Monitoring a Risk through a Selective and Sensitive HPLC–MS/MS Method. Animals 2021, 11, 1399. https://doi.org/10.3390/ani11051399
Yévenes K, Pokrant E, Trincado L, Lapierre L, Galarce N, Martín BS, Maddaleno A, Hidalgo H, Cornejo J. Detection of Antimicrobial Residues in Poultry Litter: Monitoring a Risk through a Selective and Sensitive HPLC–MS/MS Method. Animals. 2021; 11(5):1399. https://doi.org/10.3390/ani11051399
Chicago/Turabian StyleYévenes, Karina, Ekaterina Pokrant, Lina Trincado, Lisette Lapierre, Nicolás Galarce, Betty San Martín, Aldo Maddaleno, Héctor Hidalgo, and Javiera Cornejo. 2021. "Detection of Antimicrobial Residues in Poultry Litter: Monitoring a Risk through a Selective and Sensitive HPLC–MS/MS Method" Animals 11, no. 5: 1399. https://doi.org/10.3390/ani11051399
APA StyleYévenes, K., Pokrant, E., Trincado, L., Lapierre, L., Galarce, N., Martín, B. S., Maddaleno, A., Hidalgo, H., & Cornejo, J. (2021). Detection of Antimicrobial Residues in Poultry Litter: Monitoring a Risk through a Selective and Sensitive HPLC–MS/MS Method. Animals, 11(5), 1399. https://doi.org/10.3390/ani11051399









