Featured Application
The optimization of aging time for improved antioxidant activity and bacteriostatic capacity of black garlic (BG) of different ages was studied, and the results can serve as a reference for assessing the feasibility of developing BG as a functional food.
Abstract
To determine the optimization of aging time for improved antioxidant activity and bacteriostatic capacity of garlic during its aging, garlic produced in Yunlin region, Taiwan, was employed as the test material in an analysis of the allicin content, total phenol content, antioxidant activity, and bacteriostatic capacity of fresh and aged garlic extracts. Allicin content of the aging garlic decreased to a minor level, whereas total phenol content increased to 16.96 mg gallic acid equivalent (GAE)/mL after 35 days of the aging process. The results of antioxidant testing demonstrated favorable positive correlations among IC50 of DPPH scavenging capacity, Trolox equivalent antioxidant activity, and superoxide dismutase activity for both the fresh and aged garlic extracts. The analytical results showed that aging of garlic at 70 °C and 85% relative humidity for 40 days substantially increased the quantity of phenolics, DPPH scavenging capacity, Trolox equivalent antioxidant activity, and superoxide dismutase activity and enhanced the antioxidant activity. The extracts exhibited higher bacteriostatic capacity against Gram-positive bacteria such as Bacillus subtilis and Staphylococcus aureus than against Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa. In conclusion, black garlic aged under the optimum conditions exhibited favorable antioxidant activity and bacteriostatic ability.
1. Introduction
Since the 18th century, when Carl Linnaeus (1707–1778) first described the genus Allium, more than 1200 Allium species have been categorized under Alliaceae. The primary Allium species in Taiwan include A. fistulosum, A. sativum, A. cepa, A. ascalonicum, and A. tuberosum, and they are critical vegetables and seasonings in some of the world’s most popular dishes. In 1844, Theodor Wertheim (1820–1864) extracted a pungent substance from garlic and named it “allyl”, the Latin name for garlic [1]. Later, scholars studied this substance and discovered that it was a sulfur compound containing allicin. Among the types of Allium, garlic (A. sativum L.) is the most commonly used seasoning in Asia. It has various biological properties such as antioxidant, anticancer, and bacteriostatic effects.
When fresh garlic is placed in an environment with high temperature and high humidity for 15 to 90 days, it ages into natural black garlic (BG) with a sweet and sour taste and without the pungency of fresh garlic [2]. The sweet and sour taste of BG originates from its fructose and amino acids, whereas its black color is mainly caused by the Maillard reaction involving fructose, glucose, and amino acids [3]. During the aging process, allicin is rapidly transformed into various organic sulfur components. Compared with the S-allylcysteine, S-allylmercaptocysteine, and N(alpha)-fructosyl arginine in fresh garlic, those in BG are more beneficial to the human body [4]. Scholars have argued that aged BG does not have the pungent smell of allicin and has stronger biological activities such as antioxidant activity [5,6,7,8], anticancer capacity, ability to reduce obesity, anti-inflammatory ability, antiallergy ability, liver protection, and dyslipidemia alleviation [9]. Jang et al. [10] compared the biological activities and antioxidant activity of fresh garlic extract and the extract of BG aged at 70 °C and 90% humidity, discovering that the total phenol content of the BG extract is 2.5-fold greater than that of the fresh garlic extract, and the superoxide dismutase (SOD) activity of the BG extract is nearly 10-fold greater than that of fresh garlic. In addition, the crude lipid, crude protein, and total sugar content were discovered to be higher in BG than in fresh garlic in another study, with the BG extract containing various free amino acids [8]. When garlic is heated and aged, its pH decreases; furthermore, its polyphenol content and antioxidant activity increase substantially [11]. Zhang et al. [12] investigated the aging of fresh garlic into BG at temperatures of 60, 70, 80, and 90 °C and discovered that during heat treatment at different temperatures, contents of water, amino acids, and allicin progressively were reduced, whereas total phenols, total acid, and browning intensity were elevated. An aging temperature of 70 °C was concluded to be the most conducive to aging because BG formed at this temperature had outstanding quality and flavor. Animal tests found that in mice, anti-free radicals from BG extract were more efficient than those from fresh garlic extract [13]. Additionally, compared with fresh garlic, BG was more effective at improving the metabolic capacity of mice and strengthening their cardiovascular activity [14].
In our previous study, we discovered that through its own enzymatic reactions, garlic aged in a high-temperature and highly humid environment turned into darker, more tender, and sweeter BG [15]. Aged BG naturally has a higher antioxidant activity than fresh garlic. Through the aging process under high temperature and humidity, allicin is decomposed into various usable compounds, including a water-soluble sulfur-containing amino acid compound (S-allylcysteine) that substantially increases the SOD activity and total phenol content. In one study on the biochemical properties of BG formed under different aging conditions, garlic was placed in a temperature- and humidity-controlled fermentation tank to age at high temperature and humidity; the optimal temperature and humidity were discovered to be 70 °C and 85%, respectively. Therefore, the present study selected organic garlic produced in the Yunlin region of Taiwan as a material for aging at a temperature of 70 °C and humidity of 85% for different durations of 5, 10, 15, 20, 25, 30, 35, and 40 days. Subsequently, the changes in pH, free amino acid content, allicin content, antioxidant activity, and bacteriostatic capacity were analyzed and compared. The optimization of aging time for improved antioxidant activity and bacteriostatic capacity of BG of different ages was thus explored, and the results can serve as an indication for assessing the feasibility of developing BG as a functional food.
2. Materials and Methods
2.1. Materials
Allium sativum L. (hardneck garlic), grown in the Yunlin region of Taiwan and purchased from Taipei Agricultural Products Marketing Co., Ltd. (Taipei, Taiwan), was used as the test material for the aging experiments. Additionally, commercial rice wine Michiu Tou, manufactured by Taiwan Tobacco and Liquor Corporation, was used for preparation of black garlic extracts.
All chemicals and reagents used in this study, including the allicin standards, DPPH, Folin-Ciocalteu reagent, gallic acid, and catechin, were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The culture media for the test bacteria employed in this study, including nutrient agar (NA), nutrient broth (NB), and Mueller–Hinton agar (MHA), were bought from Difco Chemical Co. (Sparks, ML, USA).
Two Gram-positive bacteria (G+) strains comprising Staphylococcus aureus ATCC 6538 and Bacillus subtilis ATCC 6051 and two Gram-negative bacteria (G−) strains consisting of Escherichia coli ATCC 10536 and Pseudomonas aeruginosa ATCC 9027 were used for the study of bacteriostatic capacity of the BG extracts. These strains were all obtained from the Bioresources Collection and Research Center (BCRC) of the Food Industry Research and Development Institute (Hsinchu, Taiwan).
2.2. Preparation of BG Extracts
This study followed the optimal conditions discovered in previous studies [12,15] to obtain favorable biochemical properties: 70 °C temperature and 85% relative humidity for 35 days. Michiu Tou (MT) wine (34% alcohol, Taiwan Tobacco and Liquor Corporation) is a commercial flavored rice wine in Taiwan; it was determined to have no bacteriostatic capacity in our previous studies [15,16]. Fresh garlic and BG were peeled, chopped into small pieces, and mixed with MT wine at the ratio of 1:2 (w/v). Subsequently, the mixture was placed in a glass serum bottle for 7 days. Next, the solvent was separated using filter paper (Whatman #1) to remove residues before it was evaporated under reduced pressure at 50 °C. The extracts were dried using a vacuum oven for 24 h and then kept in the refrigerator with a temperature of 4 °C prior to analysis.
2.3. Analysis of the Biochemical Properties of the Extract
2.3.1. Measurement of pH Level
The pH value of extracts of fresh garlic and BG aged under various temperatures was determined using a Sartorius pH meter PB-11.
2.3.2. Determination of Allicin Content
The allicin content of the sample was determined by high-performance liquid chromatography (HPLC) analysis. An Agilent 1100 HPLC instrument (Waldbronn, Germany) was used, and the system consisted of the following components: a UV–Vis (DAD) detector (Heisenberg), a Finnigan LCQ Deca (CURIE) and a Phenomenex Luna C18 (2) column (150 × 4.6 mm i.d., 5 μm particle size) (Phenomenex, Taipei, Taiwan). Acetonitrile/deionized water (30:70, v/v) was used as the mobile phase for analysis of allicin at a flow rate of 1.0 mL/min. The injection volume was 1 μL, and the column temperature was maintained at 25 °C. All samples were filtered through a 0.22 μm membrane filter before being injected into the HPLC column. The UV detector was set at 195 nm for all operations.
2.3.3. Determination of Total Phenol Content
The total phenol content of the fresh garlic and BG samples was measured using the Folin–Ciocalteu phenol reagent [17]. We employed this analysis method when measuring the total phenol content in shallot extracts [15,16,18]. A total of 500 μL of the Folin–Ciocalteu reagent at the concentration of 1.0 N was mixed thoroughly with 500 μL of BG extract. After the mixture had been left at room temperature for 5 min, 1 mL of 20% Na2CO3 was added, mixed thoroughly, and placed at room temperature for 8 min. Subsequently, the mixture was centrifuged for 10 min at 12,000 rpm. The supernatant liquor was sampled and placed in a cuvette. Ultraviolet–visible (UV–Vis) spectrophotometry was performed at the absorbance wavelength of 730 nm, and the measurement was conducted three times. Additionally, gallic acid standard concentrations ranging from 0.005 to 0.16 mg/mL were used to prepare the calibration curve. The gallic acid equivalent (GAE) calibration curve was plotted using Microsoft Excel, and the total phenol compound content of the extracts was calculated from the regression equation of the calibration curve. The total phenol content in the fresh garlic and BG extract is expressed as milligram gallic acid equivalents per milliliter of the sample (mg GAE/mL).
2.3.4. Free Amino Acid Assay
Fresh garlic and BG samples were examined by the Food Industry Research and Development Institute in Hsinchu, Taiwan, where ion exchange chromatography was performed to measure the content of free amino acids (Taiwan CNS 12632:N6221 method). The limit of quantification of individual free amino acid was 0.005 mg/100 g.
2.4. Analysis of the Antioxidant Activity of Garlic and BG Extract
2.4.1. Assessing the Capacity of DPPH to Capture Free Radicals
This study measured the antioxidant activity of the extracts using the method proposed by Gyamfi et al. [19], which involves measuring the capacity of DPPH to capture free radicals. For the DPPH scavenging capacity assay, BG extract or catechin with solution was adjusted to the initial concentration of 1 g dry weight/mL. Subsequently, the BG extract or catechin solution was subjected to twofold serial dilution. Briefly, 100 μL of BG extract or catechin solution was added to the equal volume of freshly prepared 0.2 mM DPPH ethanol solution. This mixture was shaken evenly at room temperature and maintained in the dark for 30 min. Then, the absorbance was measured at 517 nm against a blank. This test was performed in triplicate. In this experiment, the 5 μg/mL catechin solution was used as the positive control for assessing the DPPH scavenging capacity of the BG extracts. Higher DPPH scavenging capacity was indicated by a decrease in absorbance at the wavelength 517 nm. The DPPH radical-scavenging activity was calculated as follows:
DPPH radical-scavenging capacity (%) = [1 − (absorbance of the sample/absorbance of the control)] × 100. The IC50 was defined as the concentration required for inhibiting 50% of the DPPH scavenging capacity.
2.4.2. Assessing the Trolox Equivalent Antioxidant Activity (TEAC)
The present study used the method employed by Re et al. [20] to evaluate the capacity of the test materials to scavenge ABTS+. ABTS+ is a blue-green water-based solution and contains highly stable free radicals. At a wavelength of 734 nm, ABTS+ exhibits specific absorbance. When ABTS+ interacts with BG extract, its absorbance is reduced, and this reduction can be used to evaluate the capacity of the sample to scavenge ABTS+; weaker absorbance indicates a stronger capacity for scavenging ABTS+. Additionally, Trolox was used as the reference standard for quantification. UV–Vis spectrophotometry was employed to measure absorbance under light of wavelength 734 nm. The correlation between absorbance and Trolox condensation was used to determine the regression equation of the calibration curve. The BG sample analysis was then conducted using BG extract samples instead of Trolox and the absorbance again measured. The absorbance of the BG extract sample was input to the regression equation of the calibration curve to determine the Trolox equivalent antioxidant activity (TEAC), which is the concentration of Trolox that has the same capacity for scavenging ABTS+ as 0.5 mg/mL of the BG sample or 1 mM of the pure compound.
2.4.3. Assessing Superoxide Dismutase (SOD) Activity
In this assessment, the xanthine/xanthine oxidase reaction system was employed to determine SOD activity. The commercial reagents for SOD analysis were produced by the British company Randox Laboratories Ltd. (Crumlin, Northern Ireland). Superoxide anions were generated in the xanthine and xanthine oxidase reaction system. The included chromogen in the system produces a water-soluble yellow dye upon reduction by superoxide anions. The activity of SOD is determined as the inhibition of chromogen reduction in the aforementioned reaction system. In this work, the SOD specific activity is expressed as SOD activity units by mg of protein. The Lowry protein assay [21] was used for determining the level of protein. One unit (U) of SOD enzyme is defined as the amount of protein that produces a 50% inhibition of the cytochrome c in a coupled xanthine and xanthine oxidase system at pH 7.8 and 25 °C.
2.5. Bacteriostatic Capacity of Fresh Garlic and BG Extracts
The tube dilution test and the disc agar diffusion test used by Fujisawa et al. [22] were used in the present study to assess the bacteriostatic capacity of the fresh garlic and BG extracts against S. aureus (ATCC 6538), B. subtilis (ATCC 6051), E. coli (ATCC 10536), and P. aeruginosa (ATCC 9027) [15]. First, the test strains were placed in an NB tube for activation and cultivation for 12–24 h. The disc agar diffusion test involved using a sterile cotton swab to inoculate the aforementioned strains (approximately 105 CFU/mL) by spreading the swab against an MHA plate. Afterwards, discs (8 mm in diameter) soaked in different fresh garlic and BG extracts were placed evenly onto the MHA medium plate with the help of sterile forceps. The plate was then incubated in a thermostatic incubator at 37 °C for 24 h. The size of the zone of inhibition around the discs (mm) was measured after incubation. Successive dilution of the fresh garlic and BG extracts was prepared for the tube dilution test. The diluted fresh garlic and BG extracts were inoculated with the bacteria inoculum (approximately 105 CFU/mL) and then incubated in a thermostatic incubator at 37 °C for 24 h. Consequently, the test bacteria growth was measured using a spectrophotometer set at a wavelength of 600 nm. The minimal inhibitory concentration (MIC) was determined, at which no visible growth of the test bacteria was seen. The minimal bactericidal concentration (MBC) is the lowest concentration of the extracts required to kill the test bacteria after 72 h of incubation. Penicillin (10 μg/mL) and tetracycline (30 μg/mL) were used as the positive control groups in this experiment.
2.6. Statistical Analysis
All experiments were performed in triplicate, and the data are expressed as the average ± standard deviation of three measurements. The triplicate data were subjected to an analysis of variance using the Statistical Package for the Social Sciences 14.0 (SPSS, SPSS INC., Hong De International Software Consulting Co., Ltd., Taipei, Taiwan). Furthermore, comparison of means was analyzed by Duncan’s multiple range test, and differences were considered significant at p < 0.05.
3. Results
3.1. Biochemical Properties of BG during the Aging Process
Table 1 contains the biochemical properties of fresh garlic and BG during the aging process. The pH of the fresh garlic extract was 6.19, whereas that of the aging garlic on the 5th and 35th day was 5.67 and 3.52; the pH thus decreased as the aging progressed. The water content of the fresh garlic and aging garlic samples was 77.50% and 30.78%, and it decreased as the aging progressed. HPLC was employed to measure the allicin content of the fresh garlic and aging garlic extracts. The allicin content of the fresh garlic was 2.688 µg/mL, whereas that of the aging garlic decreased as the aging progressed. The allicin content of the garlic aged for 5, 10, 15, and 20 days was 1.72, 0.56, 0.22, and 0.01 µg/mL, respectively. Between the 25th and 35th days of aging, allicin was undetectable. Regarding the total phenol content, the fresh garlic contained 5.87 mg GAE/mL, whereas the aging garlic contained 0.98 and 16.96 mg GAE/mL on the 5th and 35th days, respectively, indicating that the total phenol content increased as the aging progressed.

Table 1.
Biochemical properties of fresh garlic and black garlic (BG) aged for different durations.
The free amino acid content of the garlic samples aged for different durations were measured, and the results are presented in Table 2. The total free amino acid content of fresh garlic was 1843.12 mg/100 g, and it decreased as the aging progressed. On the 5th and 10th days and between the 15th and 35th days, the total amino acid content was 2415.20, 1817.05, and 505.66–82.01 mg/100 g, respectively. Regarding the individual amino acid contents, the results varied. Arginine, asparagine, aspartic, cystine, and glutamic acid were found to be most prevalent in the garlic aged for 5 days, whereas the alanine and methionine contents were highest in the garlic aged for 10 days. Glycine content was highest when aging was performed for 15 days. Histidine, isoleucine, leucine, lysine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, and valine were most prevalent in the fresh garlic. Cystine was not identified in fresh garlic, whereas 61.98 and 19.79 mg/100 g of cystine was identified in the garlic aged for 5 and 10 days, respectively, but not in the samples with other aging durations. The fresh garlic contained 15.96 mg/100 g tryptophan, but tryptophan was not detected in the other samples. Loss of tryptophan by aging is rapid with an undetectable level at 5 days. For amino acids, the cut-off for drastic decline might be 10 days (15 days exhibits a much greater decline than 10 days). If the industrial goal is to preserve specific amino acids, the data in Table 2 could be an informative reference.

Table 2.
Free amino acids in fresh garlic and garlic aged for different durations.
3.2. Antioxidant Activity of Extracts from Fresh Garlic and BG
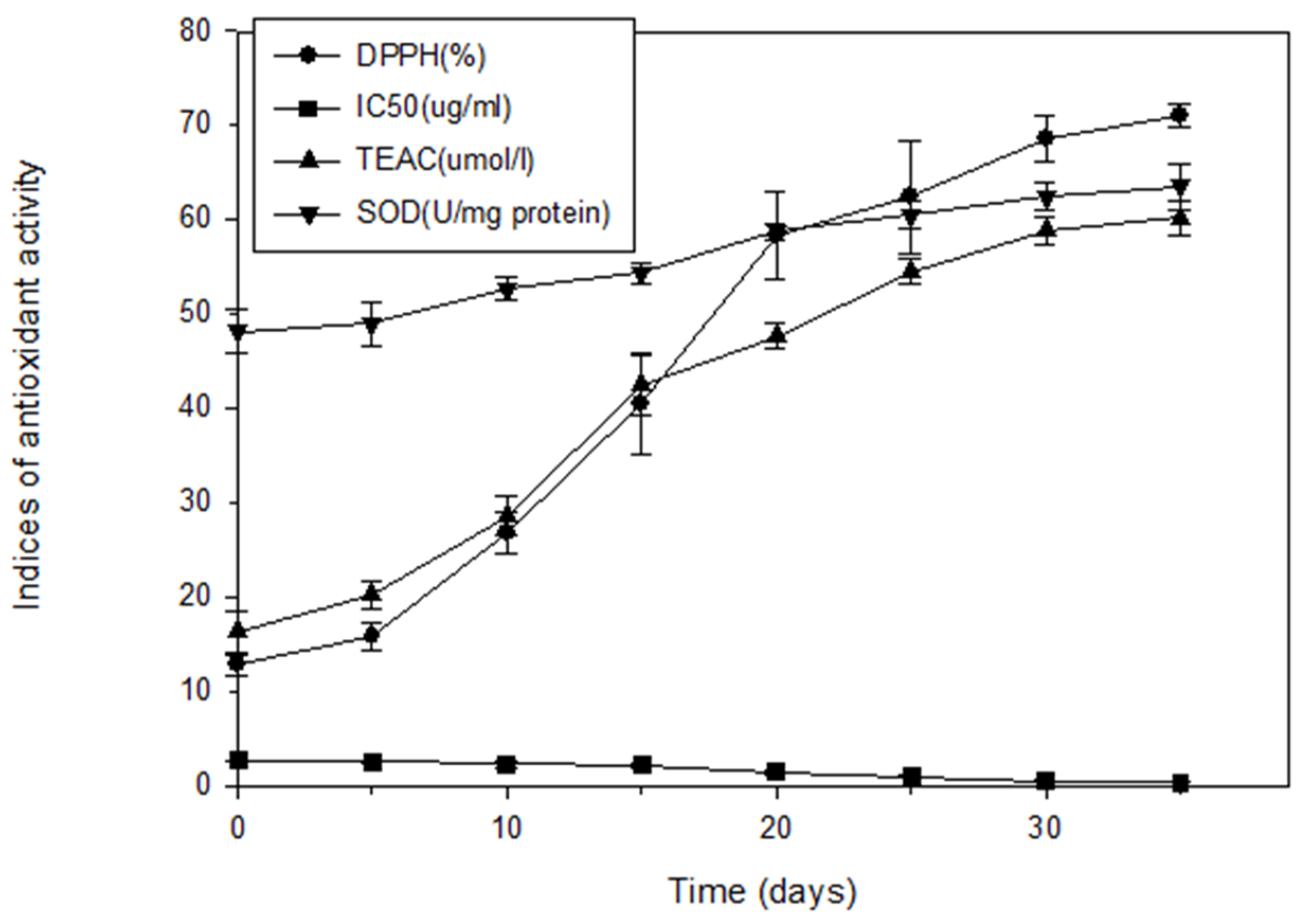
Figure 1 illustrates the antioxidant activity of extracts from fresh and aged garlic. The DPPH free-radical-scavenging capacity, TEAC, and antioxidant activity of SOD in aged garlic increased as the aging duration increased. The DPPH scavenging capacity of the fresh garlic was 12.90% and increased as the aging duration increased, from 15.88% for 5 days of aging to 70.95% for 35 days. The IC50 reduced from 2.72 μg/mL for the fresh garlic to 2.63 μg/mL after 5 days of aging and then to 0.49 μg/mL after 35 days. The TEAC of the fresh garlic was 16.34 μmol/L, and it increased as the aging duration increased, to 20.27 μmol/L after 5 days of aging, 58.85 μmol/L after 30 days, and 60.18 μmol/L after 35 days. The SOD activity of the fresh garlic was 48.14 U/mg protein, and it also increased as the aging process progressed, to 48.96 U/mg protein after 5 days, 62.48 U/mg protein after 30 days, and 63.51 U/mg protein after 35 days.
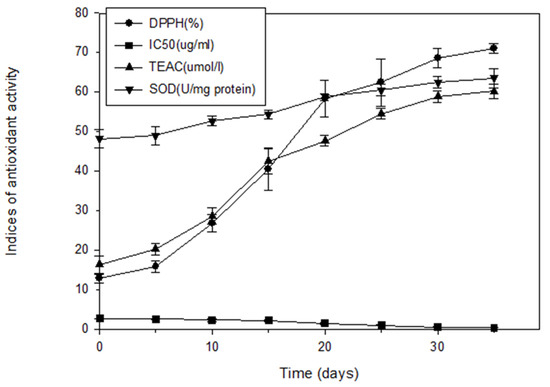
Figure 1.
Antioxidant capacity of fresh and aged garlic extracts during the aging process. Each experiment was performed in triplicate, and the results (DPPH scavenging capability (%), IC50 (μg/mL), Trolox equivalent antioxidant activity (TEAC, mmol/L), and SOD (U/mg protein)) are expressed as the average of three measurements ± standard deviation.
3.3. Bacteriostatic Capacity of Extracts from Fresh and Aged Garlic
Table 3 details the bacteriostatic capacity of extracts from fresh and aged garlic. All samples exhibited bacteriostatic capacity against B. subtilis (ATCC 6051), S. aureus (ATCC 6538), and P. aeruginosa (ATCC 9027). However, the fresh garlic and that aged for 5–10 days did not exhibit bacteriostatic capacity against E. coli (ATCC 10536); only the garlic aged for longer than 15 days was bacteriostatic against E. coli, but the diameter of the inhibition zone was only up to 8.33 mm, which showed little inhibitory effect. The diameter of the inhibition zone against B. subtilis and S. aureus for the garlic aged for 35 days was 23.00 and 25.33 mm, respectively, and the MIC and MBC were 0.2 and 0.4 mg, respectively. In contrast, the diameter of the inhibition zone against B. subtilis and S. aureus for the fresh garlic extract was 12.90 and 11.00 mm, respectively, the MIC and MBC for B. subtilis were 0.4 and 0.8 mg, and the MIC and MBC for S. aureus were 0.8 and 1.0 mg, respectively. Therefore, the bacteriostatic capacity of the BG extracts was higher against B. subtilis and S. aureus than that of the fresh garlic extract. The bacteriostatic capacity against P. aeruginosa was highest for the garlic aged for 35 days, with the diameter of the inhibition zone being 12.67 mm and the MIC and MBC being 0.4 and 0.8 mg, respectively. The diameter of the inhibition zone for the fresh garlic extract against E. coli was only 8.33 mm, and the MIC and MBC were 1.0 and 2.0 mg, respectively. These results demonstrated that the extracts from fresh garlic and BG had higher bacteriostatic capacity against Gram-positive bacteria such as B. subtilis (ATCC 6051) and S. aureus (ATCC 6538) than against Gram-negative bacteria such as P. aeruginosa (ATCC 9027) and E. coli (ATCC 10536).

Table 3.
Bacteriostatic capacity of extracts from fresh and aged garlic.
4. Discussion
4.1. Comparison of the Biochemical Properties and Antioxidant Activity of Garlic and BG Extracts
Regarding the biochemical properties of garlic and BG aged for different durations, we discovered that during the aging process from 0 to 35 days of aging, the pH decreased from 6.19 down to 3.52. According to Choi et al. [23], BG aged in an environment at 70 °C and 90% relative humidity for 35 days exhibited decreasing pH from 6.33 to 3.74. Liang et al. [24] conducted a 90-day garlic aging experiment, and the pH of the garlic decreased from 7.1 to 4.1. Kim et al. [25] analyzed giant BG, giant fresh garlic, normal BG, and normal fresh garlic and discovered their pH values to be 3.77, 5.97, 3.94, and 6.10, respectively. Yuan et al. [2] investigated aged garlic in a high-temperature environment and observed a similar trend; they deduced that the main reason for the decrease in pH during the aging process was the Maillard reaction, which increases the amount of organic acid, thus reducing the pH. During the aging process, the water content of the fresh garlic decreased from 77.5% to 30.78%, which was similar to the reduction from 64.21% to 29.88% in the study by Choi et al. [23]. Although the experimental materials were placed in a highly humid environment, the garlic’s water content was lost to the environment during the heating process. The present study demonstrated that as the aging process progressed, the allicin content decreased; from the 25th day, allicin was not detected. The optimized aging time of 25 days is compatible with the mitigation of allicin content to undetectable. This result is the same as that of Liang et al. [24], who conducted a 90-day garlic aging experiment. Zhang et al. [12] reported that during the high-temperature aging process, the allicin content of fresh garlic was 3.18 g/kg and increased to 3.53 g/kg after 2 days of aging but was undetectable from 5 days of aging. Thus, under high temperature, allicin was released into the environment and likely transformed into alliin or other compounds [12,22].
The total phenol content increased as the aging duration increased, reaching a maximum after 35 days of aging. In their studies on BG aging, scholars have reported that total phenol content was positively correlated with the antioxidant activity of the test material [13,23,26]. Choi et al. [23] maintained that after a 35-day garlic aging process, the total phenol content was 13.91 mg GAE/g, far lower than the 25.81–58.33 mg GAE/g determined using BG extract in the present study. Toledano-Medina et al. [11] aged garlic at 72, 75, and 78 °C, discovering that the total phenol content increased as the aging duration increased. Lu et al. [27] discovered that the total phenol content in BG extract was approximately 4–10 times higher than that in fresh garlic. These results are in general agreement with the results obtained in the present study; that is, the total phenol content of BG extract is higher that than in fresh garlic.
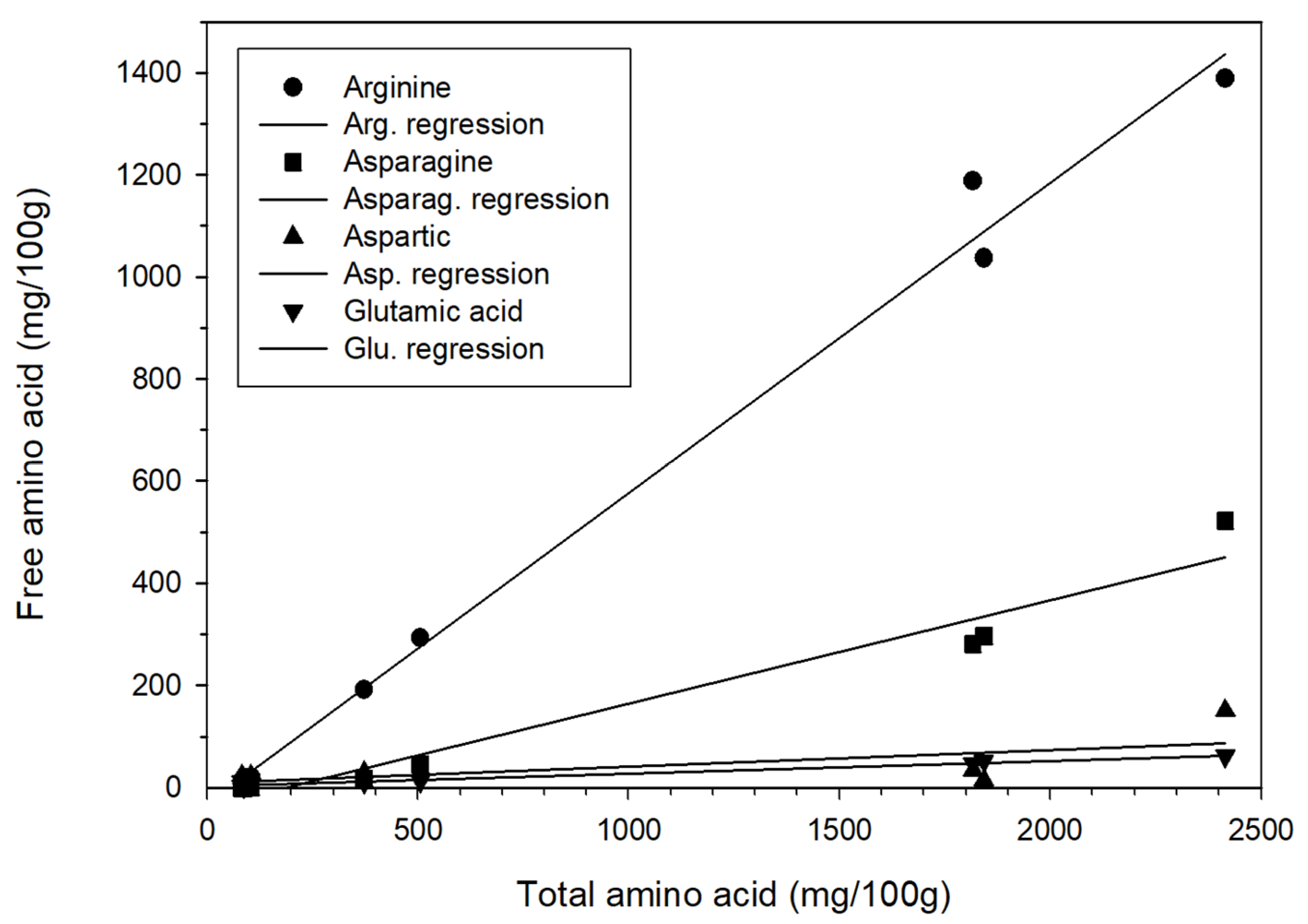
The contents of free amino acids and total amino acids both decreased as aging duration increased. Further exploration revealed that in regression analysis—with the total amino acid content represented as X and the content of arginine, asparagine, and glutamic acid represented as Y—the following relationships were obtained: Y = 0.61X − 31.15, Y = 0.20X − 37.16, and Y = 0.03X + 2.94, respectively, with r2 = 0.99, 0.96, and 0.98 (Figure 2). Therefore, highly positive linear relationships between total amino acid content and arginine, asparagine, and glutamic acid contents were determined. Aspartic, an amino acid related to aspartame, was not highly positively correlated with total amino acid content; this relationship was described by Y = 0.03X + 9.46, with r2 = 0.44. This finding has not been reported in the literature. No other significant positive correlations were identified between the content of other amino acids and the total amino acid content. The results of the present study showed that the free amino acid content of the fresh garlic (1843.12 mg/100 g) was significantly higher than that of the garlic aged for 35 days (87.42 mg/100 g). Thus, it was inferred that during the high-temperature aging process, the amino acids were denatured. Consequently, the free amino acid content of the BG was only 4.74%, a loss rate of 99.95%. Choi et al. [22] conducted a 35-day garlic aging experiment under a temperature of 70 °C and relative humidity of 90%, discovering that the total amino acid content of the fresh garlic was 1942.18 mg/100 g, whereas that of the BG was 912.64 mg/100 g; the total content of amino acids was 46.99% of that before the garlic was aged, suggesting a loss of 53.01%. Liang et al. [24] discovered that during the heating process, the content of 17 types of amino acids in garlic was higher on day 5 but substantially lower on day 25 compared with day 0. After day 90 had been reached, the amino acid content decreased even further. However, Kim et al. [25] investigated giant BG, giant fresh garlic, normal BG, and normal fresh garlic and discovered their total free amino acid contents to be 1175.64, 762.91, 1123.62, and 425.93 mg/100 g, respectively. This result of the free amino acid content of BG being greater than that of fresh garlic was different from the finding of the present study, of Choi et al. [23], and of Liang et al. [24]. This considerable difference could have resulted from different garlic varieties, experimental methods, and aging conditions.
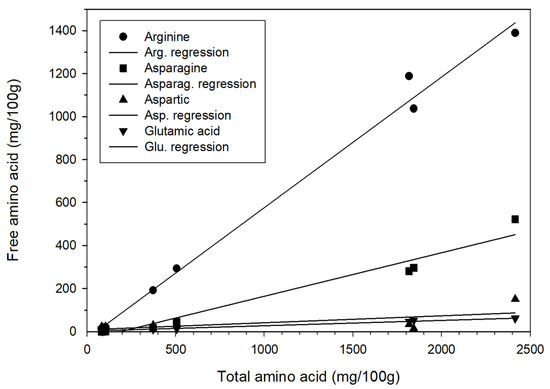
Figure 2.
Correlation between free amino acid and total amino acid content.
Regarding antioxidant activity, the present study revealed that the DPPH scavenging capacity, TEAC, and SOD capacity all increased as the aging duration increased. In their literature review, Kimura et al. [9] concluded that the DPPH scavenging capacity [13,27], TAEC [28], and SOD capacity [29] of BG were greater than those of fresh garlic. In the study of Kim et al. [13] on the physical stability and antioxidant properties of BG extract, they found that adding 10% BG extract led to a higher DPPH scavenging capacity than adding 10% fresh garlic extract. Choi et al. [23] also stated in their study on the physical and antioxidant properties of BG undergoing 35-day aging that BG had substantially higher DPPH scavenging capacity than fresh garlic, with the DPPH scavenging capacity of garlic aged for 21 days being 74.48% and that of fresh garlic being 4.65%. Jeong et al. [27] found that the BG extracts had antioxidant activity higher than that of the fresh garlic extracts. Lu et al. [26] discovered that the DPPH scavenging capacity of BG extract was stronger than that of fresh garlic. The results from the present study identified DPPH scavenging capacity of BG extract and fresh garlic to be 70.95% and 12.90%, respectively, in agreement with the aforementioned studies and revealing that garlic planted in Taiwan and extracts from BG aged for different durations have favorable DPPH scavenging capacity. Regarding the TEAC, Lee et al. [28] reported in their research on animals with type II diabetes that BG reduced the mass of free radicals by 59.2 µmol/g wet weight, which was superior to the 13.3 µmol/g wet weight reduction induced by fresh garlic. Choi et al. [23] reported that during a 35-day garlic aging process, the TEAC was between 92.43 and 249.20 mM Trolox equivalent/g and that garlic aged for 21 days exhibited the highest ABTS+ free-radical-scavenging capacity. The present study found that for aging lasting up to 35 days, the TEAC of BG extract was higher than that of fresh garlic extract. Regarding SOD activity, Sato et al. [29] conducted short-term fermentation of garlic to increase its antioxidant activity. They discovered that after placing fresh garlic in an environment of temperature 60–70 °C and relative humidity 85%–95% for 40 days, the SOD activity and scavenging capacity against hydrogen peroxide were 10 times higher than those of fresh garlic extract. In their antioxidation research on animals with type II diabetes, Lee et al. [28] discovered that the SOD activity of BG was three to nine times higher than that of fresh garlic. The results of the present study revealed that the SOD activity of the garlic aged for 35 days was 63.51 U/mg protein, 1.3 times that of the fresh garlic (48.14 U/mg protein). Therefore, the BG indeed exhibited stronger SOD activity.
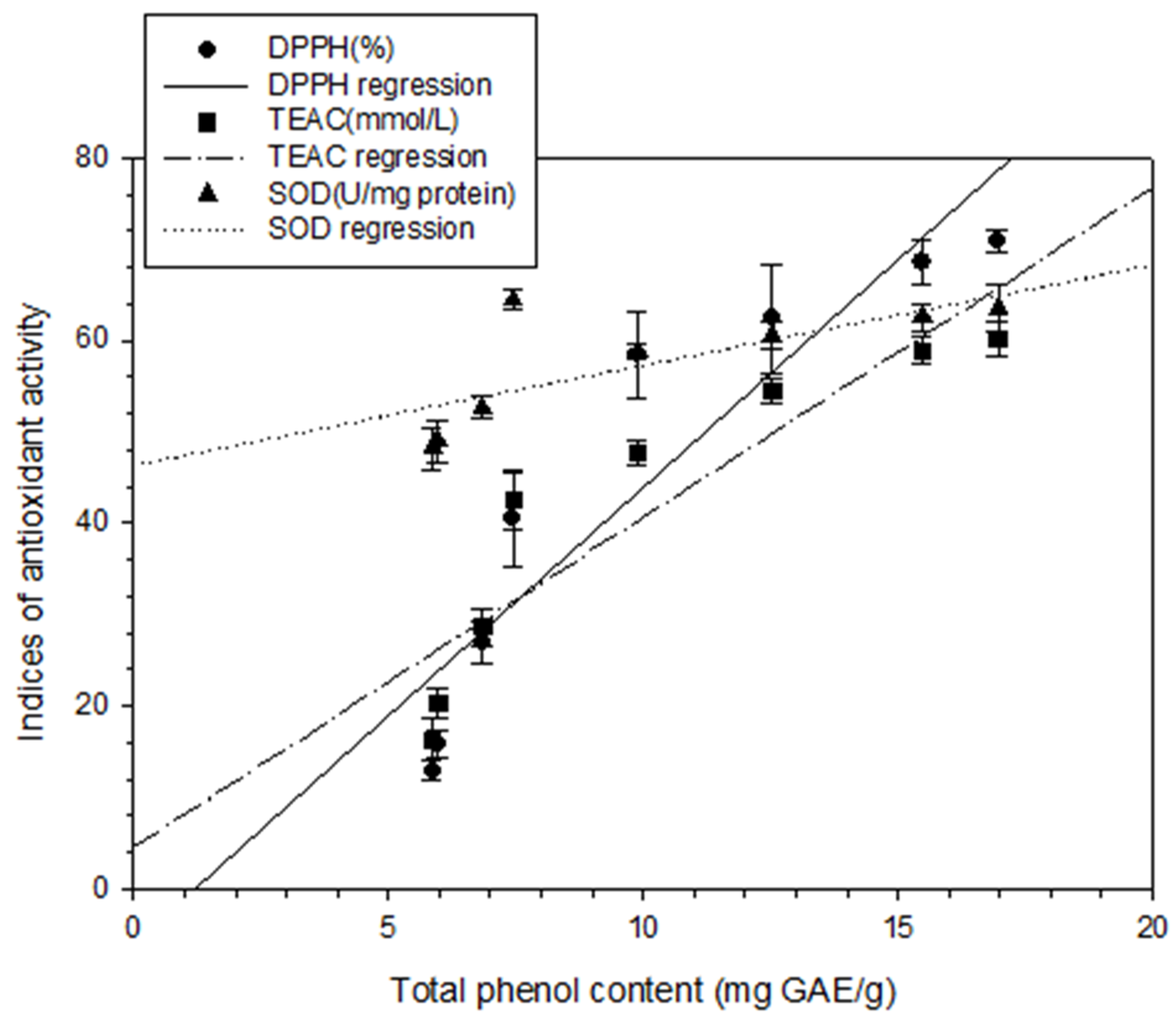
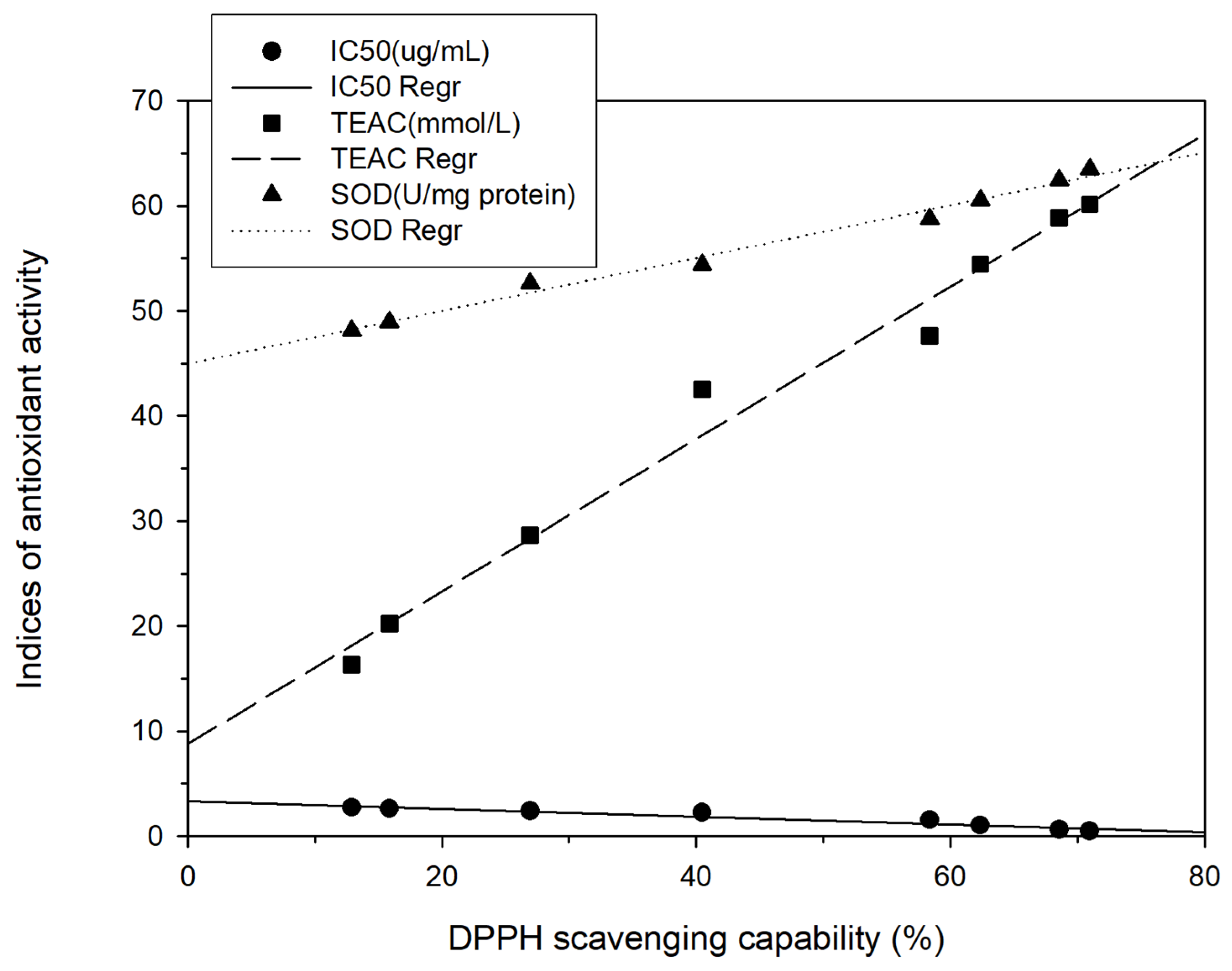
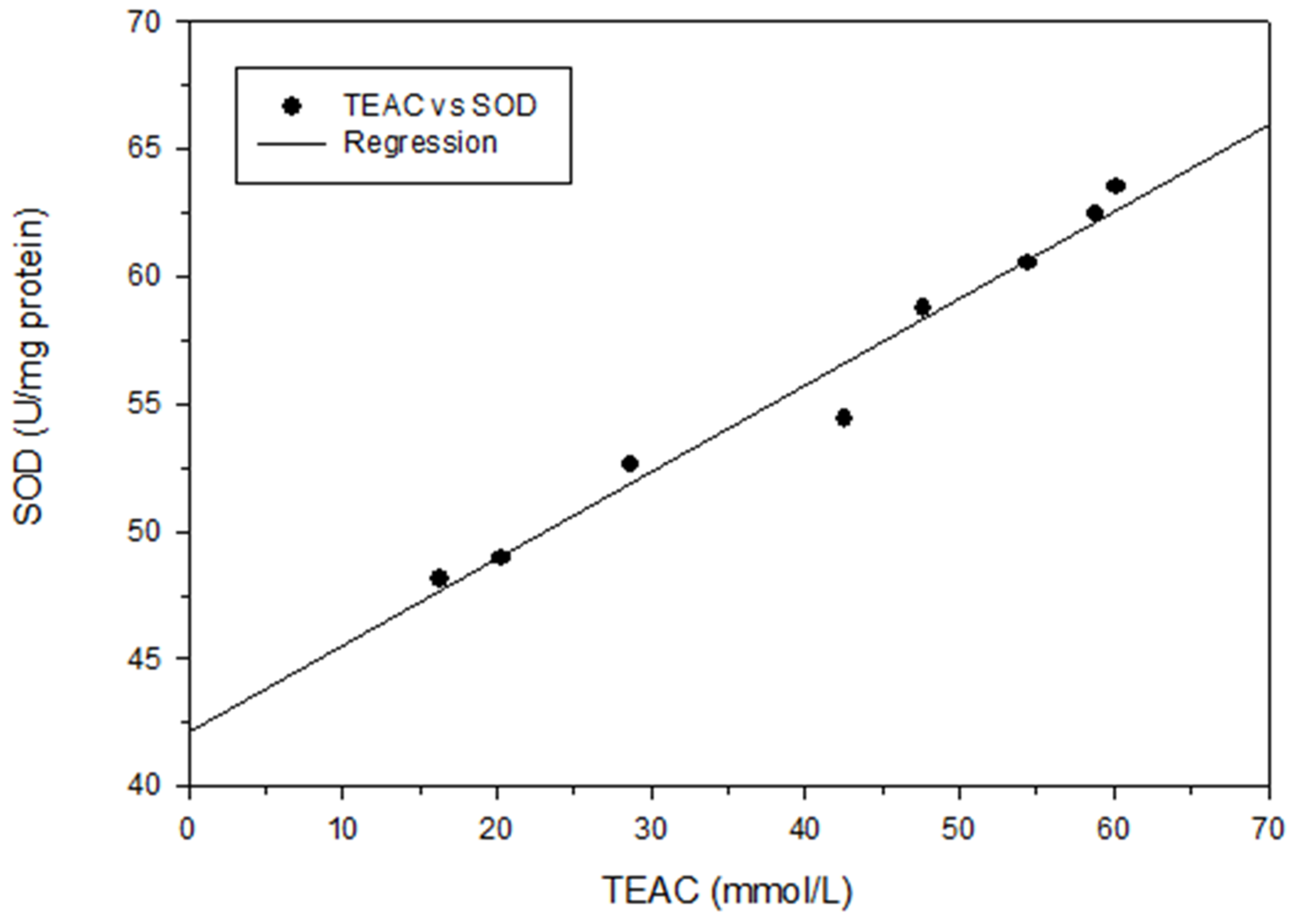
To analyze the relationship between total phenol content and different antioxidant activity indices, regression analysis was performed in this study, with the total phenol content represented as X and the antioxidant activity indices (DPPH scavenging capacity, TEAC, or SOD) represented as Y. The following correlation formulas were obtained: Y = 4.99X − 6.00, r2 = 0.85; Y = 3.61X + 4.58, r2 = 0.83; and Y = 1.10X + 46.31, r2 = 0.53, respectively (Figure 3). Positive linear correlations were thus discovered between the total phenol content and those antioxidant activity indices. These findings are in agreement with those of Chang et al. [15,17,18] for shallots and for BG aged at different temperatures. Regression analysis was also performed with DPPH scavenging capacity represented as X and antioxidant activity indices (IC50, TEAC, or SOD activity) represented as Y; the results were Y = − 0.04X + 3.34, r2 = 0.95; Y = 0.73X + 8.79, r2 = 0.98; and Y = 0.25X + 44.97, r2 = 0.99, respectively (Figure 4). Subsequently, TEAC and SOD activity were used as X and Y, respectively, and the regression analysis result was Y = 0.34X + 42.16, r2 = 0.97 (Figure 5). Therefore, the tests for measuring antioxidant capacity applied in this study—namely DPPH scavenging capacity, TEAC, and SOD activity—were positively correlated and could serve as favorable antioxidant activity indices in BG experiments. This study also demonstrated that the antioxidant activity indices of the BG were all superior to those of the fresh garlic extract, in agreement with the results of Chang et al. [15,17,18] on BG aged at different temperatures.
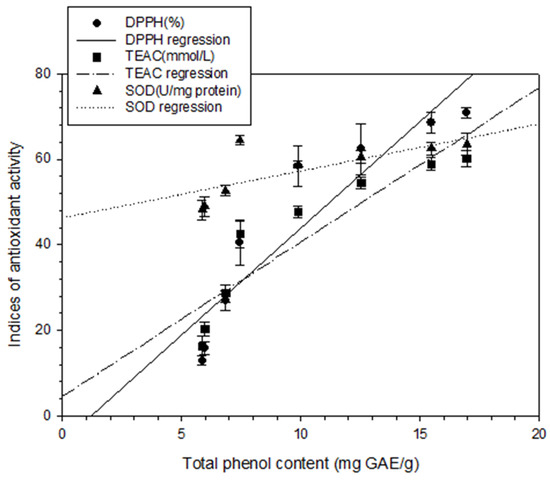
Figure 3.
Correlation between DPPH scavenging capability (%), TEAC (mmol/L) and SOD (U/mg protein) and between total phenolic content (mg GAE/g). Each test was performed in triplicate, and results are expressed as the average of three measurements ± standard deviation. The scale of error bar is too small to be visible.
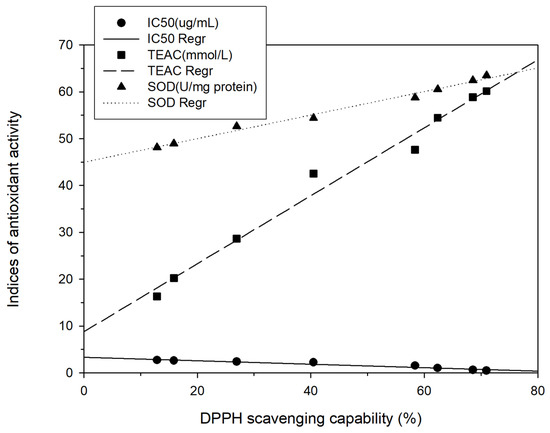
Figure 4.
Correlation between TEAC (mmol/L) and SOD (U/mg protein) and between DPPH scavenging capability (%). Each test was performed in triplicate, and results are expressed as the average of three measurements ± standard deviation. The scale of error bar is too small to be visible.
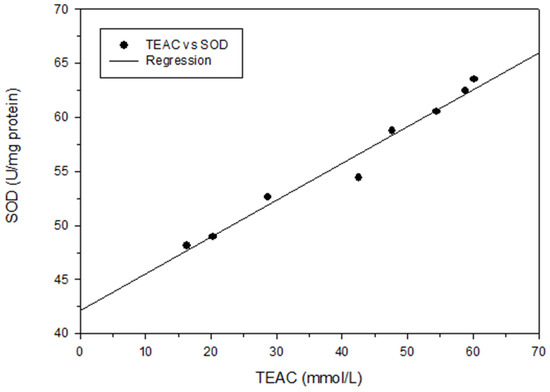
Figure 5.
Correlation between TEAC (mmol/L) and SOD (U/mg protein). Each test was performed in triplicate, and results are expressed as the average of three measurements ± standard deviation. The scale of error bar is too small to be visible.
4.2. Comparison of the Biochemical Properties and Bacteriostatic Capacity of Fresh Garlic and Garlic Aged for Different Durations
Allicin content gives garlic favorable bacteriostatic capacity [30]. Various studies have discovered that allicin exerts an antibacterial effect against S. aureus, B. subtilis, E. coli and P. aeruginosa [22,31,32]. In the present study, HPLC was used to measure the allicin content of garlic, finding that the allicin content of the fresh garlic was 2.68 µg/mL, whereas that of the aged garlic decreased with increasing aging duration. No allicin was detected in the garlic aged for 25–35 days (Table 1). The results presented in Table 3 reveal that fresh and aged garlic (including BG aged for various durations) extracts exhibited bacteriostatic capacity against B. subtilis, S. aureus and P. aeruginosa. Regardless of whether garlic was fresh or aged, the bacteriostatic capacity against Gram-positive bacteria (B. subtilis and S. aureus) was higher than that against Gram-negative bacteria (E. coli and P. aeruginosa). Several studies on the bacteriostatic capacity of garlic extract have revealed that garlic extract and its sulfur compounds inhibited the activities of bacteria such as S. aureus, P. aeruginosa, and Candida albicans [33,34,35]. The present study used penicillin and tetracycline in control experiments. Penicillins exhibited superior bacteriostatic capacity against Gram-positive bacteria (B. subtilis and S. aureus), whereas tetracyclines are broad-spectrum agents, exhibiting activity against a wide range of Gram-positive and Gram-negative bacteria. It is well known that penicillin acts by inhibiting the synthesis of the peptidoglycan layer of bacterial cell walls, whereas tetracycline inhibits bacterial growth by stopping protein and enzyme synthesis. This study revealed that extracts from fresh and aged garlic exhibited higher bacteriostatic capacity against B. subtilis and S. aureus than against E. coli and P. aeruginosa. Additionally, garlic aged for longer periods had higher bacteriostatic capacity. These results revealed that allicin was not the only antibacterial substance in the garlic and BG extracts. The bacteriostatic capacity of the fresh and aged garlic extracts could thus be related to the extracts’ antimicrobial peptide content. Reddy et al. [36] reported that antimicrobial peptides inhibited the growth of Gram-positive and Gram-negative bacteria. Papagianni et al. [37] further stated that antimicrobial peptides are peptides containing two or more arginine, lysine, and histidine molecules. The component analysis detailed in Table 2 regarding free amino acids showed that the extracts from garlic and BG contained arginine, lysine, and histidine; thus, the bacteriostatic capacity of these extracts can be inferred as related to the antimicrobial peptides they contained.
5. Conclusions
Extracts from fresh and black garlic have favorable antioxidant activity and bacteriostatic ability. The antioxidant activity and bacteriostatic ability of garlic could be increased significantly by optimizing the aging time and conditions. This study might be useful for understanding the optimum aging conditions of black garlic and to maximize antioxidant activity and bacteriostatic ability.
Author Contributions
Conception and design: T.-C.C., H.-D.J.; Analysis and interpretation of the data: T.-C.C.; Drafting of the article: T.-C.C.; Final approval of the article: T.-C.C., H.-D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the MacKay Junior College of Medicine, Nursing, and Management, Taiwan, grant number MKC105R14 and MKC106R04.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wertheim, T. Untersuchung des Knoblauchöls. Ann. Chem. Pharm. 1844, 51, 289–315. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Ríos, K.L.; Vázquez-Barrios, M.E.; Gaytán-Martínez, M.; Olano, A.; Montilla, A.; Villamiel, M. 2-furoylmethyl amino acids as indicators of Maillard reaction during the elaboration of black garlic. Food Chem. 2018, 240, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Petesch, B.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–966S. [Google Scholar] [CrossRef] [PubMed]
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Imai, J.; Ide, N.; Nagae, S.; Moriguchi, T.; Matsuura, H.; Itakura, Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994, 60, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Choi, D.J.; Lee, S.J.; Cha, J.Y.; Sung, N.J. Antioxidant activity of black garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 965–971. [Google Scholar] [CrossRef]
- Choi, D.J.; Lee, S.J.; Kang, M.J.; Cho, H.S.; Sung, N.J.; Shin, J.H. Physicochemical characteristics of black garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 465–471. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.C.; Pan, M.H.; Su, N.W.; Lai, Y.J.; Cheng, K.C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.K.; Seo, J.H.; Lee, S.P. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J. Food Sci. Technol. 2008, 40, 443–448. [Google Scholar]
- Toledano-Medina, M.A.; Pérez-Aparicio, J.; Moreno-Rojas, R.; Merinas-Amo, T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016, 199, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2015, 96, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jung, E.Y.; Kang, D.H.; Chang, U.J.; Hong, Y.H.; Suh, H.J. Physical stability, antioxidative properties, and photoprotective effects of a functionalized formulation containing black garlic extract. J. Photochem. Photobiol. B Biol. 2012, 117, 104–110. [Google Scholar] [CrossRef] [PubMed]
- García-Villalón, A.L.; Amor, S.; Monge, L. In vitro studies of an aged black garlic extract enriched in S-allylcysteine and polyphenols with cardioprotective effects. J. Funct. Foods 2016, 27, 189–200. [Google Scholar] [CrossRef]
- Chang, T.C.; Jang, H.D.; Lin, W.D. Biochemical properties of black garlic aged under different temperatures of commercial rice wine extracts in Taiwan. J. Food Meas. Charact. 2021, 15, 509–518. [Google Scholar] [CrossRef]
- Chang, T.C.; Jang, H.D.; Lin, W.D.; Duan, P.F. Antioxidant and antimicrobial activities of commercial rice wine extracts of Taiwanese Allium fistulosum. Food Chem. 2016, 190, 724–729. [Google Scholar] [CrossRef]
- Kujala, T.S.; Loponen, J.M.; Klika, K.D.; Pihlaja, K. Phenolics and betacyanins in red beetroot (Beta vulgaris) root: Distribution and effect of cold storage on the content of total phenolics and three individual compounds. J. Agric. Food Chem. 2000, 48, 5338–5342. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Chang, H.T.; Chang, S.T.; Lin, S.F.; Chang, Y.H.; Jang, H.D. A comparative study on the total antioxidant and antimicrobial potentials of ethanolic extracts from various organ tissues of Allium spp. Food Nutr. Sci. 2013, 4, 182–190. [Google Scholar]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free-radical scavenging action of medicinal herbs from Ghana Thonningia Sanguinea on experimentally-induced liver injuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; RiceEvans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Kumagai, H.; Seki, T.; Ariga, T. Biological and chemical stability of garlic-derived allicin. J. Agric. Food Chem. 2008, 56, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Wei, F.; Lu, Y.; Kodani, Y.; Nakada, M.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of compositional changes of black garlic during thermal processing. J. Agric. Food Chem. 2015, 63, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, K.H.; Yook, H.S. Analysis of active components of giant black garlic. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1672–1681. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.H. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Gweon, O.C.; Seo, Y.J. Antioxidant effect of garlic and aged black garlic in animal model of Type 2 diabetes mellitus. Nutr. Res. Pract. 2009, 3, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Kohno, M.; Hamano, H.; Niwano, Y. Increased antioxidative potency of garlic by spontaneous short-term fermentation. Plant. Foods Hum. Nutr. 2006, 61, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Cavallito, C.J.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Delaha, E.C.; Garagusi, V.F. Inhibition of mycobacterial by garlic extract (Allium sativum). Antimicrob. Agents Chemother. 1985, 27, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Marta, C.M.; Nieves, C.; Mar, V. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar]
- Tsao, S.M.; Yin, M.C. In-vitro antimicrobial activity of four diallylsulphides occurring naturally in garlic and Chinese Leek Oils. J. Med. Microbiol. 2001, 50, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.M.; Yin, M.C. In vitro activity of garlic oil and four diallyl sulphides against antibiotic-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae. J. Antimicrob. Chemother. 2001, 47, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Chang, H.C.; Tsao, S.M. Inhibitory effects of aqueous garlic extract, garlic oil and four diallyl sulphides against four enteric pathogens. J. Food Drug Anal. 2002, 10, 120–125. [Google Scholar] [CrossRef]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M. Ribosomally synthesized peptides with antimicrobial properties: Biosynthesis, structure, function, and applications. Biotechnol. Adv. 2003, 21, 465–499. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).