Impact of a Novel Nano-Protectant on the Viability of Probiotic Bacterium Lactobacillus casei K17
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterium and Culture Conditions
2.2. Preparation of Pickering Emulsions
2.3. Preparation of Compound Protectants
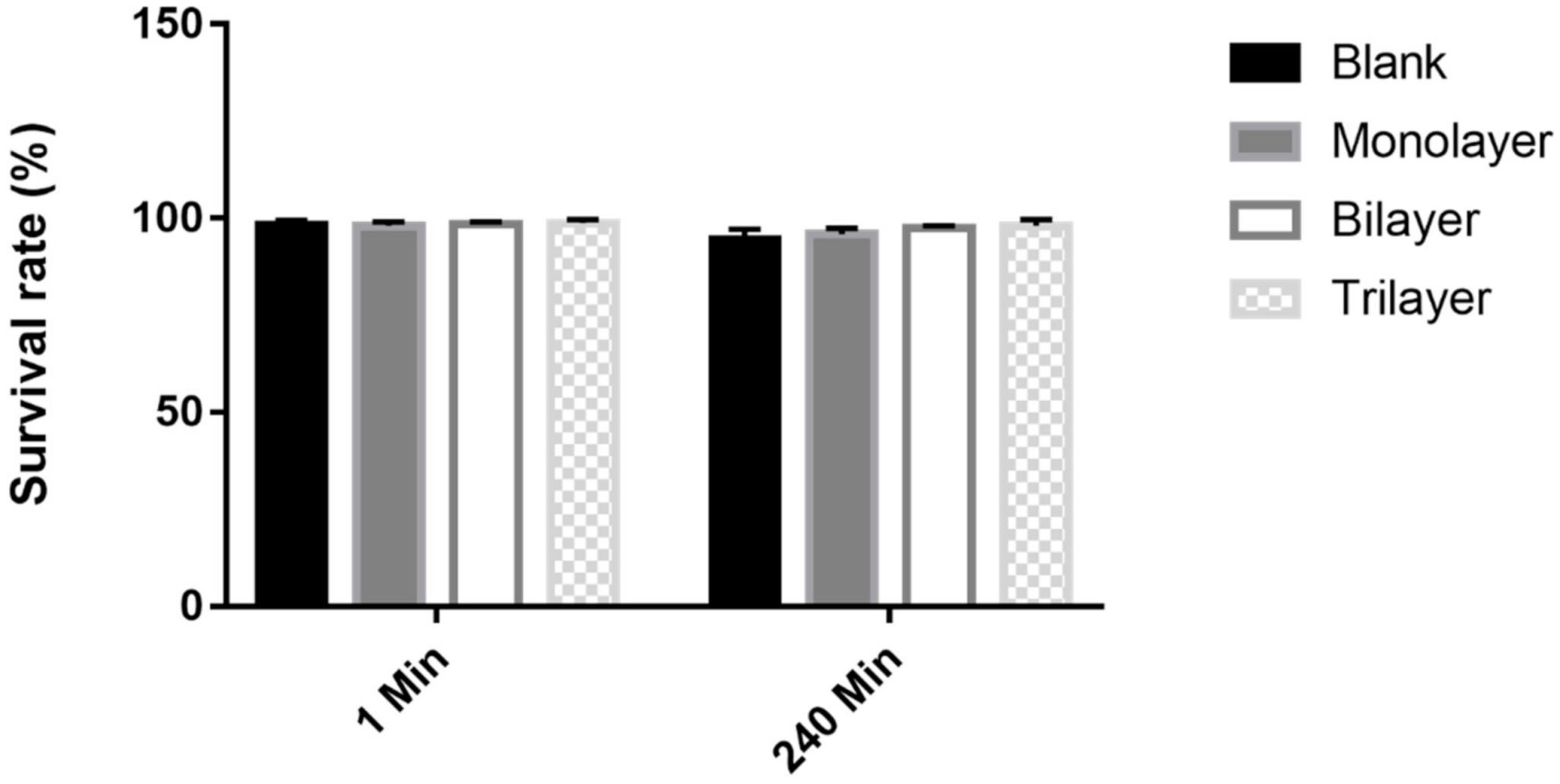
2.4. Bacterial Viability under Protectants during Feed Storage
2.5. Survival of Bacteria under Protectants during Freeze-Drying
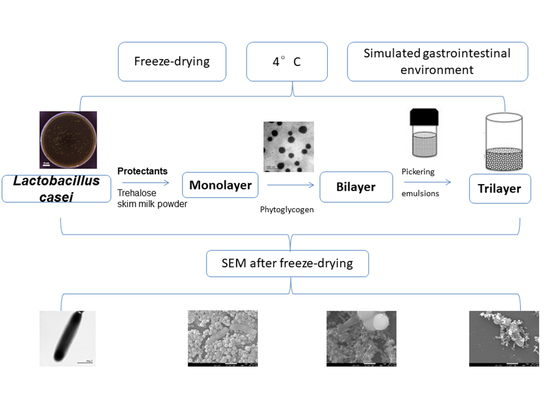
2.6. Microstructure of Lyophilized Bacterial Powder
2.7. Survival of Bacteria under Protectants in the Simulated Gastrointestinal Environment
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Colony Detection on Feed
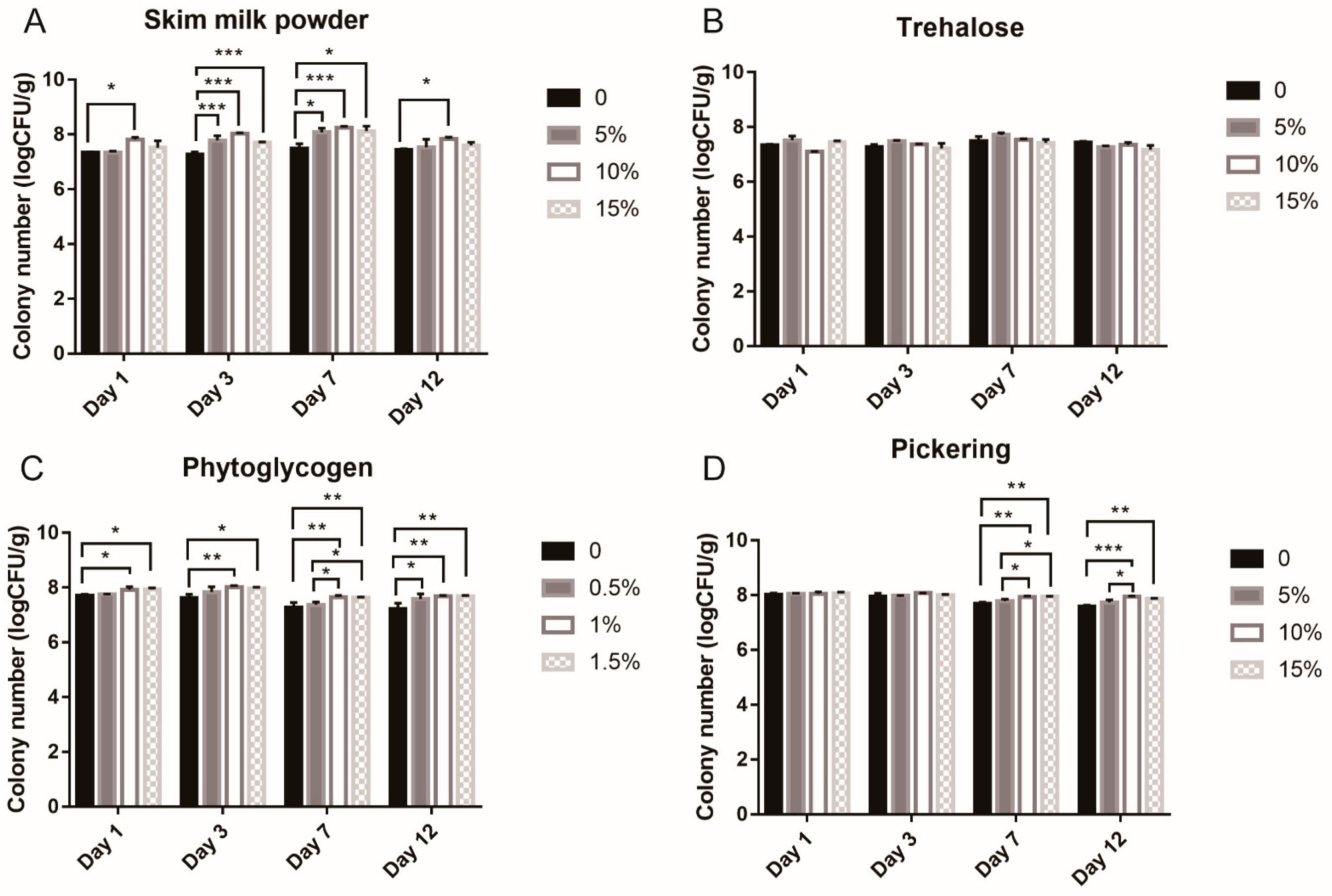
3.2. Optimization of Protectant Component on Feed
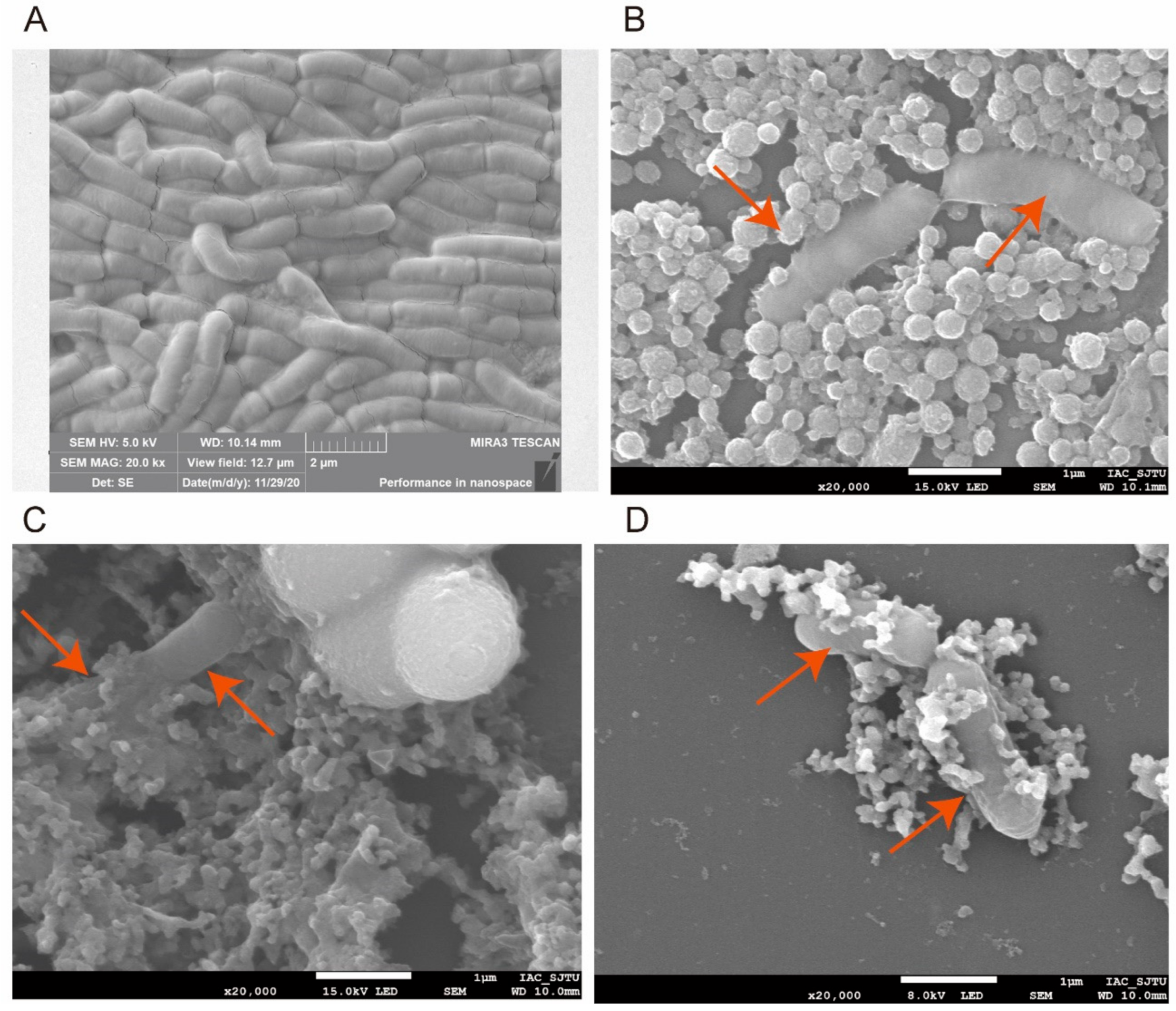
3.3. Protective Effect of Three Treatments on Feed and Evaluation of Bacterial Morphology and Shape Integrity by SEM after Freeze-Drying
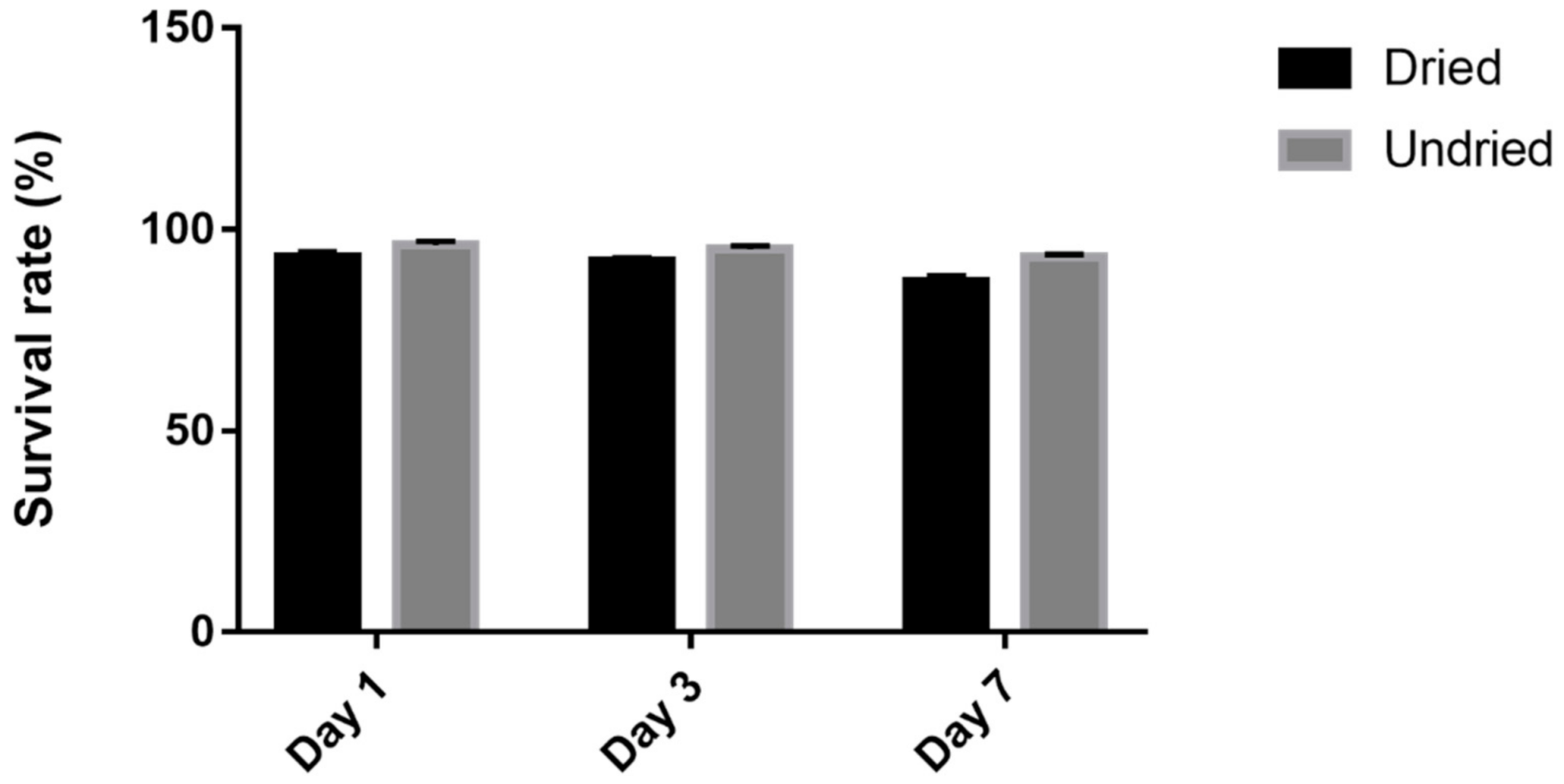
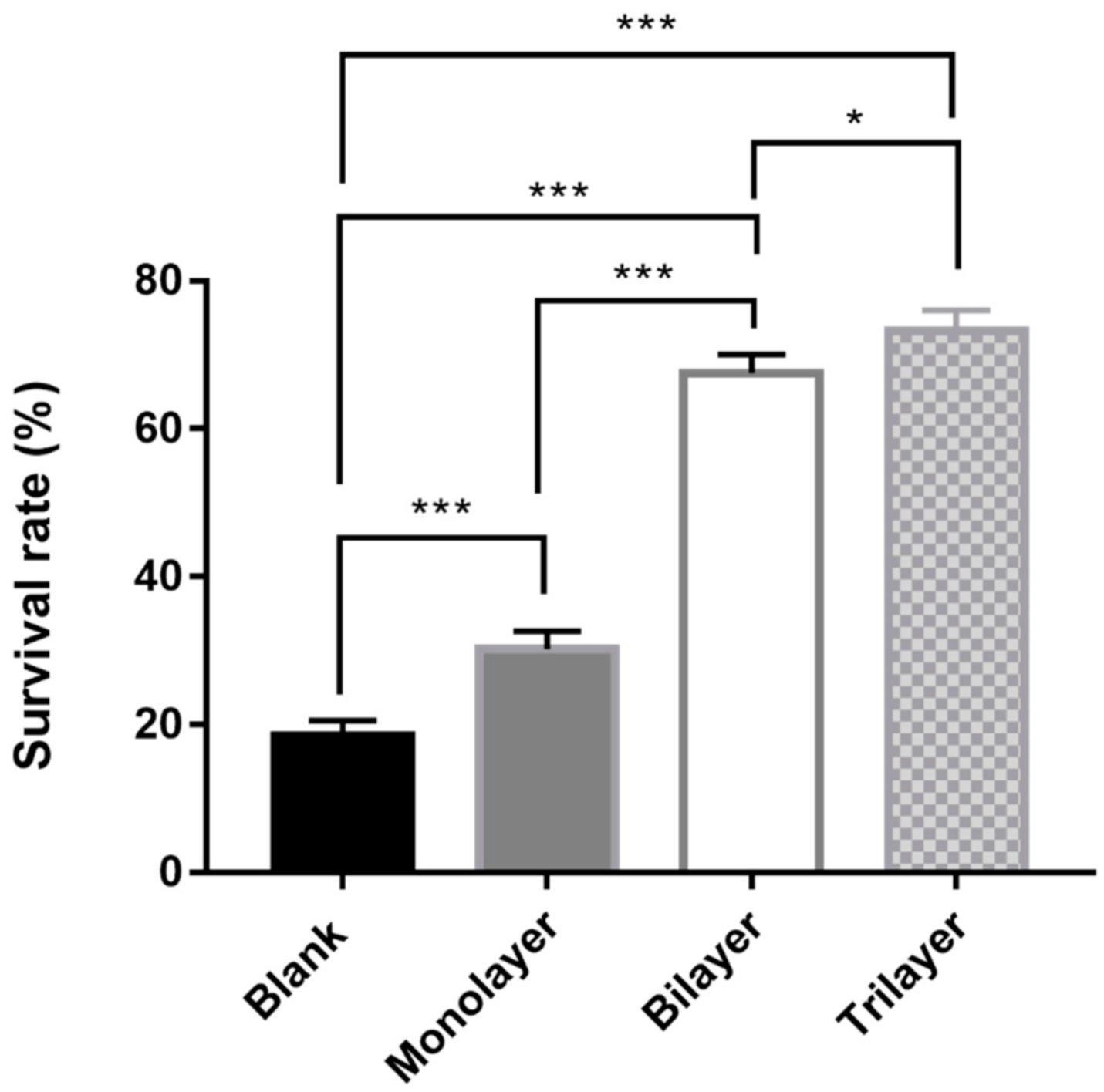
3.4. Survival of L. casei K17 under Protectants against the Freeze-Drying Process
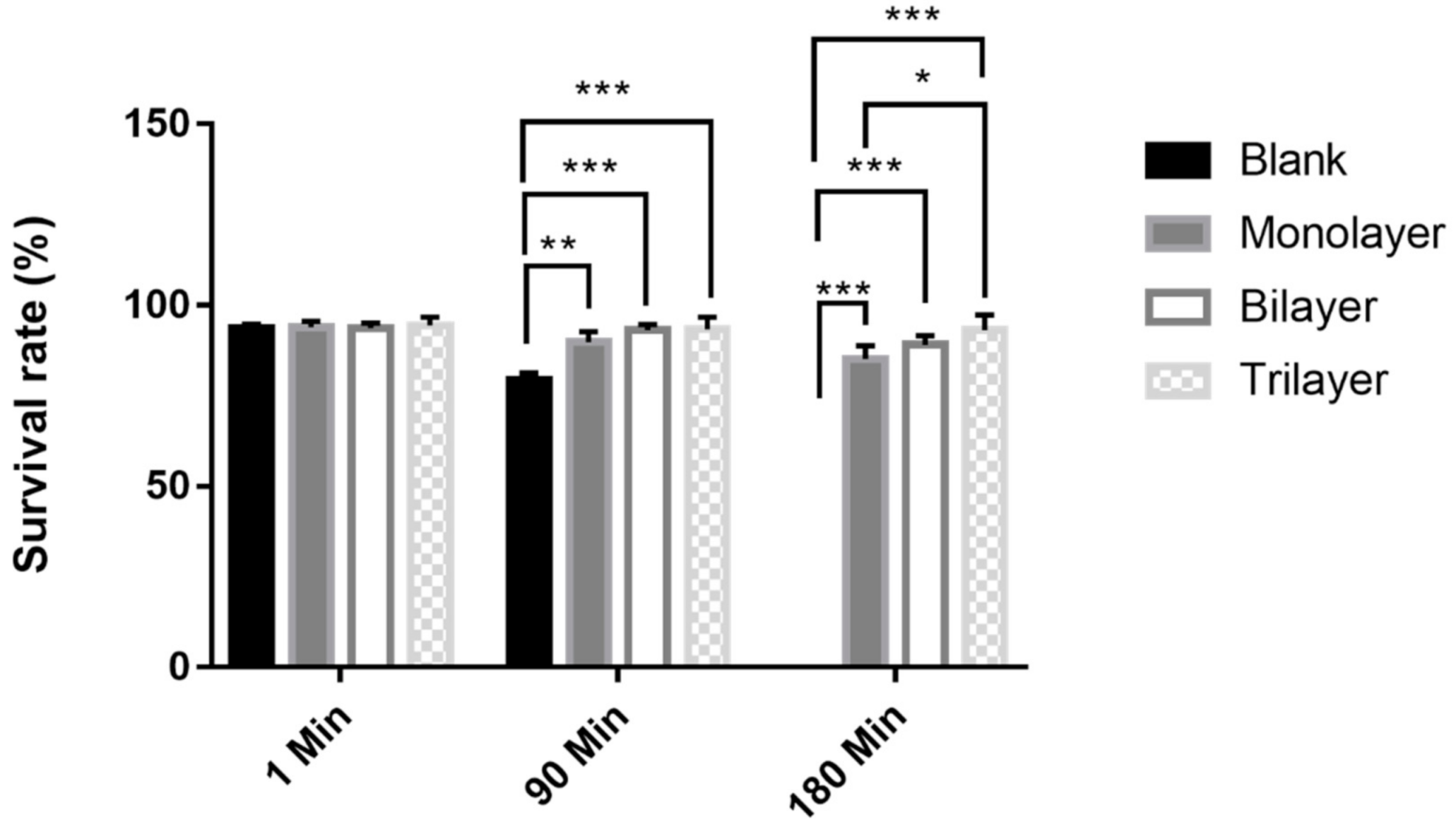
3.5. Survival of L. casei K17 under Protectants against Simulated Gastrointestinal Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuford, J.A.; Patel, R. Antimicrobial growth promoter use in livestock- implications for human health. Rev. Med. Microbiol. 2005, 16, 17–24. [Google Scholar] [CrossRef]
- Chuah, L.O.; Effarizah, M.E.; Goni, A.M.; Rusul, G. Antibiotic application and emergence of multiple antibiotic resistance (MAR) in global Catfish aquaculture. Curr. Environ. Health Rep. 2016, 3, 118–127. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X.; Qin, J.; Lu, N.; Cheng, G.; Wu, N.; Pan, Y.; Li, J.; Zhu, L.; Wang, X.; et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat Commun. 2014, 4, 2151. [Google Scholar] [CrossRef] [Green Version]
- Singer, R.S.; Roger, F.; Wegener, H.C.; Robin, B.; John, W.; Marc, L. Antibiotic resistance—The interplay between antibiotic use in animals and human beings. Lancet Infect. Dis. 2003, 3, 47–51. [Google Scholar] [CrossRef]
- Millet, S.; Maertens, L. The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet. J. 2011, 187, 143–144. [Google Scholar] [CrossRef]
- Witte, W. Selective pressure by antibiotic use in livestock. Int. J. Antimicrob. Agents. 2000, 16 (Suppl. S1), 19–24. [Google Scholar] [CrossRef]
- Mark, C.; Christian, F.; Enric, M.; Paul, M.; Ian, P. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar]
- Ren, H.; Zentek, J.; Vahjen, W. Optimization of production parameters for probiotic Lactobacillus strains as feed additive. Molecules 2019, 24, 3286. [Google Scholar] [CrossRef] [Green Version]
- Sathyabama, S.; Kumar, M.R.; Devi, P.B.; Vijayabharathi, R.; Priyadharisini, V.B. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci. Technol. 2013, 57, 419–425. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P. Efficacy of species-specific probiotic Pediococcus acidilactici FT28 on blood biochemical profile, carcass traits and physicochemical properties of meat in fattening pigs. Res. Vet. Sci. 2018, 117, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Mahnert, A.; Moissl-Eichinger, C.; Zojer, M.; Bogumil, D.; Mizrahi, I.; Rattei, T.; Martinez, J.L.; Berg, G. Man-made microbial resistances in built environments. Nat. Commun. 2019, 10, 968. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ni, X.; Qing, X.; Zeng, D.; Luo, M.; Liu, L.; Li, G.; Pan, K.; Jing, B. Live probiotic Lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers. Front. Microbiol. 2017, 8, 1073. [Google Scholar] [CrossRef] [Green Version]
- Abdou, A.M.; Hedia, R.H.; Omara, S.T.; Mahmoud, M.A.E.-F.; Kandil, M.M.; Bakry, M.A. Interspecies comparison of probiotics isolated from different animals. Vet. World 2018, 11, 227–230. [Google Scholar] [CrossRef] [Green Version]
- No. 2045 notice of the ministry of agriculture of the People’s Republic of China. China Feed. 2014, 7, 39–42.
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Gibbs, P. Effect of various growth media upon survival during storage of freeze-dried Enterococcus faecalis and Enterococcus durans. J. Appl. Microbiol. 2003, 94, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Augustynowicz, J.; Kaszycki, P.; Kus, M.; Bialecka, A.; Koloczek, H. Optimized methods for stabilization of microbial communities specializing in biodegradation of organic environmental contaminants. Brit. J. Oral Max. Surg. 2008, 17, 655–664. [Google Scholar]
- Jalali, M.; Abedi, D.; Varshosaz, J.; Najjarzadeh, M.; Mirlohi, M.; Tavakoli, N. Stability evaluation of freeze-dried Lactobacillus paracasei subsp. tolerance and Lactobacillus delbrueckii subsp. bulgaricus in oral capsules. Res. Pharm. Sci. 2012, 7, 31–36. [Google Scholar] [PubMed]
- Wróblewski, R.; Wróblewski, J. Freeze drying and freeze substitution combined with low temperature-embedding. Histochem Cell Biol. 1984, 81, 469–475. [Google Scholar]
- Saarela, M.; Virkajrvi, I.; Alakomi, H.-L.; Sigvart-Mattila, P.; Mättö, J. Stability and functionality of freeze-dried probiotic Bifidobacterium cells during storage in juice and milk. Int. Dairy J. 2006, 16, 1477–1482. [Google Scholar] [CrossRef]
- Schoug, A.; Olsson, J.; Carlfors, J.; Schnürer, J.; Hakansson, S. Freeze-drying of Lactobacillus coryniformis Si3—effects of sucrose concentration, cell density, and freezing rate on cell survival and thermophysical properties. Cryobiology 2006, 53, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Polo, L.; Mañes-Lázaro, R.; Olmeda, I.; Cruz-Pio, L.E.; Medina, Á.; Ferrer, S.; Pardo, I. Influence of freezing temperatures prior to freeze-drying on viability of yeasts and lactic acid bacteria isolated from wine. J. Appl. Microbiol. 2017, 122, 1603–1614. [Google Scholar] [CrossRef] [Green Version]
- Siaterlis, A.; Deepika, G.; Charalampopoulos, D. Effect of culture medium and cryoprotectants on the growth and survival of probiotic lactobacilli during freeze drying. Lett. Appl. Microbiol. 2009, 48, 295–301. [Google Scholar] [CrossRef]
- Juárez Tomás, M.S.; Bru, E.; Martos, G.; Nader-Macías, M.E. Stability of freeze-dried vaginal Lactobacillus strains in the presence of different lyoprotectors. Can. J. Microbiol. 2009, 55, 544–552. [Google Scholar] [CrossRef]
- Abadias, M.; Benabarre, A.; Teixidó, N.; Usall, J.; Vias, I. Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. Int. J. Food Microbiol. 2001, 65, 173–182. [Google Scholar] [CrossRef]
- Bechtle, R.M.; Claydon, T.J. Glucose-residue polymers as protectants against heat denaturation of whey proteins. J. Dairy Sci. 1971, 54, 1410–1416. [Google Scholar] [CrossRef]
- Lynch, A.L.; Dury, B.A.P.; Guyader, C.P.E.; Slater, N.K.H. Sugars comparable to glutathione as hemoglobin oxidation protectants during vacuum drying. Biopreserv. Biobank. 2011, 9, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Pu, T.; Wang, X.; Liu, B.; Wang, Y.; Feng, J.; Zhang, X. Optimization of a protective medium for enhancing the viability of freeze-dried Bacillus amyloliquefaciens B1408 based on response surface methodology. Cryobiology 2018, 81, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.C.; Stanton, C.; Fitzgerald, G.F.; Daly, C.; Ross, R.P. Anhydrobiotics: The challenges of drying probiotic cultures. Food Chem. 2008, 106, 1406–1416. [Google Scholar] [CrossRef]
- WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Minelli, E.B.; Benini, A.; Marzotto, M.; Sbarbati, A.; Ruzzenente, O.; Ferrario, R.; Hendriks, H.; Dellaglio, F. Assessment of novel probiotic Lactobacillus casei strains for the production of functional dairy foods. Int. Dairy J. 2004, 14, 723–736. [Google Scholar] [CrossRef]
- Chen, K.N.; Chen, M.J.; Lin, C.W. Optimal combination of the encapsulating materials for probiotic microcapsules and its experimental verification (R1). J. Food Eng. 2006, 76, 313–320. [Google Scholar] [CrossRef]
- Gisela, G.; Leonardo, A.E.; Lucia, P.; Rodrigo, V.; Eduard, G.; Angeles, C.M. Enhacement of the viability of during the preservation and storage process based on the response surface methodology. Food Sci. Nutr. 2014, 5, 1746–1755. [Google Scholar]
- Kapka-Skrzypczak, L.; Niedźwiecka, J.; Wojtyła, A.; Kruszewski, M. Probiotics and prebiotics as a bioactive component of functional food. Pediatr. Endocrinol. Diabetes Metab. 2012, 18, 79–83. [Google Scholar] [PubMed]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Xu, S.; Liu, T.; Radji, C.A.; Yang, J.; Chen, L. Isolation, identification, and evaluation of new lactic acid bacteria strains with both cellular antioxidant and bile salt hydrolase activities in Vitro. J. Food Prot. 2016, 79, 1919–1928. [Google Scholar] [CrossRef]
- Brugger, S.D.; Baumberger, C.; Jost, M.; Jenni, W.; Brugger, U.; Mühlemann, K. Automated counting of bacterial colony forming units on agar plates. PLoS ONE 2012, 7, e33695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch. Food Chem. 2014, 151, 154–160. [Google Scholar] [CrossRef]
- Fan, Y.; Ming, M.; Cui, S.W.; Bo, J.; Jin, Z.; Li, X. Characterisations of oil-in-water Pickering emulsion stabilized hydrophobic phytoglycogen nanoparticles. Food Hydrocoll. 2018, 76, 78–87. [Google Scholar]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage in the presence of protectants. Biotechnol. Lett. 2002, 24, 1587–1591. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Heifer, V.E.; Velho, R.V.; Lopes, F.C.; Brandelli, A. Probiotic potential of Lactobacillus spp. isolated from Brazilian regional ovine cheese. J Dairy Res. 2012, 79, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Wang, A.; Lin, H. Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 72, 399–409. [Google Scholar]
- Chan, E.S.; Wong, S.L.; Lee, P.P.; Lee, J.S.; Ti, T.B.; Zhang, Z.; Poncelet, D.; Ravindra, P.; Phan, S.H.; Yim, Z.H. Effects of starch filler on the physical properties of lyophilized calcium–alginate beads and the viability of encapsulated cells. Carbohyd. Polym. 2011, 83, 225–232. [Google Scholar] [CrossRef]
- Buitink, J.; Van Den Dries, I.J.; Hoekstra, F.A.; Alberda, M.; Hemminga, M.A. High critical temperature above T(g) may contribute to the stability of biological systems. Biophys. J. 2000, 79, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Mensink, M.A.; Frijlink, H.W.; Van Der Voort Maarschalk, K.; Hinrichs, W.L. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef]
- Herdeiro, R.S.; Pereira, M.D.; Panek, A.D.; Eleutherio, E.C. Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim. Biophys. Acta 2006, 1760, 340–346. [Google Scholar] [CrossRef]
- Tao, T.; Ding, Z.; Hou, D.; Prakash, S.; Zhao, Y.; Fan, Z.; Zhang, D.; Wang, Z.; Liu, M.; Han, J. Influence of polysaccharide as co-encapsulant on powder characteristics, survival and viability of microencapsulated Lactobacillus paracasei Lpc-37 by spray drying. J. Food Eng. 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.; Huang, Q. Kafirin nanoparticle-stabilized Pickering emulsions as oral delivery vehicles: Physicochemical stability and in vitro digestion profile. J. Agric. Food Chem. 2015, 63, 10263–10270. [Google Scholar] [CrossRef]
- Kargar, M.; Fayazmanesh, K.; Alavi, M.; Spyropoulos, F.; Norton, I.T. Investigation into the potential ability of Pickering emulsions (food-grade particles) to enhance the oxidative stability of oil-in-water emulsions. J. Colloid Interf. Sci. 2012, 366, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bellali, S.; Bou Khalil, J.; Fontanini, A.; Raoult, D.; Lagier, J.C. A new protectant medium preserving bacterial viability after freeze drying. Microbiol. Res. 2020, 236, 126454. [Google Scholar] [CrossRef] [PubMed]
- Selmer-Olsen, E.; Birkeland, S.; Sorhaug, T. Effect of protective solutes on leakage from and survival of immobilized lactobacillus subjected to drying, storage and rehydration. J. Appl. Microbiol. 1999, 87, 429–437. [Google Scholar] [CrossRef]
- Liao, L.K.; Wei, X.Y.; Gong, X.; Li, J.H.; Huang, T.; Xiong, T. Microencapsulation of Lactobacillus casei LK-1 by spray drying related to its stability and in vitro digestion. LWT-Food Sci. Technol. 2017, 82, 82–89. [Google Scholar] [CrossRef]
- Gul, O. Microencapsulation of Lactobacillus casei Shirota by spray drying using different combinations of wall materials and application for probiotic dairy dessert. J. Food Process. Preserv. 2017, 41, e13198. [Google Scholar] [CrossRef]
- Verruck, S.; De Carvalho, M.W.; De Liz, G.R.; Amante, E.R.; Vieira, C.R.W.; Amboni, R.D.D.M.C.; Prudencio, E.S. Survival of Bifidobacterium BB-12 microencapsulated with full-fat goat’s milk and prebiotics when exposed to simulated gastrointestinal conditions and thermal treatments. Small Rumin. Res. 2017, 153, 48–56. [Google Scholar] [CrossRef]
- Samedi, L.; Charles, A.L. Viability of 4 probiotic bacteria microencapsulated with arrowroot starch in the simulated gastrointestinal tract (GIT) and yoghurt. Foods 2019, 8, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Treatment Group | Protectants Formula of L. casei K17 |
|---|---|
| Blank | 100% sterile normal saline + 109 CFU/mL L. casei K17 |
| Monolayer | 10% sterile skim milk powder or trehalose + 90% sterile normal saline + 109 CFU/mL L. casei K17 |
| Bilayer | 10% sterile skim milk powder + 1% sterile water-soluble phytoglycogen nanoparticles + 89% sterile normal saline + 109 CFU/mL L. casei K17 |
| Trilayer | 10% sterile Pickering emulsions + 10% skim milk powder + 1% sterile water-soluble phytoglycogen nanoparticles + 79% sterile normal saline + 109 CFU/mL L. casei K17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Chen, L. Impact of a Novel Nano-Protectant on the Viability of Probiotic Bacterium Lactobacillus casei K17. Foods 2021, 10, 529. https://doi.org/10.3390/foods10030529
Wang J, Chen L. Impact of a Novel Nano-Protectant on the Viability of Probiotic Bacterium Lactobacillus casei K17. Foods. 2021; 10(3):529. https://doi.org/10.3390/foods10030529
Chicago/Turabian StyleWang, Jinsong, and Lanming Chen. 2021. "Impact of a Novel Nano-Protectant on the Viability of Probiotic Bacterium Lactobacillus casei K17" Foods 10, no. 3: 529. https://doi.org/10.3390/foods10030529