Hypothiocyanite and Hypothiocyanite/Lactoferrin Mixture Exhibit Virucidal Activity In Vitro against SARS-CoV-2
Abstract
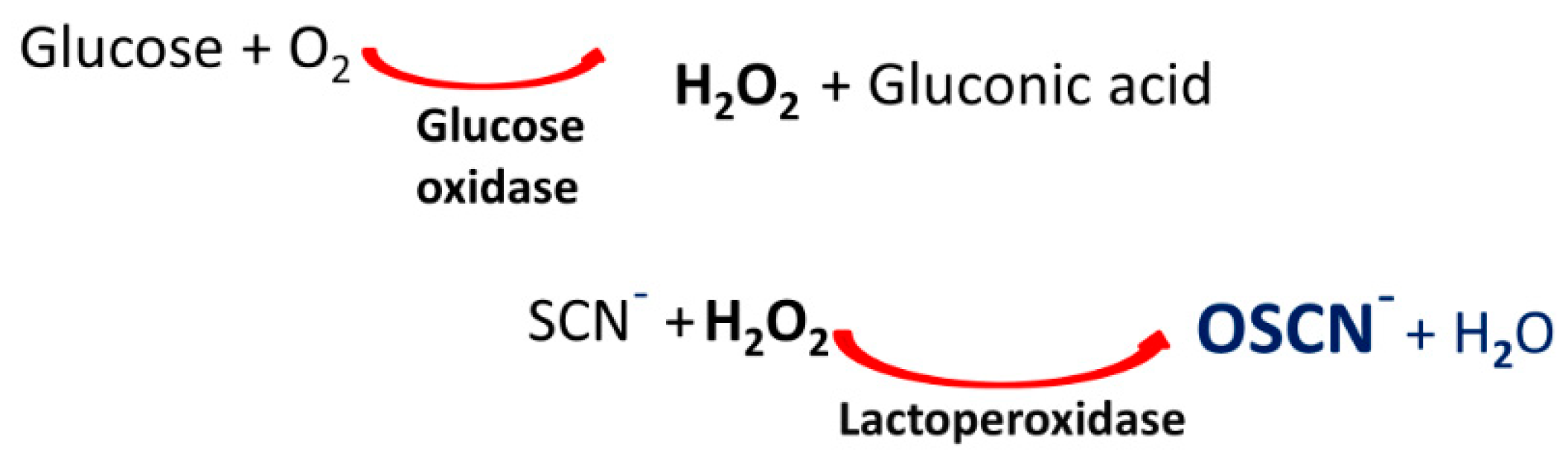
1. Introduction
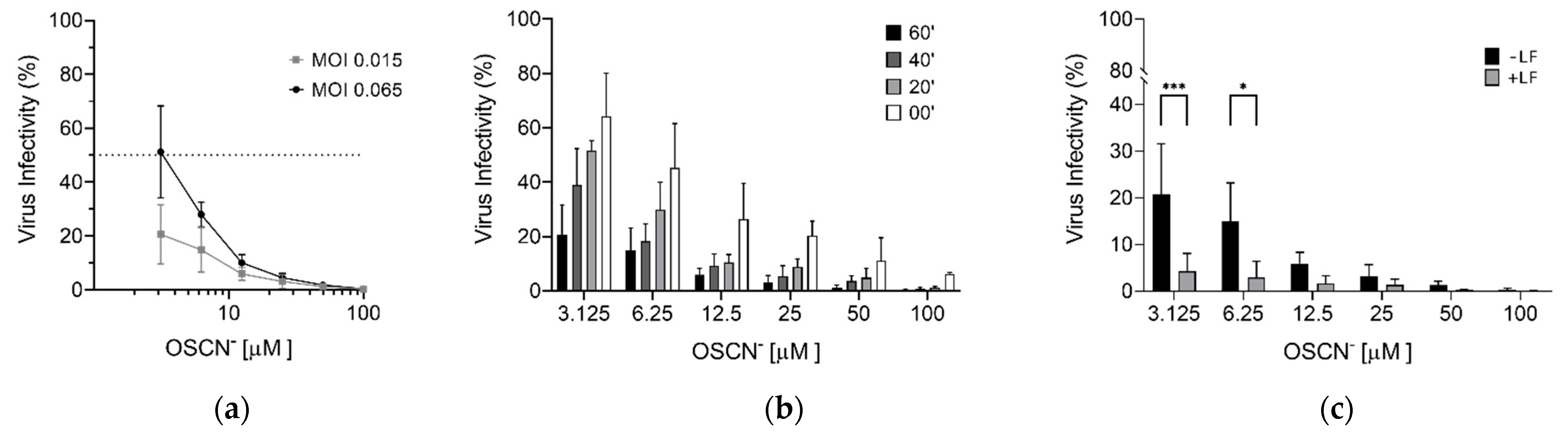
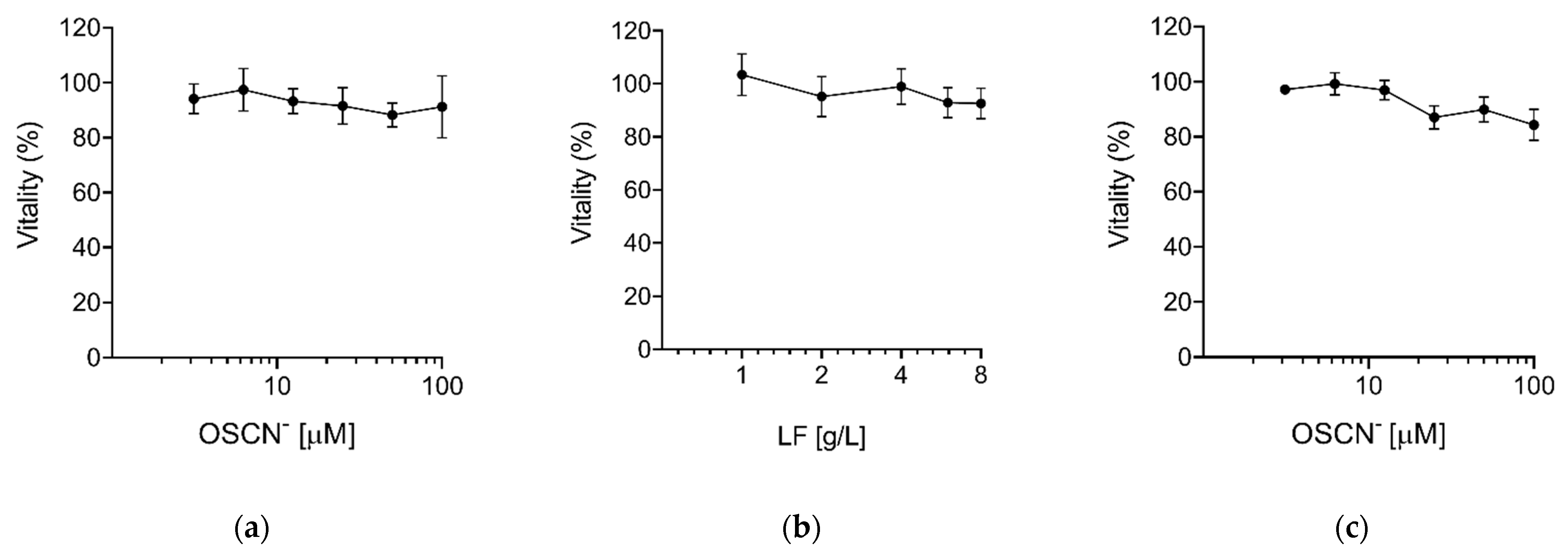
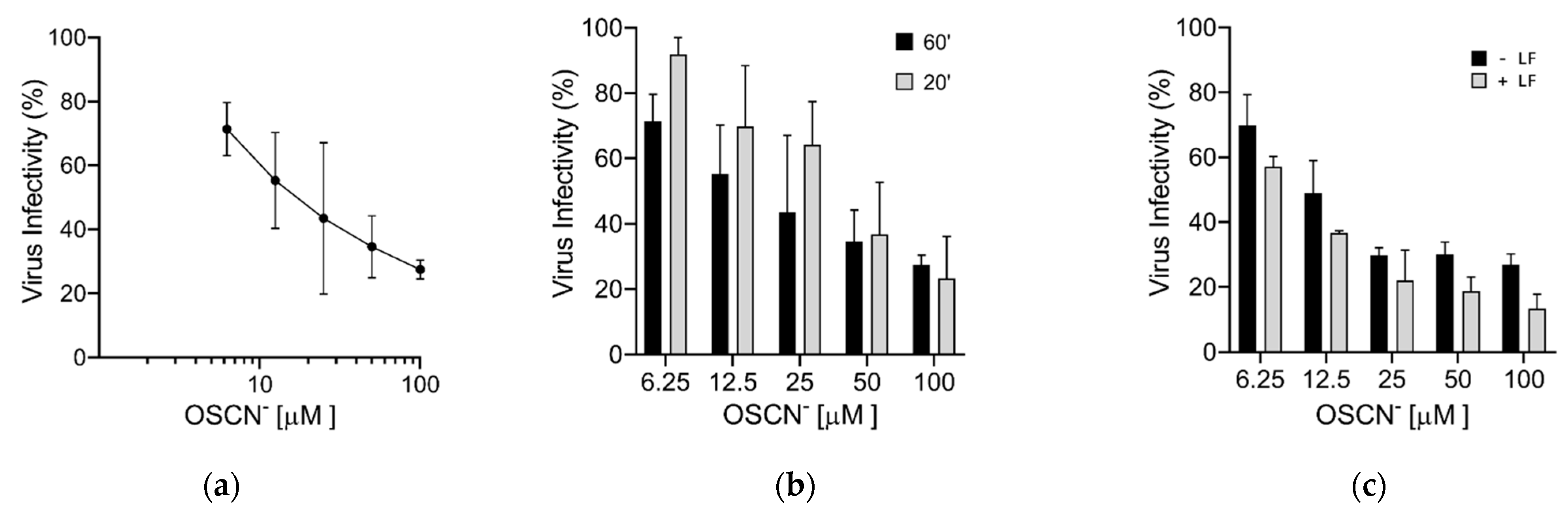
2. Results
Virucidal Activity of OSCN− and OSCN−/LF
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Viruses
4.2. Virus Stock Preparation and Titration
4.3. Compounds Preparation and Cytotoxicity Assay
4.4. Virucidal Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salata, C.; Calistri, A.; Parolin, C.; Palù, G. Coronaviruses: A paradigm of new emerging zoonotic diseases. Pathog. Dis. 2019, 77. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Liu, Z. Expression of ACE2 in airways: Implication for COVID-19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin. Exp. Allergy 2020, 50, 1313–1324. [Google Scholar] [CrossRef]
- Mason, R.J. Thoughts on the alveolar phase of COVID-19. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L115–L120. [Google Scholar] [CrossRef]
- Prescott, H.C.; Rice, T.W. Corticosteroids in COVID-19 ARDS: Evidence and Hope During the Pandemic. JAMA 2020, 324, 1292–1295. [Google Scholar] [CrossRef]
- Kim, P.S.; Read, S.W.; Fauci, A.S. Therapy for Early COVID-19: A Critical Need. JAMA 2020, 324, 2149–2150. [Google Scholar] [CrossRef]
- Cegolon, L. Investigating hypothiocyanite against SARS-CoV-2. Int. J. Hyg. Environ. Health 2020, 227, 113520. [Google Scholar] [CrossRef]
- Cegolon, L.; Javanbakht, M.; Mastrangelo, G. Nasal disinfection for the prevention and control of COVID-19: A scoping review on potential chemo-preventive agents. Int. J. Hyg. Environ. Health 2020, 230, 113605. [Google Scholar] [CrossRef] [PubMed]
- Tunney, M.M.; Payne, J.E.; McGrath, S.J.; Einarsson, G.G.; Ingram, R.J.; Gilpin, D.F.; Juarez-Perez, V.; Elborn, J.S. Activity of hypothiocyanite and lactoferrin (ALX-009) against respiratory cystic fibrosis pathogens in sputum. J. Antimicrob. Chemother. 2018, 73, 3391–3397. [Google Scholar] [CrossRef]
- Moreau-Marquis, S.; Coutermarsh, B.; Stanton, B.A. Combination of hypothiocyanite and lactoferrin (ALX-109) enhances the ability of tobramycin and aztreonam to eliminate Pseudomonas aeruginosa biofilms growing on cystic fibrosis airway epithelial cells. J. Antimicrob. Chemother. 2015, 70, 160–166. [Google Scholar] [CrossRef]
- Salata, C.; Calistri, A.; Alvisi, G.; Celestino, M.; Parolin, C.; Palù, G. Ebola Virus Entry: From Molecular Characterization to Drug Discovery. Viruses 2019, 11, 274. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef]
- Whitt, M.A. Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods 2010, 169, 365–374. [Google Scholar] [CrossRef]
- Conner, G.E.; Salathe, M.; Forteza, R. Lactoperoxidase and Hydrogen Peroxide Metabolism in the Airway. Am. J. Respir. Crit. Care Med. 2002, 166, S57–S61. [Google Scholar] [CrossRef] [PubMed]
- Gerson, C.; Sabater, J.; Scuri, M.; Torbati, A.; Coffey, R.; Abraham, J.W.; Lauredo, I.; Forteza, R.; Wanner, A.; Salathe, M.; et al. The Lactoperoxidase System Functions in Bacterial Clearance of Airways. Am. J. Respir. Cell Mol. Biol. 2000, 22, 665–671. [Google Scholar] [CrossRef]
- Jay, R.R.; Howard, R. A Plausible “Penny” Costing Effective Treatment for Corona Virus—Ozone Therapy. J. Infect. Dis. Epidemiol. 2020, 6. [Google Scholar] [CrossRef]
- Izadi, M.; Cegolon, L.; Javanbakht, M.; Sarafzadeh, A.; Abolghasemi, H.; Alishiri, G.; Zhao, S.; Einollahi, B.; Kashaki, M.; Jonaidi-Jafari, N.; et al. Ozone therapy for the treatment of COVID-19 pneumonia: A scoping review. Int. Immunopharmacol. 2021, 92, 107307. [Google Scholar] [CrossRef]
- Tizaoui, C. Ozone: A Potential Oxidant for COVID-19 Virus (SARS-CoV-2). Ozone Sci. Eng. 2020, 42, 378–385. [Google Scholar] [CrossRef]
- Gavazza, A.; Marchegiani, A.; Rossi, G.; Franzini, M.; Spaterna, A.; Mangiaterra, S.; Cerquetella, M. Ozone Therapy as a Possible Option in COVID-19 Management. Front. Public Health 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.K.; Ohmine, S.; Tomer, D.P.; Jensen, K.J.; Johnson, F.B.; Kirsi, J.J.; Robison, R.A.; O’Neill, K.L. Virion disruption by ozone-mediated reactive oxygen species. J. Virol. Methods 2008, 153, 74–77. [Google Scholar] [CrossRef]
- Ashby, M.T.; Kreth, J.; Soundarajan, M.; Sivuilu, L.S. Influence of a model human defensive peroxidase system on oral streptococcal antagonism. Microbiology 2009, 155, 3691–3700. [Google Scholar] [CrossRef]
- Tenovuo, J. Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme and lactoferrin in xerostomia: Efficacy and safety. Oral Dis. 2002, 8, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jalil, R.A. Concentrations of Thiocyanate and Hypothiocyanite in the Saliva of Young Adults. J. Nihon Univ. Sch. Dent. 1994, 36, 254–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cegolon, L.; Salata, C.; Piccoli, E.; Juarez, V.; Palu’, G.; Mastrangelo, G.; Calistri, A. In vitro antiviral activity of hypothiocyanite against A/H1N1/2009 pandemic influenza virus. Int. J. Hyg. Environ. Health 2014, 217, 17–22. [Google Scholar] [CrossRef]
- Patel, U.; Gingerich, A.; Widman, L.; Sarr, D.; Tripp, R.A.; Rada, B. Susceptibility of influenza viruses to hypothiocyanite and hypoiodite produced by lactoperoxidase in a cell-free system. PLoS ONE 2018, 13, e0199167. [Google Scholar] [CrossRef]
- Gingerich, A.; Pang, L.; Hanson, J.; Dlugolenski, D.; Streich, R.; Lafontaine, E.R.; Nagy, T.; Tripp, R.A.; Rada, B. Hypothiocyanite produced by human and rat respiratory epithelial cells inactivates extracellular H1N2 influenza A virus. Inflamm. Res. 2015, 65, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nat. Cell Biol. 2002, 417, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglio, L.G.V.; Borcherding, J.A.; Frommelt, M.; Parker, G.J.; Duchman, B.; Calderón, O.G.V.; Fernandez-Ruiz, R.; Noriega, J.E.; Stone, E.A.; Gerke, A.K.; et al. Airway surface liquid from smokers promotes bacterial growth and biofilm formation via iron-lactoferrin imbalance. Respir. Res. 2018, 19, 1–11. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Salata, C.; Baritussio, A.; Munegato, D.; Calistri, A.; Ha, H.R.; Bigler, L.; Fabris, F.; Parolin, C.; Palù, G.; Mirazimi, A. Amiodarone and metabolite MDEA inhibit Ebola virus infection by interfering with the viral entry process. Pathog. Dis. 2015, 73. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegolon, L.; Mirandola, M.; Salaris, C.; Salvati, M.V.; Mastrangelo, G.; Salata, C. Hypothiocyanite and Hypothiocyanite/Lactoferrin Mixture Exhibit Virucidal Activity In Vitro against SARS-CoV-2. Pathogens 2021, 10, 233. https://doi.org/10.3390/pathogens10020233
Cegolon L, Mirandola M, Salaris C, Salvati MV, Mastrangelo G, Salata C. Hypothiocyanite and Hypothiocyanite/Lactoferrin Mixture Exhibit Virucidal Activity In Vitro against SARS-CoV-2. Pathogens. 2021; 10(2):233. https://doi.org/10.3390/pathogens10020233
Chicago/Turabian StyleCegolon, Luca, Mattia Mirandola, Claudio Salaris, Maria Vittoria Salvati, Giuseppe Mastrangelo, and Cristiano Salata. 2021. "Hypothiocyanite and Hypothiocyanite/Lactoferrin Mixture Exhibit Virucidal Activity In Vitro against SARS-CoV-2" Pathogens 10, no. 2: 233. https://doi.org/10.3390/pathogens10020233
APA StyleCegolon, L., Mirandola, M., Salaris, C., Salvati, M. V., Mastrangelo, G., & Salata, C. (2021). Hypothiocyanite and Hypothiocyanite/Lactoferrin Mixture Exhibit Virucidal Activity In Vitro against SARS-CoV-2. Pathogens, 10(2), 233. https://doi.org/10.3390/pathogens10020233






