Improving the Phloroglucinolysis Protocol and Characterization of Sagrantino Wines Proanthocyanidins
Abstract
1. Introduction
2. Results
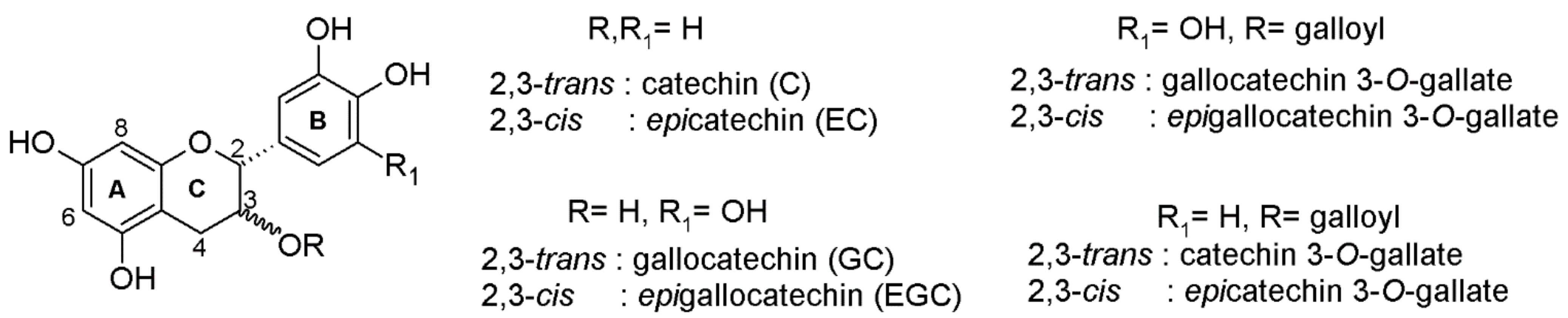
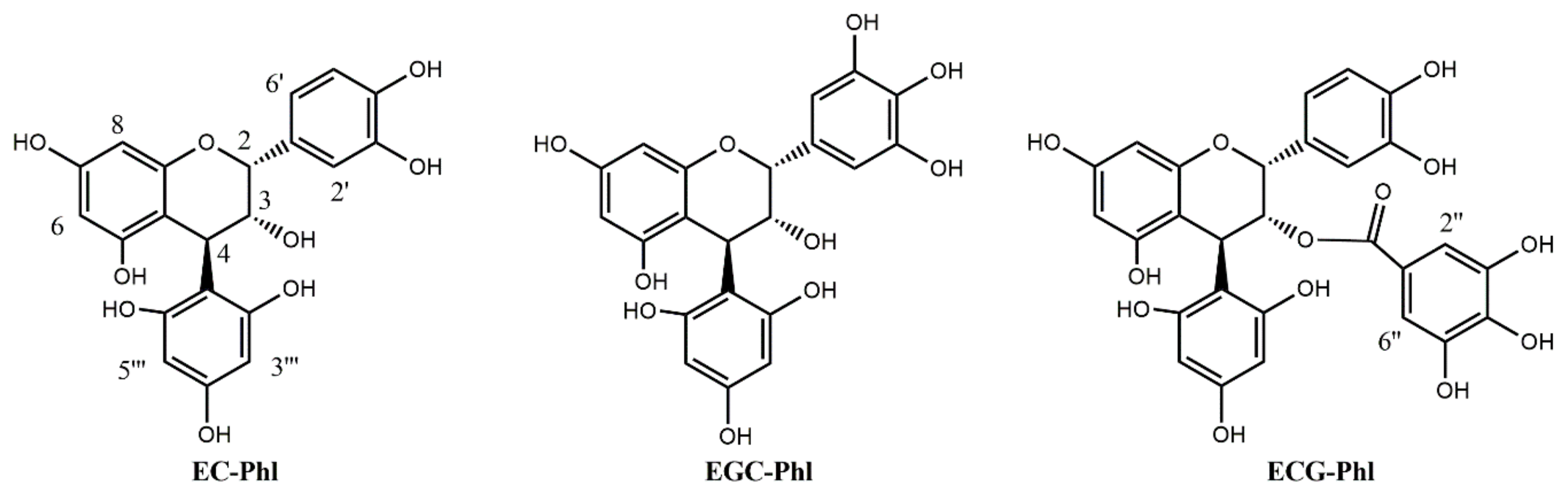
2.1. Semi-Synthesis
2.2. UHPLC-MS/MS Method
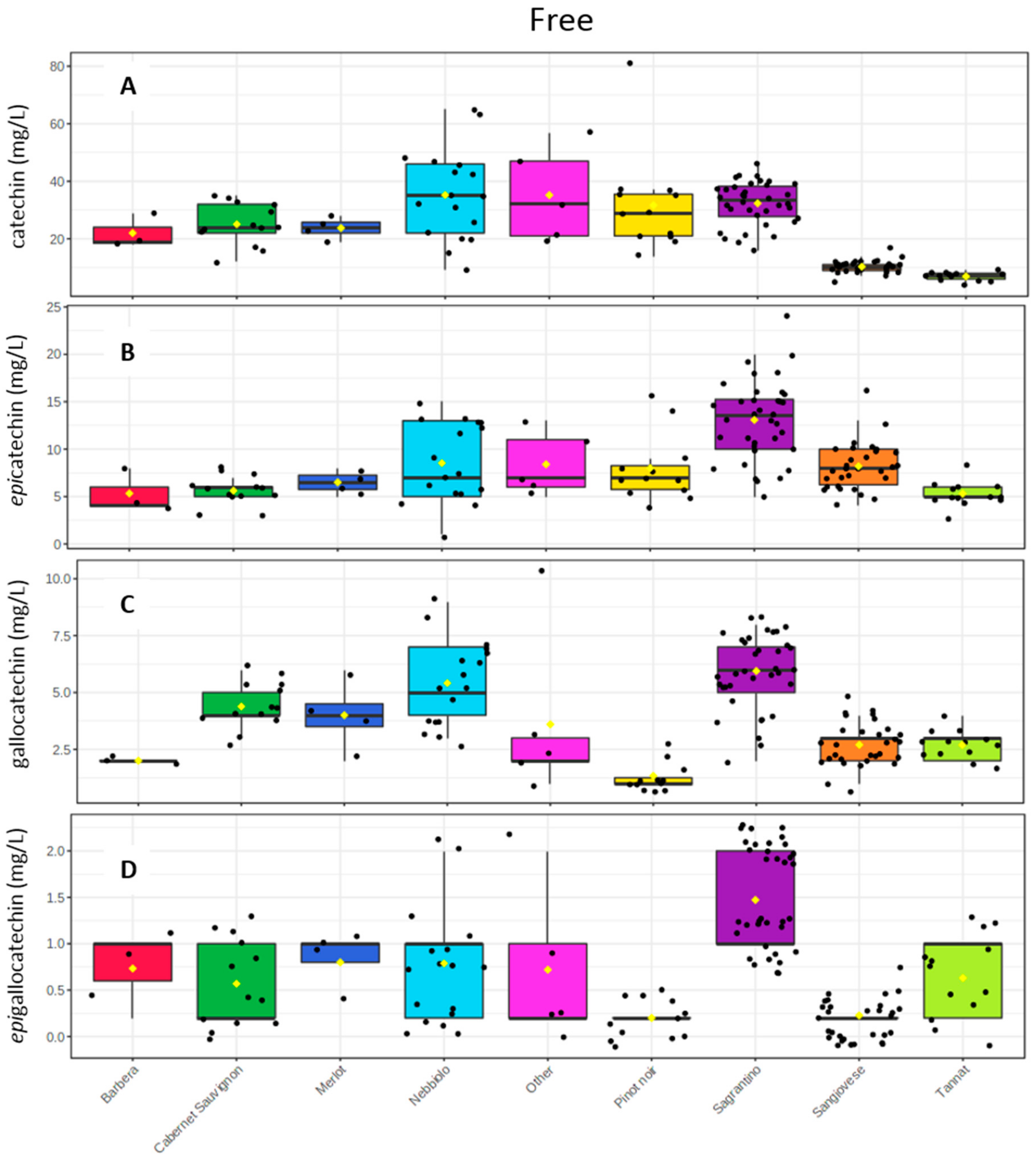
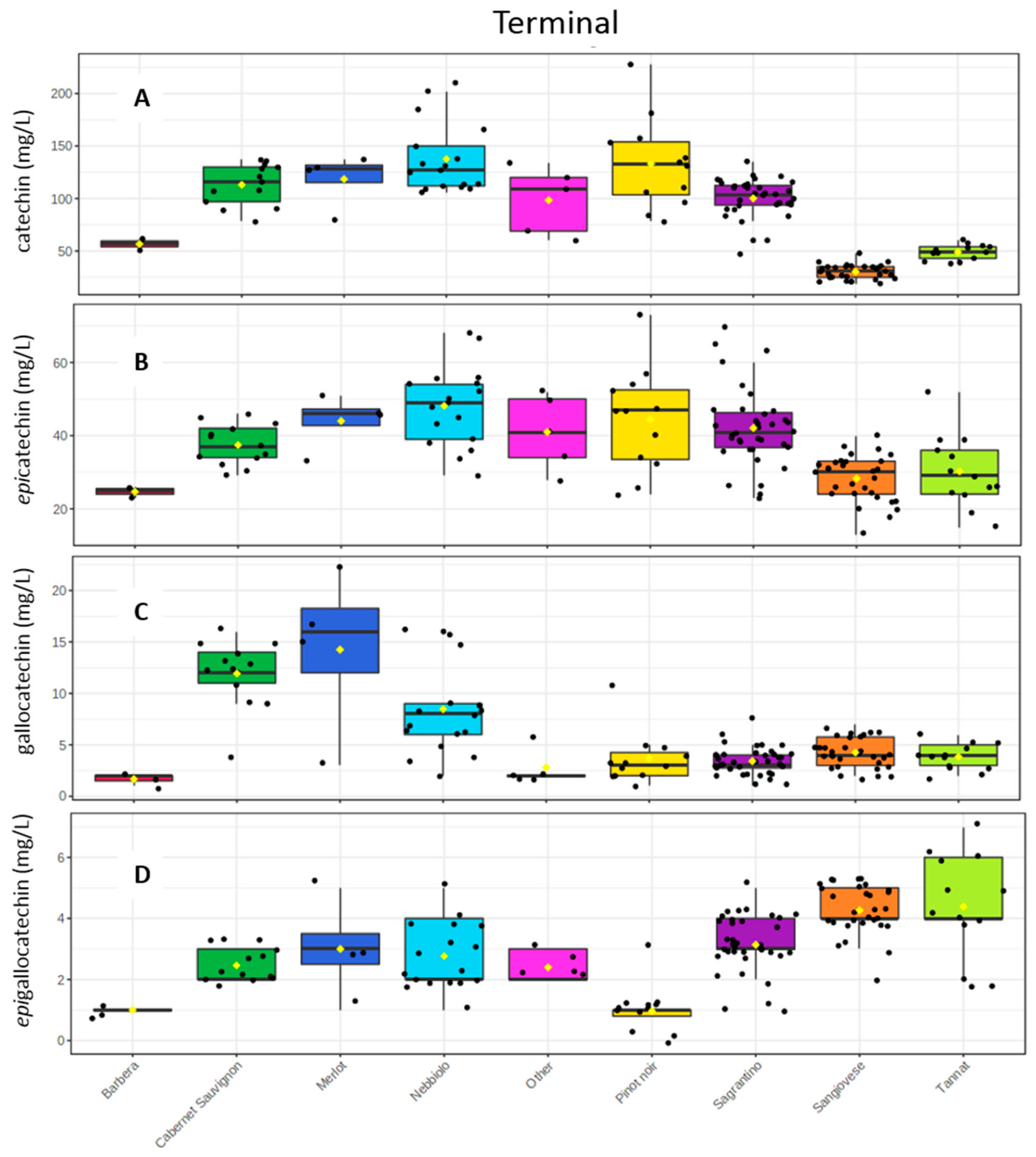
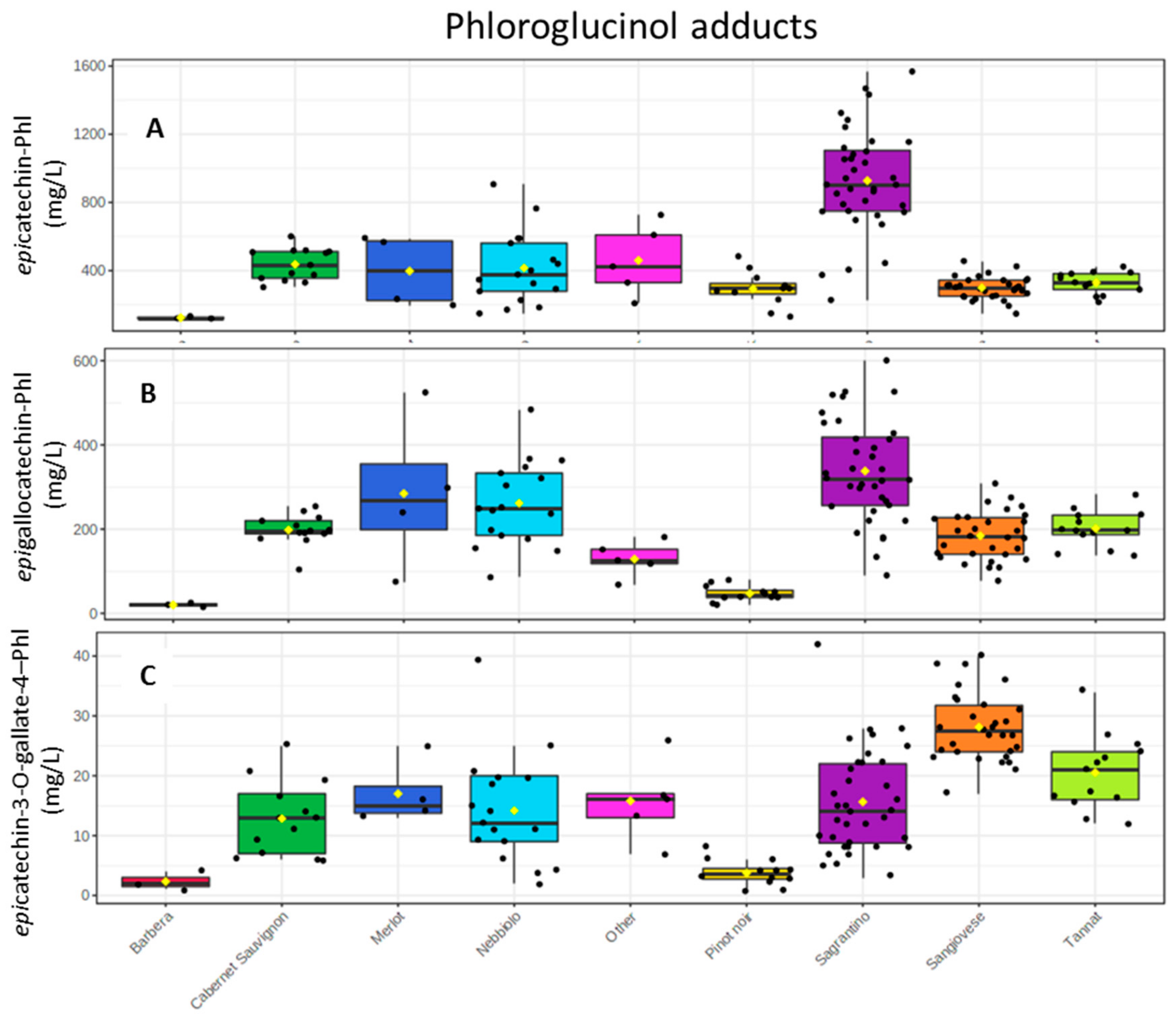
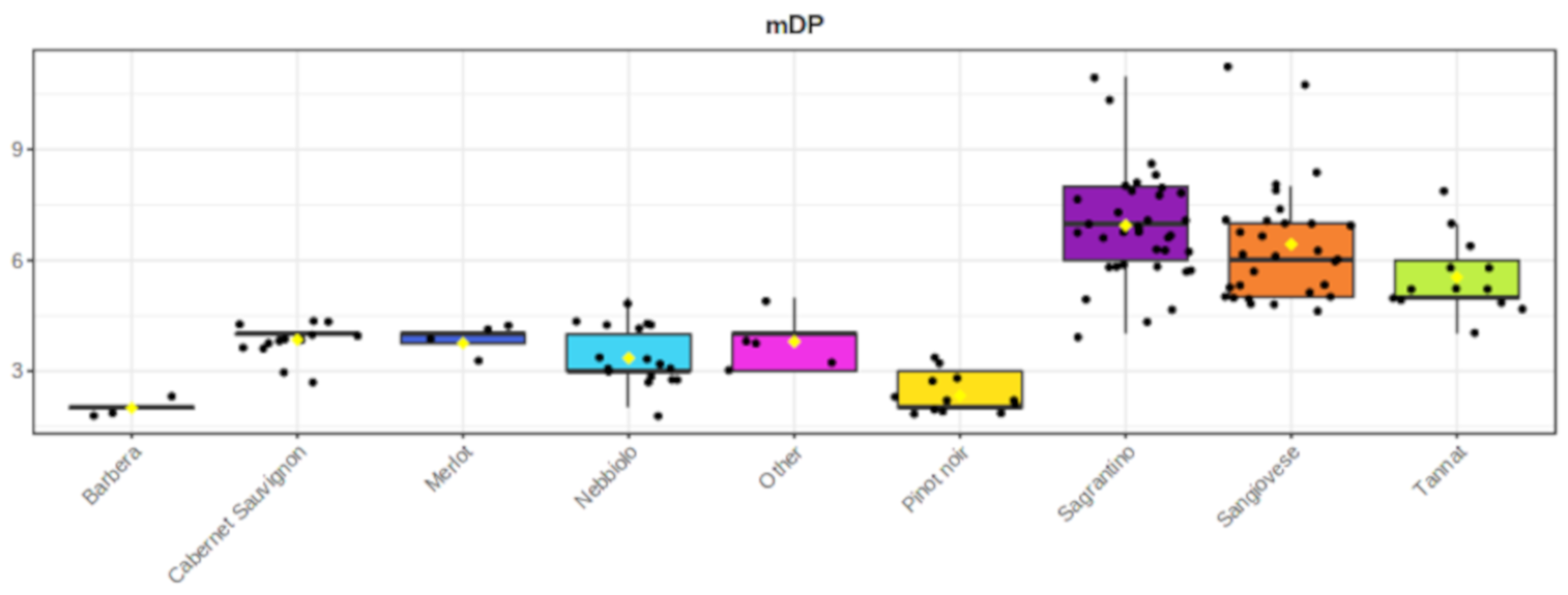
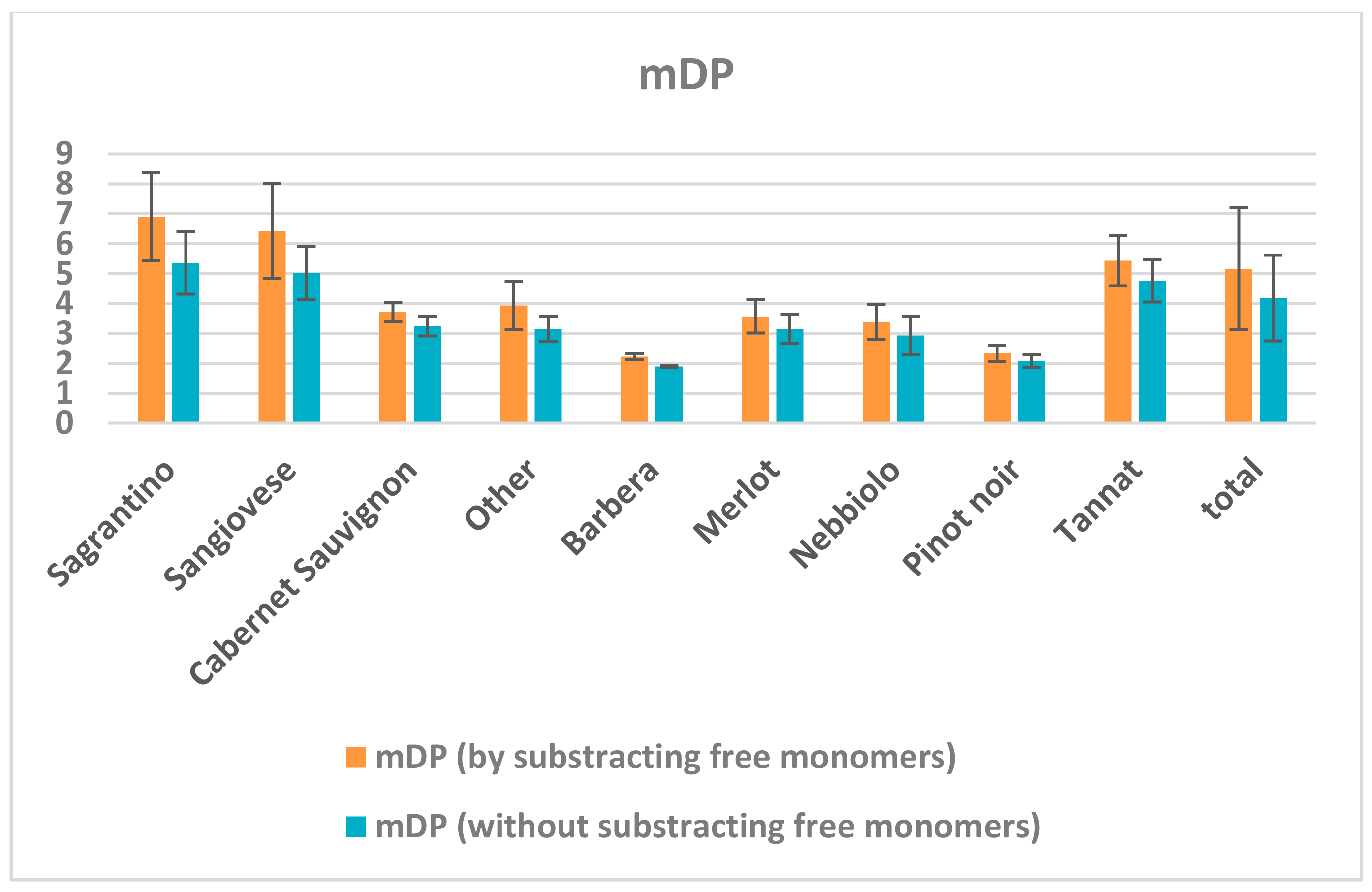
2.3. Wine Analysis
3. Discussion
- The high mDP values have an impact on its “dry” astringent character.
- The high epicatechin amounts (and monomeric flavan-3-ols in general) should be correlated with its astringent and bitter character.
- The high oligomeric procyanidins concentration (Sagrantino wines have the highest mean value with 1.2 g/L and the second rich group is Nebbiolo with 0.8 g/L. (Table S2)), which should also explain the strong tannic character.
- The low % of galloylated proanthocyanidins do not allow the decrease of the “coarse” perception.
4. Materials and Methods
4.1. Wine Samples
4.2. Chemicals
4.3. Epicatechin 4-Phloroglucinol
4.4. Epicatechin 3-O-Gallate 4-Phloroglucinol
4.5. Epigallocatechin 4-Phloroglucinol
4.6. Quantitative 1H-NMR Spectroscopy
4.7. Sample Preparation
4.8. UHPLC-MS/MS Analysis
4.9. Data Processing and Statistical Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited; Cambridge University Press: Cambridge, UK, 1989; ISBN 0521321891. [Google Scholar]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- German, J.B.; Walzem, R.L. The health benefits of wine. Annu. Rev. Nutr. 2000, 20, 561–593. [Google Scholar] [CrossRef]
- Goñi, I.; Hernández-Galiot, A. Intake of nutrient and non-nutrient dietary antioxidants. Contribution of macromolecular antioxidant polyphenols in an elderly mediterranean population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, P.; Maujean, A.; Glories, Y.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology; John Wiley: Trenton, NJ, USA, 2006; Volumes 1 and 2, ISBN 0470010371. [Google Scholar]
- Ontañón, I.; Sánchez, D.; Sáez, V.; Mattivi, F.; Ferreira, V.; Arapitsas, P. Liquid chromatography–mass spectrometry-based metabolomics for understanding the compositional changes induced by oxidative or anoxic storage of red wines. J. Agric. Food Chem. 2020, 68, 13367–13379. [Google Scholar] [CrossRef]
- Mattivi, F.; Arapitsas, P.; Perenzoni, D.; Guella, G. Influence of storage conditions on the composition of red wines. In Advances in Wine Research; American Chemical Society: Washington DC, USA, 2015; ISBN 9780841230101. [Google Scholar]
- Arapitsas, P.; Perenzoni, D.; Nicolini, G.; Mattivi, F. Study of Sangiovese wines pigment profile by UHPLC-MS/MS. J. Agric. Food Chem. 2012, 60, 10461–10471. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Speri, G.; Angeli, A.; Perenzoni, D.; Mattivi, F. The influence of storage on the “chemical age” of red wines. Metabolomics 2014, 10, 816–832. [Google Scholar] [CrossRef]
- Longo, E.; Rossetti, F.; Jouin, A.; Teissedre, P.-L.; Jourdes, M.; Boselli, E. Distribution of crown hexameric procyanidin and its tetrameric and pentameric congeners in red and white wines. Food Chem. 2019, 299, 125125. [Google Scholar] [CrossRef]
- Arapitsas, P.; Guella, G.; Mattivi, F. The impact of SO2 on wine flavanols and indoles in relation to wine style and age. Sci. Rep. 2018, 8, 858. [Google Scholar] [CrossRef]
- Arapitsas, P.; Ugliano, M.; Marangon, M.; Piombino, P.; Rolle, L.; Gerbi, V.; Versari, A.; Mattivi, F. Use of untargeted liquid chromatography–mass spectrometry metabolome to discriminate Italian Monovarietal red wines, produced in their different terroirs. J. Agric. Food Chem. 2020, 68, 13353–13366. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, F.; Guella, G.; Mattivi, F.; Catorci, D.; Arapitsas, P. Kinetic investigations of sulfite addition to flavanols. Sci. Rep. 2020, 10, 12792. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 179–193. [Google Scholar] [CrossRef]
- Moro, L.; Da Ros, A.; da Mota, R.V.; Purgatto, E.; Mattivi, F.; Arapitsas, P. LC–MS untargeted approach showed that methyl jasmonate application on Vitis labrusca L. grapes increases phenolics at subtropical Brazilian regions. Metabolomics 2020, 16, 18. [Google Scholar] [CrossRef]
- Lemut, M.S.; Trost, K.; Sivilotti, P.; Arapitsas, P.; Vrhovsek, U. Early vs. late leaf removal strategies for ‘Pinot Noir’ (Vitis vinifera L.): Effect on colour-related phenolics in young wines following alcoholic fermentation. J. Sci. Food Agric. 2013, 93, 3670–3681. [Google Scholar] [CrossRef] [PubMed]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Filho, D.W.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Phenolic profile and effect of regular consumption of Brazilian red wines on in vivo antioxidant activity. J. Food Compos. Anal. 2013, 31, 31–40. [Google Scholar] [CrossRef]
- Drinkine, J.; Lopes, P.; Kennedy, J.A.; Teissedre, P.-L.; Saucier, C. Analysis of Ethylidene-Bridged Flavan-3-ols in Wine. J. Agric. Food Chem. 2007, 55, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Seddon, T.J.; Downey, M.O. Comparison of analytical methods for the determination of condensed tannins in grape skin. Aust. J. Grape Wine Res. 2008, 14, 54–61. [Google Scholar] [CrossRef]
- Quaglieri, C.; Prieto-Perea, N.; Berrueta, L.A.; Gallo, B.; Rasines-Perea, Z.; Jourdes, M.; Teissedre, P.L. Comparison of aquitaine and Rioja red wines: Characterization of their phenolic composition and evolution from 2000 to 2013. Molecules 2017, 22, 192. [Google Scholar] [CrossRef]
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P.-L. Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem. 2011, 126, 1971–1977. [Google Scholar] [CrossRef]
- Pinasseau, L.; Verbaere, A.; Roques, M.; Meudec, E.; Vallverdú-Queralt, A.; Terrier, N.; Boulet, J.-C.; Cheynier, V.; Sommerer, N.; Pinasseau, L.; et al. A fast and robust UHPLC-MRM-MS method to characterize and quantify grape skin tannins after chemical depolymerization. Molecules 2016, 21, 1409. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust. J. Grape Wine Res. 2003, 9, 15–27. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.; Kennedy, J.A. Direct method for determining seed and skin proanthocyanidin extraction into red wine. J. Agric. Food Chem. 2003, 51, 5877–5881. [Google Scholar] [CrossRef]
- Robinson, S.P.; Pezhmanmehr, M.; Speirs, J.; McDavid, D.A.J.; Hooper, L.C.; Rinaldo, A.R.; Bogs, J.; Ebadi, A.; Walker, A.R. Grape and wine flavonoid composition in transgenic grapevines with altered expression of flavonoid hydroxylase genes. Aust. J. Grape Wine Res. 2019, 25, 293–306. [Google Scholar] [CrossRef]
- Billerach, G.; Rouméas, L.; Dubreucq, E.; Fulcrand, H. Furanolysis with Menthofuran: A new depolymerization method for analyzing condensed tannins. J. Agric. Food Chem. 2020, 68, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Köhler, N.; Winterhalter, P. Large-scale isolation of flavan-3-ol phloroglucinol adducts by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1072, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Proanthocyanidin profile and antioxidant capacity of Brazilian Vitis vinifera red wines. Food Chem. 2011, 126, 213–220. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine Res. 2009, 15, 27–35. [Google Scholar] [CrossRef]
- Mattivi, F.; Zulian, C.; Nicolini, G.; Valenti, L. Wine, biodiversity, technology, and antioxidants. Ann. N.Y. Acad. Sci. 2002, 957, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Kyraleou, M.; Kallithraka, S.; Gkanidi, E.; Koundouras, S.; Mannion, D.T.; Kilcawley, K.N. Discrimination of five Greek red grape varieties according to the anthocyanin and proanthocyanidin profiles of their skins and seeds. J. Food Compos. Anal. 2020, 92, 103547. [Google Scholar] [CrossRef]
- Chira, K.; Lorrain, B.; Ky, I.; Teissedre, P.-L. Tannin composition of Cabernet-Sauvignon and Merlot grapes from the Bordeaux area for different vintages (2006 to 2009) and comparison to tannin profile of five 2009 vintage Mediterranean grapes varieties. Molecules 2011, 16, 1519. [Google Scholar] [CrossRef] [PubMed]
- Gawel, R.; Oberholster, A.; Francis, I.L. A “Mouth-feel Wheel”: Terminology for communicating the mouth-feel characteristics of red wine. Aust. J. Grape Wine Res. 2000, 6, 203–207. [Google Scholar] [CrossRef]
- Piombino, P.; Pittari, E.; Gambuti, A.; Curioni, A.; Giacosa, S.; Mattivi, F.; Parpinello, G.P.; Rolle, L.; Ugliano, M.; Moio, L. Preliminary sensory characterisation of the diverse astringency of single cultivar Italian red wines and correlation of sub-qualities with chemical composition. Aust. J. Grape Wine Res. 2020, 26, 233–246. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Arapitsas, P.; Curioni, A.; Gerbi, V.; Parpinello, G.P.; Ugliano, M.; Piombino, P. Exploring olfactory–Oral cross-Modal interactions through sensory and chemical characteristics of Italian red wines. Foods 2020, 9, 1530. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [CrossRef]
- Pinasseau, L.; Vallverdú-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Péros, J.-P.; Ageorges, A.; Sommerer, N.; Boulet, J.-C.; et al. Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Front. Plant Sci. 2017, 8, 1826. [Google Scholar] [CrossRef] [PubMed]
- McRae, J.M.; Dambergs, R.G.; Kassara, S.; Parker, M.; Jeffery, D.W.; Herderich, M.J.; Smith, P.A. Phenolic compositions of 50 and 30 year sequences of Australian red wines: The impact of wine age. J. Agric. Food Chem. 2012, 60, 10093–10102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Wang, Y.; Xu, M.; Hu, X. UV-Vis spectroscopy combined with chemometric study on the interactions of three dietary flavonoids with copper ions. Food Chem. 2018, 26, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Prieur, C.; Guyot, S.; Rigaud, J.; Moutounet, M. The structures of tannins in grapes and wines and their interaction with proteins. In Wine: Nutritional and Therapeutic Benefits; Watkins, T.R., Ed.; American Chemical Society: Washington, DC, USA, 1997; pp. 81–93. [Google Scholar]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 2006, 57, 289. [Google Scholar]
- Kallithraka, S.; Bakker, J.; Clifford, M.N. Evaluation of bitterness and astringency of (+)-catechin and (−)-epicatechin in red wine and model solution. J. Sens. Stud. 1997, 12, 25–37. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Guyot, S.; Marnet, N.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of grape and apple proanthocyanidins in a wine-like medium. J. Sci. Food Agric. 2003, 83, 564–573. [Google Scholar] [CrossRef]
- Liang, N.N.; He, F.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Optimization of sample preparation and phloroglucinol analysis of Marselan grape skin proanthocyanidins using HPLC-DADESI- MS/MS. South Afr. J. Enol. Vitic. 2016, 33, 122–131. [Google Scholar] [CrossRef]

| Compound 1 | RT (min) | Cone Voltage (V) | ESI Mode | Quantifier MRM (Collision Energy/V) | Qualifier MRM (Collision Energy/V) |
|---|---|---|---|---|---|
| B1 | 3.06 | 32 | − | 577.1 → 289.0 (26) | 577.1 → 425.1 (16) |
| B2 | 3.67 | 30 | − | 577.1 → 289.0 (24) | 577.1 → 425.1 (16) |
| C | 3.50 | 32 | − | 289.0 → 203.0 (20) | 289.0 → 123.0 (32) |
| EC | 4.00 | 34 | − | 289.0 → 203.0 (20) | 289.0 → 123.0 (30) |
| GC | 2.56 | 32 | − | 305.0 → 124.9 (26) | 305.0 → 179.0 (19) |
| EGC | 3.15 | 32 | − | 305.0 → 124.9 (22) | 305.0 → 179.0 (16) |
| ECG | 5.25 | 32 | − | 441.0 → 289.0 (18) | 441.0 → 169.0 (26) |
| CG | 5.35 | 32 | − | 441.0 → 289.0 (18) | 441.0 → 169.0 (27) |
| EC-Phl | 3.02 | 40 | − | 413.3 → 125.0 (20) | 413.3 → 261.1 (14) |
| EGC-Phl | 2.28 | 25 | − | 429.4 → 125.0 (25) | 429.4 → 177.0 (22) |
| ECG-Phl | 3.88 | 28 | − | 565.5 → 125.0 (38) | 565.5 → 395.2 (16) |
| Compound 1 | LOD–LOQ 2 | Range (mg/L) | Calibration Curve | Correlation Coefficient |
|---|---|---|---|---|
| B1 | 0.039–0.132 | 0.006–16.20 | y = 826.07x + 0.96 | r = 0.987 |
| B2 | 0.028–0.078 | 0.013–32.42 | y = 1498.46x − 9.94 | r = 0.986 |
| C | 0.063–0.387 | 0.020–101.85 | y = 930.83x + 70.44 | r = 0.994 |
| EC | 0.069–0.386 | 0.069–68.70 | y = 888.96x + 116.86 | r = 0.982 |
| GC | 0.027–0.076 | 0.019–46.75 | y = 2023.71x − 12.14 | r = 0.985 |
| EGC | 0.024–0.068 | 0.011–28.12 | y = 2345.22x − 12.26 | r = 0.987 |
| ECG | 0.012–0.037 | 0.011–27.50 | y = 7623.12x − 7.09 | r = 0.998 |
| CG | 0.032–0.078 | 0.025–63.12 | y = 1793.95x − 21.87 | r = 0.985 |
| EC-Phl | 0.322–0.442 | 0.322–321.6 | y = 1202.57x + 92.00 | r = 0.990 |
| EGC-Phl | 0.153–0.198 | 0.153–153.0 | y = 861.83x − 77.76 | r = 0.985 |
| ECG-Phl | 0.083–0.141 | 0.083–432.2 | y = 716.06x + 24.53 | r = 0.991 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arapitsas, P.; Perenzoni, D.; Guella, G.; Mattivi, F. Improving the Phloroglucinolysis Protocol and Characterization of Sagrantino Wines Proanthocyanidins. Molecules 2021, 26, 1087. https://doi.org/10.3390/molecules26041087
Arapitsas P, Perenzoni D, Guella G, Mattivi F. Improving the Phloroglucinolysis Protocol and Characterization of Sagrantino Wines Proanthocyanidins. Molecules. 2021; 26(4):1087. https://doi.org/10.3390/molecules26041087
Chicago/Turabian StyleArapitsas, Panagiotis, Daniele Perenzoni, Graziano Guella, and Fulvio Mattivi. 2021. "Improving the Phloroglucinolysis Protocol and Characterization of Sagrantino Wines Proanthocyanidins" Molecules 26, no. 4: 1087. https://doi.org/10.3390/molecules26041087
APA StyleArapitsas, P., Perenzoni, D., Guella, G., & Mattivi, F. (2021). Improving the Phloroglucinolysis Protocol and Characterization of Sagrantino Wines Proanthocyanidins. Molecules, 26(4), 1087. https://doi.org/10.3390/molecules26041087








