Prospective Ultrasonographic Evaluation of Adrenal Glands in a Population of Beagle Puppies and Functional Analysis of Basal Cortisol Levels in Blood
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Samples and Serum Cortisol Levels
2.3. Sonographic Measurements of the Adrenal Gland
2.4. Statistics
3. Results
3.1. General Observations
3.2. Sonographic Parameters
3.3. Serum Cortisol Levels
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| ACTH | Adrenocorticotropic hormone |
| ANOVA | Analysis of variance |
| ECLIA | Electrochemiluminescence immunoassay |
| ECVDI | European College of Veterinary Diagnostic Imaging |
| kg | Kilogram |
| MA | Mean average |
| max. | Maximum |
| min. | Minimum |
| mm | Millimeter |
| nmol/L | Nanomoles per liter |
| r | Spearman’s correlation coefficient |
| SD | Standard deviation |
| e.g. | For example |
References
- Bovens, C.; Tennant, K.; Reeve, J.; Murphy, K.F. Basal Serum Cortisol Concentration as a Screening Test for Hypoadrenocorticism in Dogs. J. Vet. Intern. Med. 2014, 28, 1541–1545. [Google Scholar] [CrossRef]
- Peterson, M.E.; Kinzter, P.P. Diagnosis and management of hypoadrenocorticism (Addisons disease). Vet. Clin. North Am. Small Anim. Pract. 1995, 25, 1283–1301. [Google Scholar]
- Kintzer, P.P.; Peterson, M.E. Primary hypoadrenocorticisem in dogs. Clin. Tech. Small Anim. Pract. 1997, 12, 236–242. [Google Scholar]
- Heilmann, R.M.; Heilmann, R.B. Atypischer Hypoadrenokortizismus(Morbus Addison) beim Hund. Veterinärspiegel 2019, 29, 92–99. [Google Scholar] [CrossRef]
- Scott-Moncrieff, J.; Ettinger, S.; Feldmann, E.; Cote, E. Hypoadrenocorticism in dogs. In Textbook of Veterinary Internal Medicine; Elsevier Health Sciences: Maryland Heights, MO, USA, 2017; pp. 1795–1810. [Google Scholar]
- Frank, L.A.; Oliver, J.W. Prevalence and clinical features of hypoadrenocorticism in dogs in the United States: A retrospective study. J. Am. Anim. Hosp. Assoc. 1998, 34, 395–403. [Google Scholar]
- Besso, J.G.; Penninck, D.G.; Gliatto, J.M. Rettropsective Ultrasonographic Evaluation of Adrenal Lesions in 26 Dogs. Vet. Radiol. Ultrascound 1997, 448–455. [Google Scholar] [CrossRef]
- Wenger, M.; Mueller, C.; Kook, P.H.; Reusch, C.E. Ultrasonographic evaluation of adrenal glands in dogs with primary hypoadrenocorticism or mimicking diseases. Vet. Rec. 2010, 167, 207–210. [Google Scholar] [CrossRef]
- Ramos, P.J.G.; Bennaim, M.; Shiel, R.E.; Mooney, C.T. Diagnosis of canine spontaneous hypoadrenocorticism. Canine Med. Genet. 2022, 9, 6. [Google Scholar] [CrossRef]
- Barthez, P.Y.; Nyland, T.G.; Feldman, E.C. Ultrasonographic evaluation of the adrenal glands in dogs. J. Am. Vet. Med. Assoc. 1995, 207, 1180–1183. [Google Scholar] [CrossRef]
- Buchanan, J.; Bucheler, J. Radiographic size of the adrenal glands in dogs. J. Am. Vet. Med. Assoc. 1995, 206, 53–57. [Google Scholar]
- Agut, A.; Martinez, M.; Anson, A.; Soler, M. Ultrasonographic measurement of adrenal gland-to-aorta ratio as a method of estimating adrenal size in dogs. Vet Rec. 2020, 186, e27. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.P.; Berry, C.R.; James, S.B. Ultrasonographic adrenal gland measurements in dogs without evidence of adrenal disease. Vet. Radiol. Ultrasound 1997, 38, 124–130. [Google Scholar] [CrossRef]
- Bento, P.L.; Center, S.A.; Randolph, J.F.; Yeager, A.E.; Bicalho, R.C. Associations between sex, body weight, age, and Ultrasonographically determined adrenal gland thickness in dogs with non-adrenal gland illness. J. Am. Vet. Med. Assoc. 2016, 248, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Chalifoux, N.V.; Burgess, H.J.; Feng, C.X.; Kong, L.R.; Snead, E.C.R. Article Canine hypoadrenocorticism: Insights into the Addisonian crisis. Can. Vet. J. 2023, 64, 457–464. [Google Scholar]
- Mills, D.S.; Karagiannis, C.I. Improving the welfare of animals in veterinary practice: Practical approaches to reducing stress and fear. J. Vet. Behav. 2018, 23, 19–26. [Google Scholar]
- China, L.; Mills, D.S.; Cooper, J.J. Efficacy of Dog Training With and Without Remote Electronic Collars vs. a Focus on Positive Reinforcement. Front Vet. Sci. 2020, 7, 547533. [Google Scholar] [CrossRef] [PubMed]
- Köhler, B.; Stengel, C.; Neiger, R. Comparison of cortisol concentrations in serum, plasma and saliva in healthy dogs and dogs with hypercortisolism or hypoadrenocorticism. J. Vet. Intern. Med. 2011, 25, 1244–1251. [Google Scholar]
- Tidwell, A.S.; Johnson, K.L. Diagnostic imaging of the adrenal glands in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2012, 42, 1329–1345. [Google Scholar] [CrossRef]
- Gold, A.J.; Langlois, D.K.; Refsal, K.R. Evaluation of Basal Serum or Plasma Cortisol Concentrations for the Diagnosis of Hypoadrenocorticism in Dogs. J. Vet. Intern. Med. 2016, 30, 1798–1805. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlation analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Palazzo, P.; Quadri, S.K. Postnatal differentiation of adrenal cortex in the dog. Anat. Histol. Embryol. 1987, 16, 122–130. [Google Scholar]
- Soulsby, S.N.; Holland, M.; Hudson, J.A.; Behrend, E.N. Ultrasonographic evaluation of adrenal gland size compared to body weight in normal dogs. Vet. Radiol. Ultrasound 2015, 56, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Perfetti, S.; Diana, A.; Toaldo, M.B.; Cipone, M.; Quinci, M.; Pey, P. CT measures of adrenal gland length and caudal pole diameter are reproducible in large breed dogs: A pilot study. Vet. Radiol. Ultrasound 2021, 62, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, H.; Yoon, J. Ultrasonographic Adrenal Gland Measurements in Clinically Normal Small Breed Dogs and Comparison with Pituitary-Dependent Hyperadrenocorticism. J. Vet. Med. Sci. 2011, 73, 985–989. [Google Scholar] [CrossRef]
- D’Anjou, M.A.; Penninch, D.G.; Cornejo, L. Utrasonographic adrenal lnad measurements in dogs: Correlation with weight and age. Vetrinary Radiol. Ultrasound 2009, 50, 548–553. [Google Scholar]
- Kooistra, H.; Galac, S. Advances in the understanding of the pathogenesis of hypoadrenocorticism in dogs. Vet. J. 2012, 192, 8–15. [Google Scholar]
- Wenger, M.; Anrold, P.; Rütten, M. Ultrasonographic evaluation of adrenal gland size in dogs with primary hypoadrenocorticism. Vet. Radiol. Ultrasound 2006, 47, 32–37. [Google Scholar]
- Nivy, R.; Segev, G.; Kass, P.H.; Aroch, I. Breed-related variability in ultrasonographic measurements of the adrenal glands in dogs. Vet. Radiol. Ultrasound 2015, 56, 276–281. [Google Scholar] [CrossRef]
- Ando, I.; Karasawa, K.; Shioya, T.; Matsuda, H.; Tanaka, A. Evaluation of stress status using the stress map for guide dog candidates in the training stage using variations in the serum cortisol with nerve growth factor and magnesium ions. Vet. Anim. Sci. 2020, 10, 100129. [Google Scholar] [CrossRef]
- Palazzolo, D.L.; Quadri, S.K. Plasma Thyroxine and Cortisol under Basal Conditions and during Cold Stress in the Aging Dog. Proc. Soc. Exp. Biol. Med. 1987, 185, 305–311. [Google Scholar] [CrossRef]
- Rothuizen, J.; Reul, J.M.; van Sluijs, F.J.; Mol, J.A.; van Eerdenburg, F.J. Increased cortisol resonse to stress in dogs with pituitary-dependent hyperadrenocorticism. Res. Vet. Sci. 1991, 50, 306–311. [Google Scholar]
- McGowan, R.T.S.; Rehn, T.; Norling, Y.; Keeling, L.J. Positive affect and learning: Exploring the ‘Eureka Effect’ in dogs. Anim. Cogn. 2014, 17, 577–587. [Google Scholar] [CrossRef]
- Boves, C.; Tennant, K.; Reusch, C.E.; Schwarz, T. Evaluation of basal serum cortisol concentrations for the diagnosis of hypoadrenocorticism in dogs. J. Vet. Intern. Med. 2010, 24, 282–289. [Google Scholar]
- Schoeman, J.P.; Goddard, A.; Herrtage, M.E. Serum cortisol and thyroxine concentrations as predictors of death in critically ill puppies with parvoviral diarrhea. JAVMA, 2007; 231, 1534–1539. [Google Scholar] [CrossRef]
- Palazzolo, D.L.; Quadri, S.K. The effects of aging on the circadian rhythm of serum cortisol in the dog. Exp. Gerontol. 1987, 22, 379–387. [Google Scholar] [CrossRef]
- Khalid, M. Sex steroid hormone receptors in the adrenal gland of the dog: An immunohistochemical study. Res. Vet. Sci. 2008, 84, 199–204. [Google Scholar]
- Kutzler, M.A.; Wood, A. Non-Surgical methods of contraception and sterillastion. Theriogenology 2006, 66, 514–525. [Google Scholar] [CrossRef]
- Kiiroja, L.; Stewart, S.H.; Gadbois, S. Can scent-detection dogs detect the stress associated with trauma cue exposure in people with trauma histories? A proof-of-concept study. Front. Allergy 2024, 5, 1352840. [Google Scholar] [CrossRef] [PubMed]
- Gazit, I.; Terkel, J. Canine olfactory detection of cortisol levels in humans. Horm. Behav. 2017, 93, 67–74. [Google Scholar] [CrossRef]
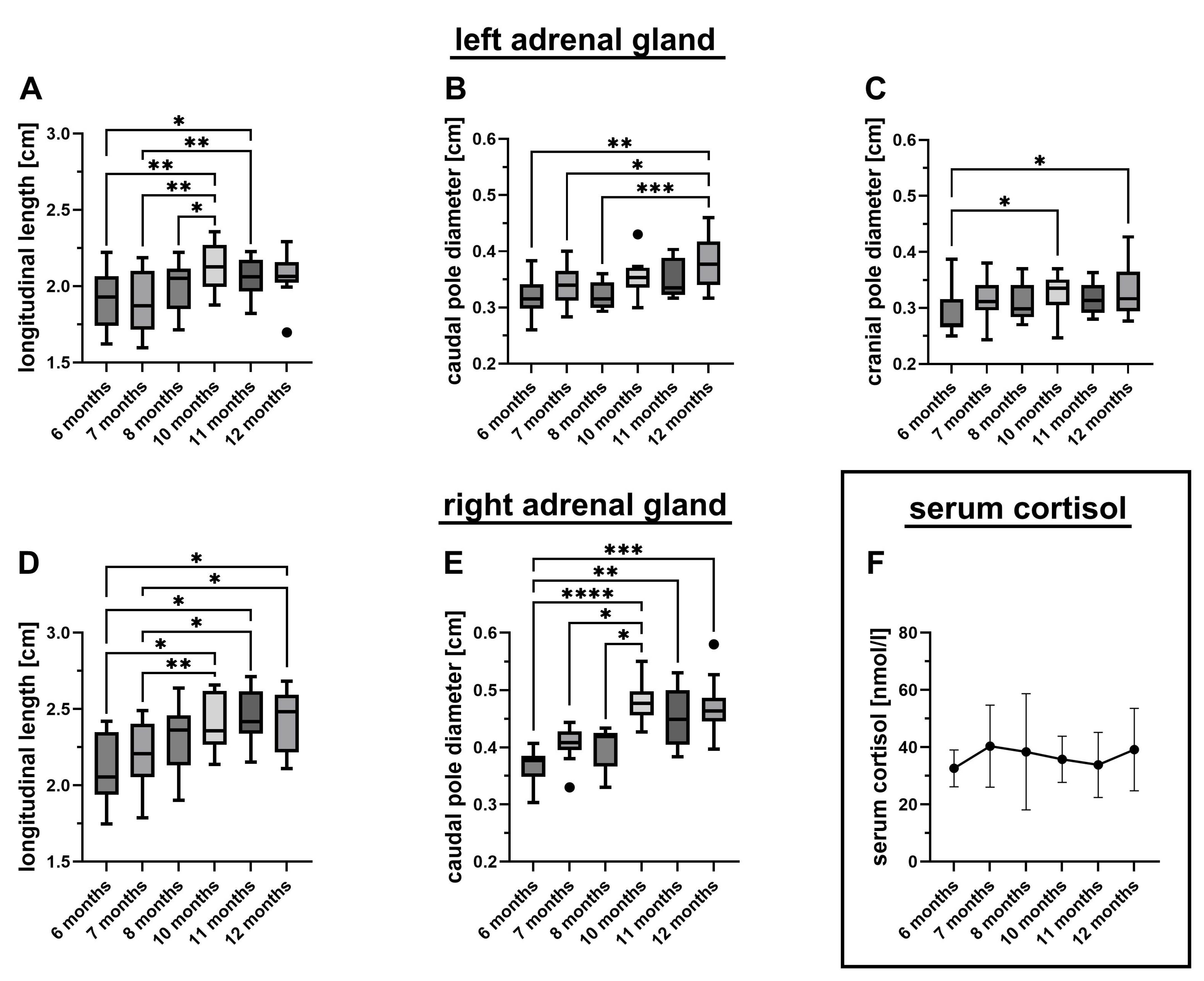
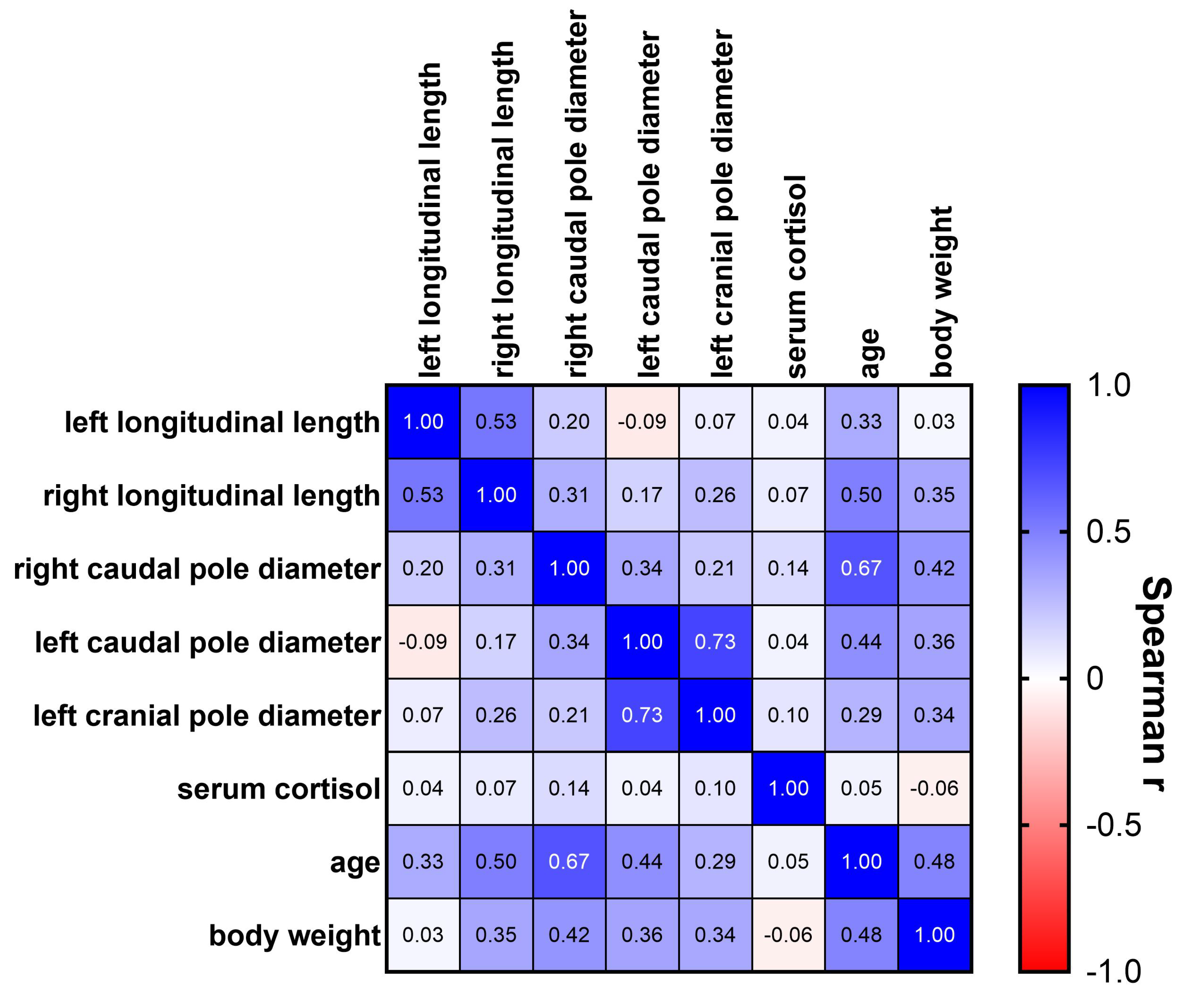
| Age [months] | Mean [cm] | Median [cm] | Range [cm] | Growth Rate | |
|---|---|---|---|---|---|
| Longitudinal length (left) | 6 | 1.92 | 1.93 | 1.62–2.22 | 8.3% |
| 10–12 | 2.08 | 2.08 | 1.70–2.36 | ||
| Longitudinal length (right) | 6 | 2.11 | 2.06 | 1.75–2.42 | 15.1% |
| 10–12 | 2.42 | 2.42 | 2.11–2.71 | ||
| Diameter caudal pole (left) | 6 | 0.32 | 0.32 | 0.26–0.38 | 13.5% |
| 10–12 | 0.36 | 0.36 | 0.30–0.46 | ||
| Diameter caudal pole (right) | 6 | 0.37 | 0.38 | 0.30–0.41 | 27.7% |
| 10–12 | 0.47 | 0.47 | 0.38–0.58 | ||
| Diameter cranial pole (left) | 6 | 0.29 | 0.27 | 0.25–0.39 | 11.7% |
| 10–12 | 0.32 | 0.32 | 0.25–0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topmöller, J.; Merhof, K.; Packeiser, E.; Schmicke, M.; Volk, H.A.; Rieder, J. Prospective Ultrasonographic Evaluation of Adrenal Glands in a Population of Beagle Puppies and Functional Analysis of Basal Cortisol Levels in Blood. Vet. Sci. 2025, 12, 472. https://doi.org/10.3390/vetsci12050472
Topmöller J, Merhof K, Packeiser E, Schmicke M, Volk HA, Rieder J. Prospective Ultrasonographic Evaluation of Adrenal Glands in a Population of Beagle Puppies and Functional Analysis of Basal Cortisol Levels in Blood. Veterinary Sciences. 2025; 12(5):472. https://doi.org/10.3390/vetsci12050472
Chicago/Turabian StyleTopmöller, Julia, Kristina Merhof, Eva Packeiser, Marion Schmicke, Holger Andreas Volk, and Johanna Rieder. 2025. "Prospective Ultrasonographic Evaluation of Adrenal Glands in a Population of Beagle Puppies and Functional Analysis of Basal Cortisol Levels in Blood" Veterinary Sciences 12, no. 5: 472. https://doi.org/10.3390/vetsci12050472
APA StyleTopmöller, J., Merhof, K., Packeiser, E., Schmicke, M., Volk, H. A., & Rieder, J. (2025). Prospective Ultrasonographic Evaluation of Adrenal Glands in a Population of Beagle Puppies and Functional Analysis of Basal Cortisol Levels in Blood. Veterinary Sciences, 12(5), 472. https://doi.org/10.3390/vetsci12050472






