Small-Lungworm (Protostrongylidae) Infections in Relation to Meat Sheep Breeds, Mediterranean Climates, and Anthelmintic Regimens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farms and Sampling
2.2. Climate Parameters
2.3. Statistical Analysis
3. Results
3.1. Regional Differences in Infection with Protostrongylids (Table 1)
3.2. Climate and Protostrongylid Infection
3.3. Sheep Breeds and Protostrongylid Infections
3.4. Anthelmintic Practices and Protostrongylid Infections
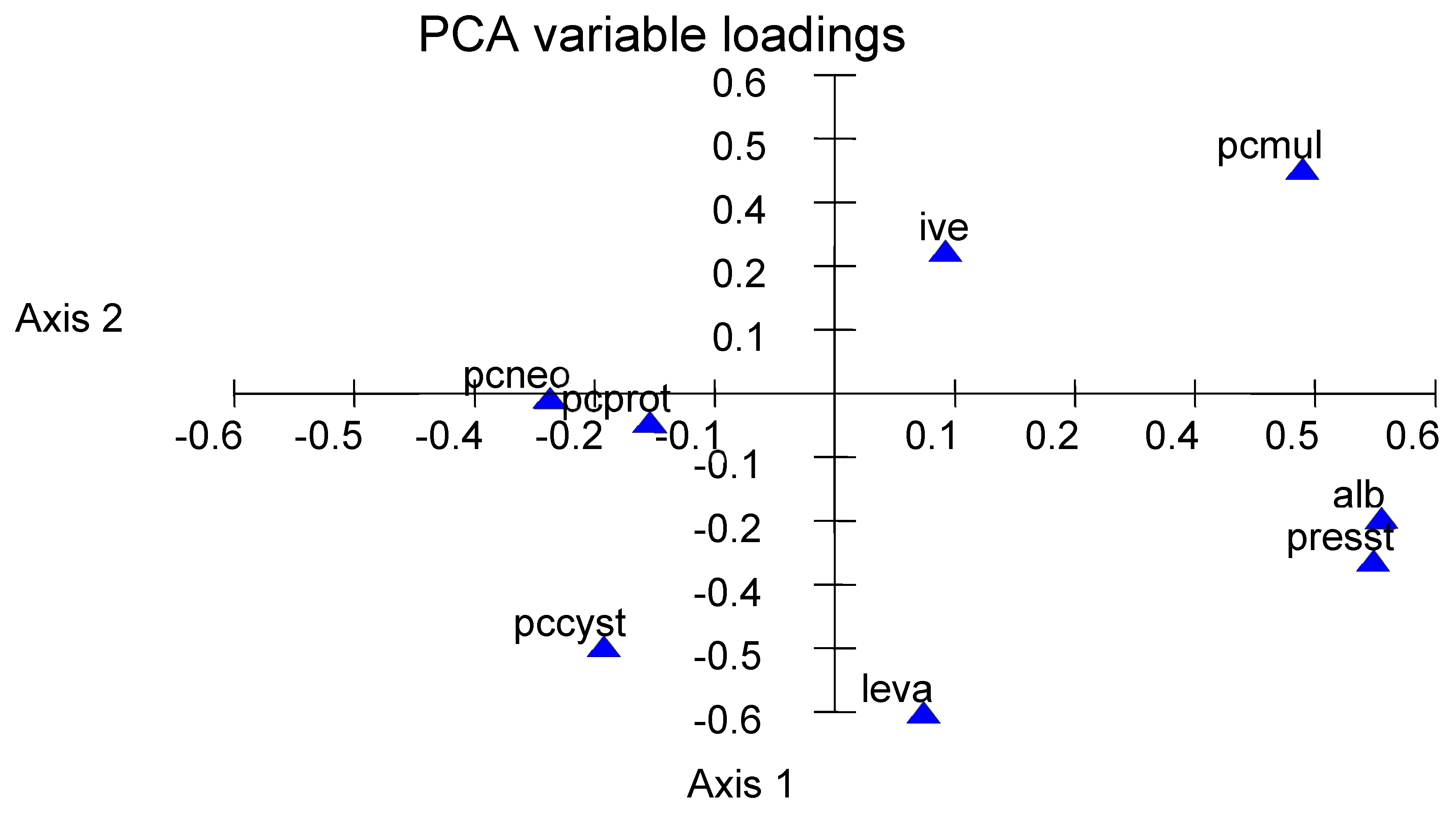
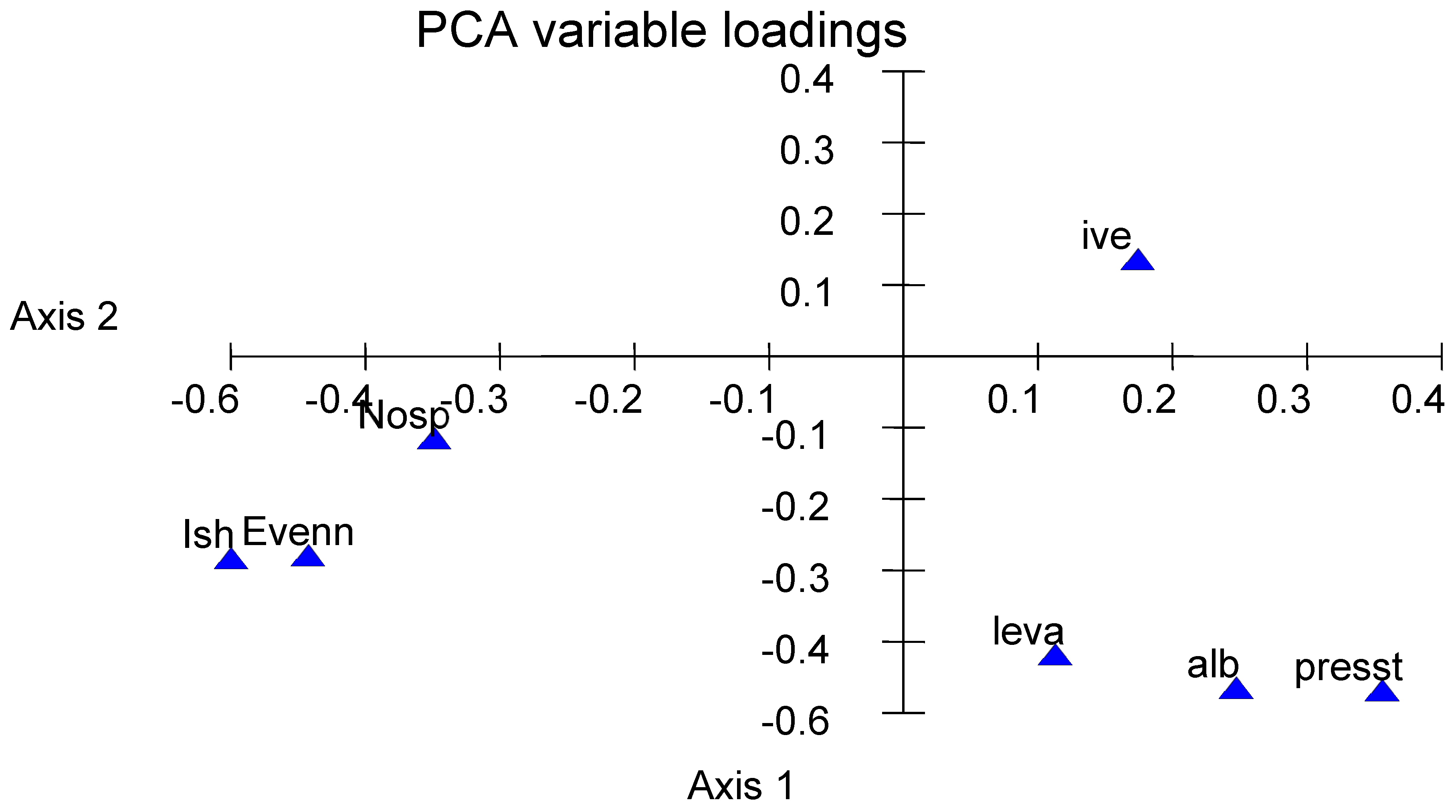
3.5. A Global Interpretation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boev, S.N. Protostrongylids. In Fundamentals of Nematology; Oxonian Press: New Delhi, India, 1984; Volume 25. [Google Scholar]
- Bentounsi, B.; Cabaret, J. (Eds.) Parasitologie Vétérinaire—Helminthoses des Herbivores en Afrique du Nord; HAL Id: Hal-04085813; HAL: Bengaluru, India, 2023; p. 204. Available online: https://hal.science/hal-04085813v3 (accessed on 1 January 2023).
- Giangaspero, M.; Gruner, L.; Nishikawa, H.; Tabbaa, D.; Vacirca, G. Lungworms, Maedi Visna and mixed infections with respiratory viruses in Syrian Awassi sheep. Vet. Res. Commun. 1993, 17, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Court, R.; Saquenet, A. Liste préliminaire des nématodes parasites des moutons d’Algérie. Bul. Soc. Hist. Nat. Afrique Nord 1945, 36, 75–78. [Google Scholar]
- Joyeux, C.; Gaud, J. La pneumonie vermineuse au Maroc. Bul. Soc. Path. Exo. 1943, 36, 232–235. [Google Scholar]
- Gerichter, C.B. Studies on the lung nematodes of sheep and goats in the Levant. Parasitology 1951, 41, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Manga-Gonzalez, Y.; Morrondo-Pelayo, P.; Cordero del Campillo, M. Moluscos Hospedadores Intermediarios de Protostrongylidae Ovinos; Universidad de Leon: Leon, Spain, 1986; p. 136. [Google Scholar]
- Gruner, L.; Cabaret, J.; Sauve, C.; Pailhories, R. Comparative susceptibility of Romanov and Lacaune sheep to gastrointestinal nematodes and small lungworms. Vet. Parasitol. 1986, 19, 85–93. [Google Scholar] [CrossRef]
- Gruner, L.; Bouix, J.; Cabaret, J.; Boulard, C.; Cortet, J.; Sauve, C.; Molenat, G.; Calamel, M. Effect of genetic type, lactation and management on helminth infection of ewes in an intensive grazing system on irrigated pasture. Int. J. Parasitol. 1992, 22, 919–925. [Google Scholar] [CrossRef]
- Cabaret, J.; Dakkak, A.; Bahaida, B. On some factors influencing the output of the larvae of Protostrongylids of sheep in natural conditions. Vet. Q. 1980, 2, 115–120. [Google Scholar] [CrossRef]
- López, C.M.; Cienfuegos, S.; Dacal, V.; Vazquez, L.; Panadero, R.; Fernandez, G.; Diaz, P.; Lago, N.; Diez-Banos, P.; Morrondo-Pelayo, M.P. Efficacy of anthelmintic control programs against natural Muellerius capillaris infection in sheep in the North-west of Spain. Effect on blood gases and pH in venous blood samples. Parasite 2010, 17, 167–171. [Google Scholar] [CrossRef]
- Pandey, V.S. Effect of tetramisole on protostrongylid lungworms of sheep. Ann. Soc. Belge Med. Trop. 1980, 60, 103–106. [Google Scholar]
- McCraw BMMenzies, P.I. Muellerius capillaris: Resumption of shedding larvae in feces following anthelmintic treatment and prevalence in housed goats. Can. Vet. J. 1988, 29, 453–454. [Google Scholar]
- Pereira, M.A.; Vila-Viçosa, M.J.; Coelho, C.; Santos, C.; Esteves, F.; Cruz, R.; Gomes, L.; Henriques, D.; Vala, H.; Nóbrega, C.; et al. Pulmonary and Gastrointestinal Parasitic Infections in Small Ruminant Autochthonous Breeds from Centre Region of Portugal—A Cross-Sectional Study. Animals 2024, 14, 1241. [Google Scholar] [CrossRef] [PubMed]
- Cabaret, J. Natural infection of land-snails by protostrongylids on a pasture grazed by sheep in the Rabat area of Morocco. Vet. Parasitol. 1988, 26, 3–4, 297–304. [Google Scholar] [CrossRef]
- Lahmar, S.; Cabaret, J.; Cheniti, T. Land snails and periods at high risk for protostrongylid infection on a sheep-grazed pasture of northeast Tunisia. Vet. Parasitol. 1990, 36, 105–115. [Google Scholar] [CrossRef]
- López, C.; Panadero, R.; Díez, P.; Morrondo, P. Development of Neostrongylus linearis in Cernuella (Cernuella) virgata experimentally infected and maintained in the subhumid climate of Galicia in northwest Spain. J. Helm. 1997, 71, 211–216. [Google Scholar] [CrossRef]
- Georgiev, D.M.; Kostadinova, A.; Georgiev, B.B. Land snails in the transmission of Protostrongylids on pastures in southern Bulgaria: Variability of infection levels related to environmental factors. Acta Parasitol. 2003, 48, 208–217. [Google Scholar]
- Forrester, D.J.; Littel, R.C. Influence of rainfall on lungworm infection in bighorn sheep. J. Wildl. Dis. 1976, 12, 48–51. [Google Scholar] [CrossRef]
- Díez-Baños, P.; Morrondo-Pelayo, P.; Feijoo-Penela, A.; Carrillo-González, B.; López-Sández, C. Relationship between the excretion of protostrongylid larvae in sheep in North-west Spain and climatic conditions. J. Helm. 1994, 68, 197–201. [Google Scholar] [CrossRef]
- Kouidri, M.; Selles, S.S.M.; Boulkaboul, A.; Khellil, C.-R.; Belkacem, H.; Nouar, Z. Study on the seasonal dynamics of lungworm infections in small ruminants slaughtered in Tiaret (Algeria). Bulg. J. Agric. Sci. 2017, 23, 142. [Google Scholar]
- Baermann, G. Eine einfach Methode zur auffindung von Ankylostomum (Nematoden) in erdproben. T. Tiergeneeskde Nederl. 1917, 57, 134–137. [Google Scholar]
- Emberger, L. Une classification biogéographique des climats. Recueil Trav. Labo. Botan. Géol. Zool Fac. Sci. 1955, 7, 3–43. [Google Scholar]
- Côte, M. Les régions bioclimatiques de l’est algérien. Rev. Rhumel 1998, 6, 57–69. [Google Scholar]
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–874. [Google Scholar] [CrossRef]
- Kovach Computing Service. MVSP (Multivariate Statistical Package), Version 3.1; Kovach Computing Service: Pentraeth Wales, UK, 2001. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; Urbana III. University Illinois Press: Champaign, IL, USA, 1940; p. 117. [Google Scholar]
- Pandey, V.S.; Cabaret, J.; Fikri, A. The effect of strategic anthelmintic treatment on the breeding performance and survival of ewes naturally infected with gastro-intestinal strongyles and protostrongylids. Ann. Rech. Vét. 1984, 1, 491–496. [Google Scholar]
- Regassa, A.; Toyeb, M.; Abebe, R.; Megersa, B.; Mekibib, B.; Mekuria, S.; Debela, E.; Abunna, F. Lungworm infection in small ruminants: Prevalence and associated risk factors in Dessie and Kombolcha districts, northeastern Ethiopia. Vet. Parasitol. 2010, 169, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Moussaoui, M.; Kouidri, M.; Selles, S.S.M.; Hemida, H.; Benallou, B. Ovine lungworms infection in Tiaret (Algeria): Prevalence, species involved, and pathological findings. Folia Vet. 2024, 68, 85–92. [Google Scholar]
- Elati, K.; Aloui, S.; Dhibi, M.; Rekik, M.; Gharbi, M. Variation saisonnière de l’infestation de brebis par les strongles respiratoires à l’abattoir de Sidi Bouzid (Tunisie centrale). Rev. Elevage Méd. Vét. Pays Trop. 2018, 70, 137–141. [Google Scholar] [CrossRef][Green Version]
- Reguera, A.; Cordero del Campillo, M.; Rojo-Vázquez, F.A. Variations in the numbers of protostrongylid larvae excreted by sheep in relation to the climate. In Libro Jubilar en Honor del Profesor Dr. Carlos Sanchez Botija—Ofrecido por sus Discipulos, Colaboradores y Amigos; Facultad Veterinaria Leon: Leon, Spain, 1983; pp. 209–220. [Google Scholar]
- Chartier, C.; Bushu, M.; Lubingo, M. Principaux helminthes des petits ruminants en Ituri. Ann. Soc. Belge Méd trop. 1990, 70, 75–85. [Google Scholar]
- Eiríksdóttir, H.; Skírnisson, K. Lung nematodes of sheep (Ovis aries) in Iceland-prevalence, intensity and geographic distribution in 1992 and 1993. Icel. Agric. Sci. 2021, 34, 3–14. [Google Scholar] [CrossRef]
- Alendal, E.; Helle, O. Helminth parasites of muskoxen Ovibos moschatus in Norway incl. Spitsbergen and in Sweden, with a synopsis of parasites reported from this host. Fauna Nor. 1983, 4, 41–52. [Google Scholar] [CrossRef]
- Dakkak, A.; Cabaret, J.; Ouhelli, H. Efficacité comparée du Fenbendazole et du Tetramisole sur les helminthes parasites du mouton au Maroc. I. Protostrongylidés et Dictyocaulus filaria. Rec. Med. Vét. 1979, 155, 703–711. [Google Scholar]
- Hamel, D.; Bosco, A.; Rinaldi, L.; Cringoli, G.; Kaulfuss, K.H.; Kellerman, H.; Fisher, J.; Wang, H.; Klei, K.; Mayr, S.; et al. Eprinomectin pour-on (EPRINEX® Pour-on, Merial): Efficacy against gastrointestinal and pulmonary nematodes and pharmacokinetics in sheep. BMC Vet. Res. 2017, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Rehbein, S.; Visser, M. Efficacy of Ivermectin Delivered via a Controlled-Release Capsule against Small Lungworms (Protostrongylidae) in Sheep. J. Vet. Med. Ser. B 2002, 49, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.J.; Sligo, J.R. Empirical comparison of univariate and multivariate analysis of variance procedures. Psychol. Bull. 1971, 76, 49–57. [Google Scholar] [CrossRef]
- Huberty, C.J.; Morris, J.D. Multivariate analysis versus multiple univariate analyses. Psychol. Bull. 1989, 105, 302–308. [Google Scholar] [CrossRef]
- Trikalinos, T.A.; Hoaglin, D.C.; Schmid, C.H. An empirical comparison of univariate and multivariate meta-analyses for categorical outcomes. Stat. Med. 2014, 33, 1441–1459. [Google Scholar] [CrossRef]
- Kuchboev, A.E.; Krücken, J.; Ruziev, B.H. von Samson-Himmelstjerna. Molecular phylogeny and diagnosis of species of the family Protostrongylidae from caprine hosts in Uzbekistan. Parasitol. Res. 2015, 114, 1355–1364. [Google Scholar] [CrossRef]
- Jabbar, A.; Mohandas, N.; Jex, A.R.; Gasser, R.B. The mitochondrial genome of Protostrongylus rufescens—implications for population and systematic studies. Parasites Vectors 2013, 6, 263. [Google Scholar] [CrossRef]
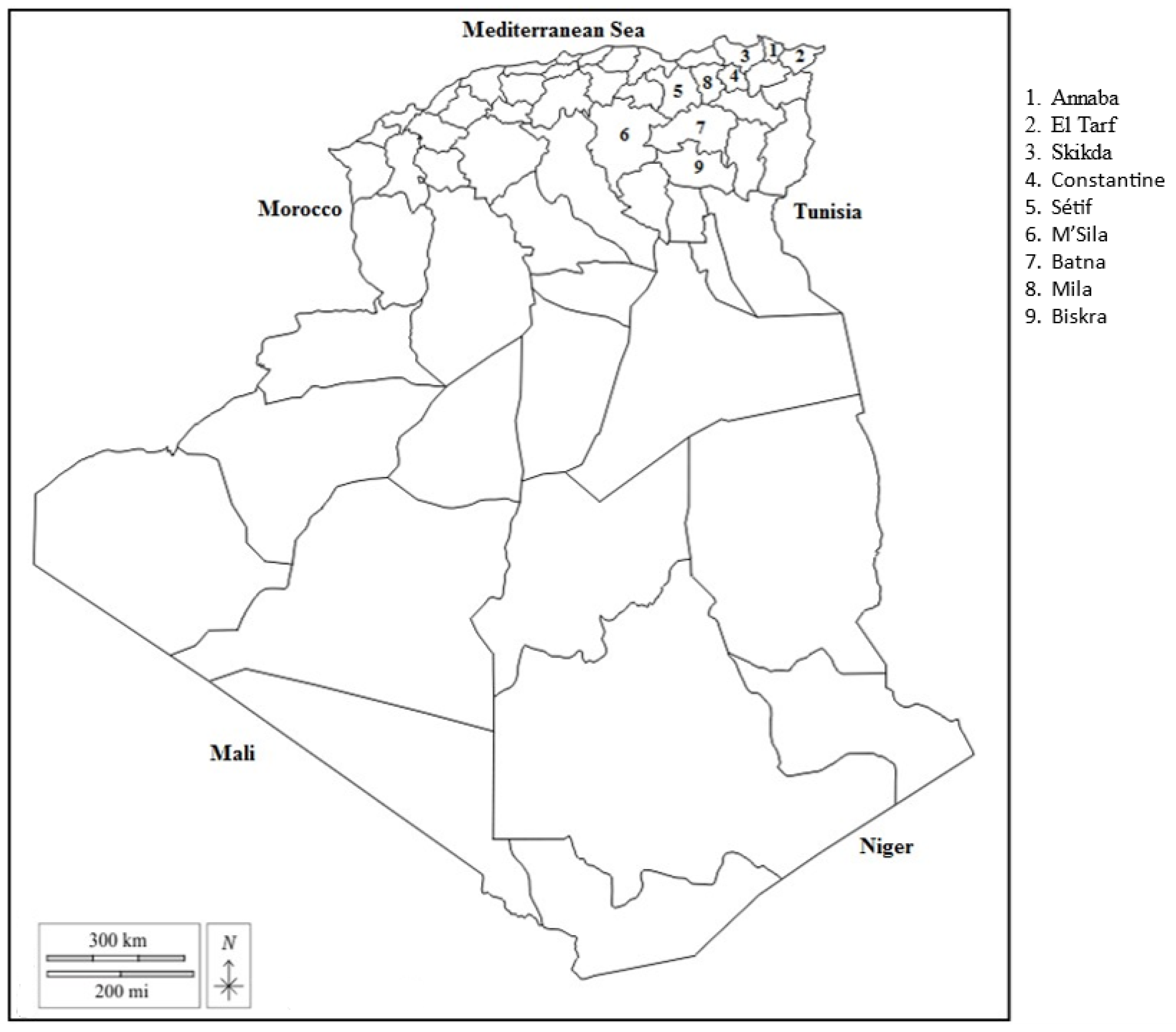


| Region (Number of Villages) | Climate (Number of Farms) | Average LPG per Farm (SD) | Prevalence (LPG) per Region and Climate | ||||
|---|---|---|---|---|---|---|---|
| Humidity | Winter Temperatures | Muellerius capillaris | Cystocaulus ocreatus | Neostrongylus linearis | Protostrongylus rufescens | ||
| Annaba (6) | Subhumid (7) | Mild | 202 (139) | 100 (176) | 75 (3) | 88 (22) | 0 (0) |
| El Tarf (4) | Subhumid (6) | Mild | 268 (542) | 100 (187) | 67 (5) | 83 (76) | 17 (0.2) |
| Skikda (6) | Subhumid (7) | Mild | 82 (110) | 100 (66) | 57 (1) | 100 (15) | 0 (0) |
| Constantine (5) | Subhumid (3) | Fresh | 126 (214) | 100 (15) | 83 (84) | 100 (28) | 0 (0) |
| Semi-arid (3) | Mild | ||||||
| Sétif (9) | Subhumid (9) | Fresh | 138 (289) | 56 (7) | 89 (14) | 89 (108) | 22 (9) |
| M’Sila (1) | Sub-arid (3) | Fresh | 2 (3) | 67 (1) | 100 (1) | 100 (0,4) | 100 (0.4) |
| Batna(5) | Sub-arid (8) | Fresh | 9 (9) | 20 (1) | 80 (5) | 90 (2) | 20 (0.8) |
| Semi-arid (1) | Fresh | ||||||
| Mila (7) | Semi-arid (5) | Fresh | 521 (640) | 38 (1) | 100 (83) | 100 (437) | 0 (0) |
| Semi-arid (3) | Mild | ||||||
| Biskra (6) | Arid (5) | Mild | 71 (107) | 67 (8) | 50 (8) | 50 (61) | 17 (0.6) |
| Sub-arid (1) | Fresh | ||||||
| Climate (No of Farms) | Muellerius capillaris | Cystocaulus ocreatus | Neostrongylus linearis | Protostrongylus rufescens |
|---|---|---|---|---|
| Subhumid mild (20) | 142a * | 3a | 35a | 0a |
| Subhumid fresh (12) | 12b | 48b | 101a | 7a |
| Semi-arid fresh (5) | 3c | 77b | 279b | 0a |
| Semi-arid mild (7) | 2c | 25a | 448c | 0a− |
| Sub-arid fresh (12) | 1c | 7a | 18a | 1a |
| Arid mild (5) | 1c | 1a | 1d | 1a |
| Significance (Kruskall and Wallis test) | p = 0.001 | p = 0.01 | p = 0.002 | p = 0.19 |
| Sheep Breed (Number of Farms) | Number of Treatments | Muellerius capillaris | Cystocaulusocreatus | Neostrongylus linearis | Protostrongylus rufescens |
|---|---|---|---|---|---|
| Ouled Djellal (26) | 1.29 a | 114 a | 21 a | 33 a | 0 a |
| Rembi (7) | 1.65 a | 1 b | 83 b | 57 a | 0 a |
| Crossbreed (18) | 0.89 b | 3 b | 4 a | 437 b | 3 b |
| Significance (Kruskal and Wallis test) | p = 0.05 | p= 0.00 | p = 0.05 | p = 0.00 | p = 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentounsi, B.; Cabaret, J. Small-Lungworm (Protostrongylidae) Infections in Relation to Meat Sheep Breeds, Mediterranean Climates, and Anthelmintic Regimens. Vet. Sci. 2025, 12, 471. https://doi.org/10.3390/vetsci12050471
Bentounsi B, Cabaret J. Small-Lungworm (Protostrongylidae) Infections in Relation to Meat Sheep Breeds, Mediterranean Climates, and Anthelmintic Regimens. Veterinary Sciences. 2025; 12(5):471. https://doi.org/10.3390/vetsci12050471
Chicago/Turabian StyleBentounsi, Bourhane, and Jacques Cabaret. 2025. "Small-Lungworm (Protostrongylidae) Infections in Relation to Meat Sheep Breeds, Mediterranean Climates, and Anthelmintic Regimens" Veterinary Sciences 12, no. 5: 471. https://doi.org/10.3390/vetsci12050471
APA StyleBentounsi, B., & Cabaret, J. (2025). Small-Lungworm (Protostrongylidae) Infections in Relation to Meat Sheep Breeds, Mediterranean Climates, and Anthelmintic Regimens. Veterinary Sciences, 12(5), 471. https://doi.org/10.3390/vetsci12050471





