A Multidrug-Resistant Escherichia coli Caused the Death of the Chinese Soft-Shelled Turtle (Pelodiscus sinensis)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal
2.2. Pathogen Isolation
2.3. Morphological Characteristic
2.4. Biochemical Characterization
2.5. Molecular Biology Analysis
2.6. Drug Susceptibility and Drug Resistance Genes Testing
2.7. Artificial Infection Experiment
2.8. Serum Physiologic and Biochemical Analysis
2.9. Histopathologic Observation
3. Results
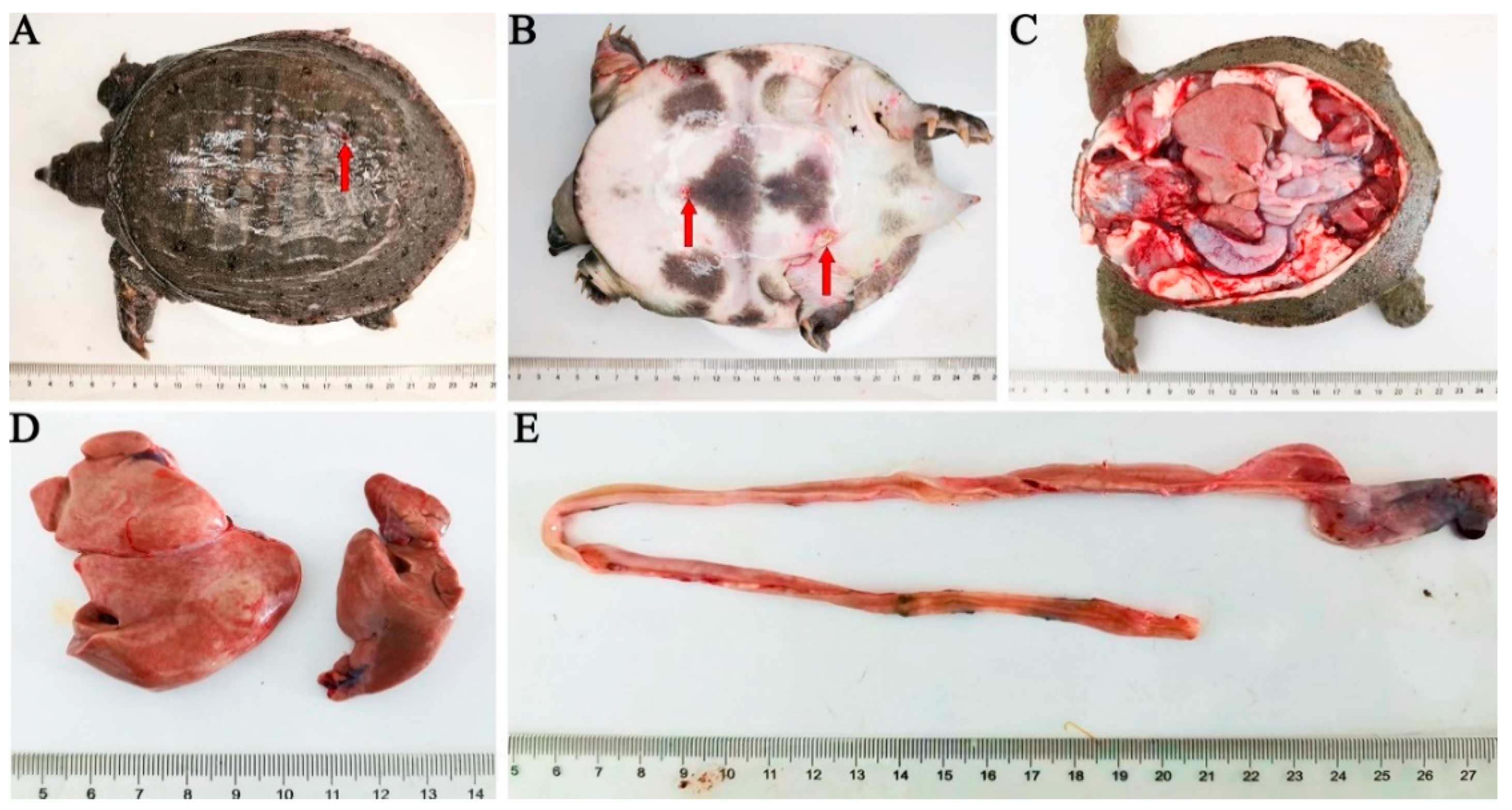
3.1. Clinical Symptom and Histopathological Findings
3.2. Morphological Features
3.3. Physiological and Biochemical Characterization of Bacteria
3.4. 16S rRNA Gene Sequences Analysis
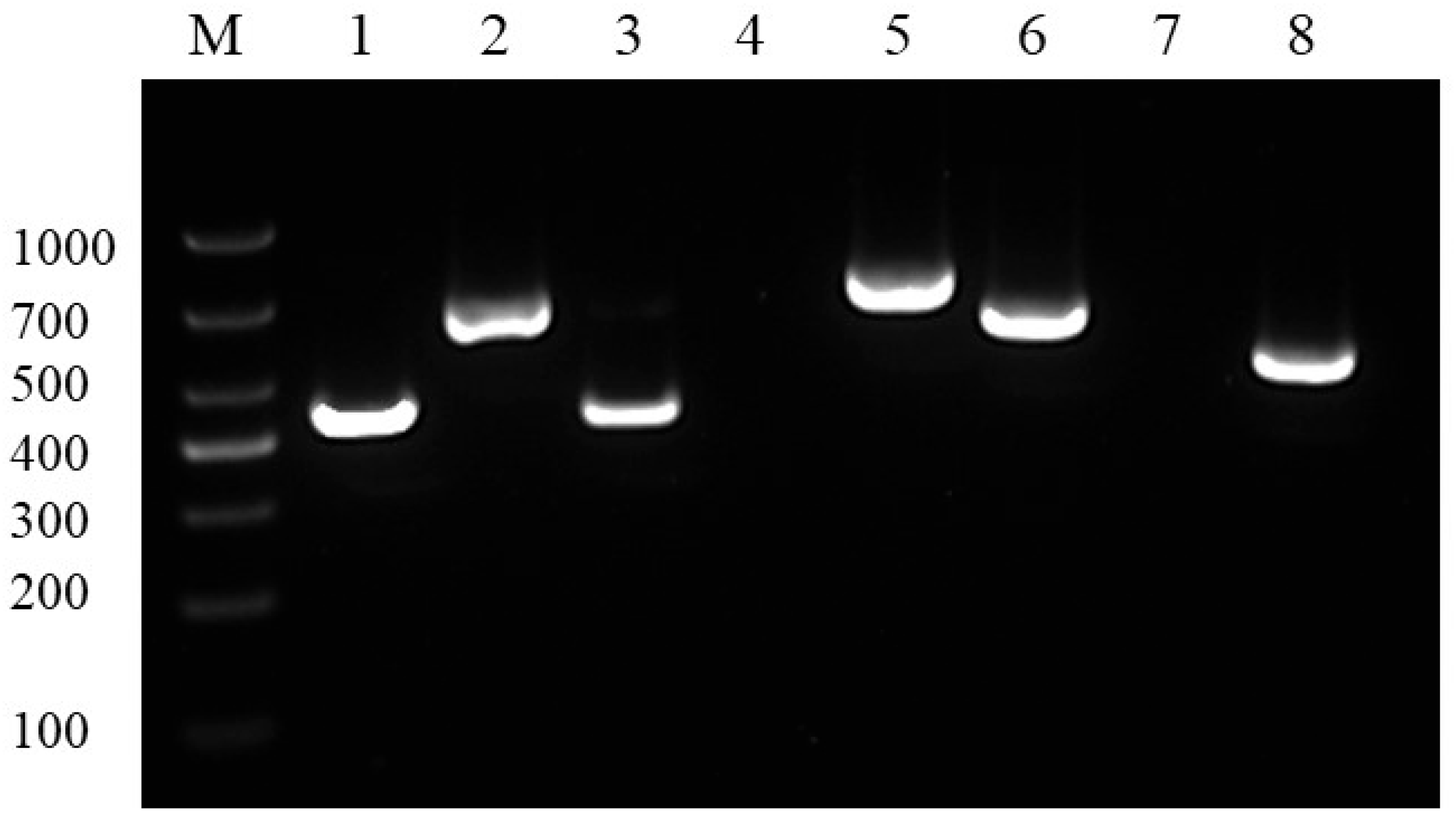
3.5. Drug Sensitivity and Drug Resistance Genes Tests
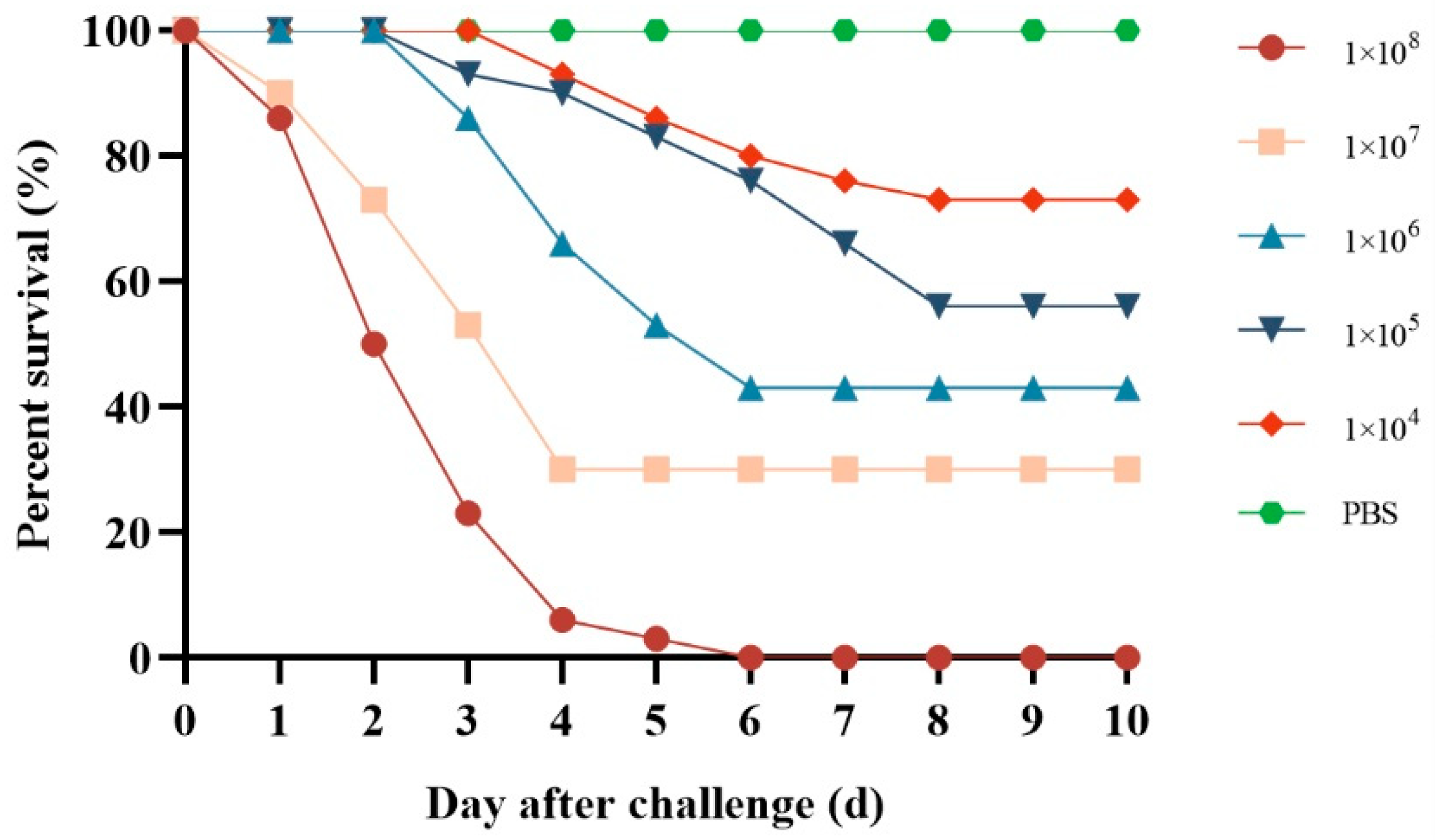
3.6. Pathogenicity
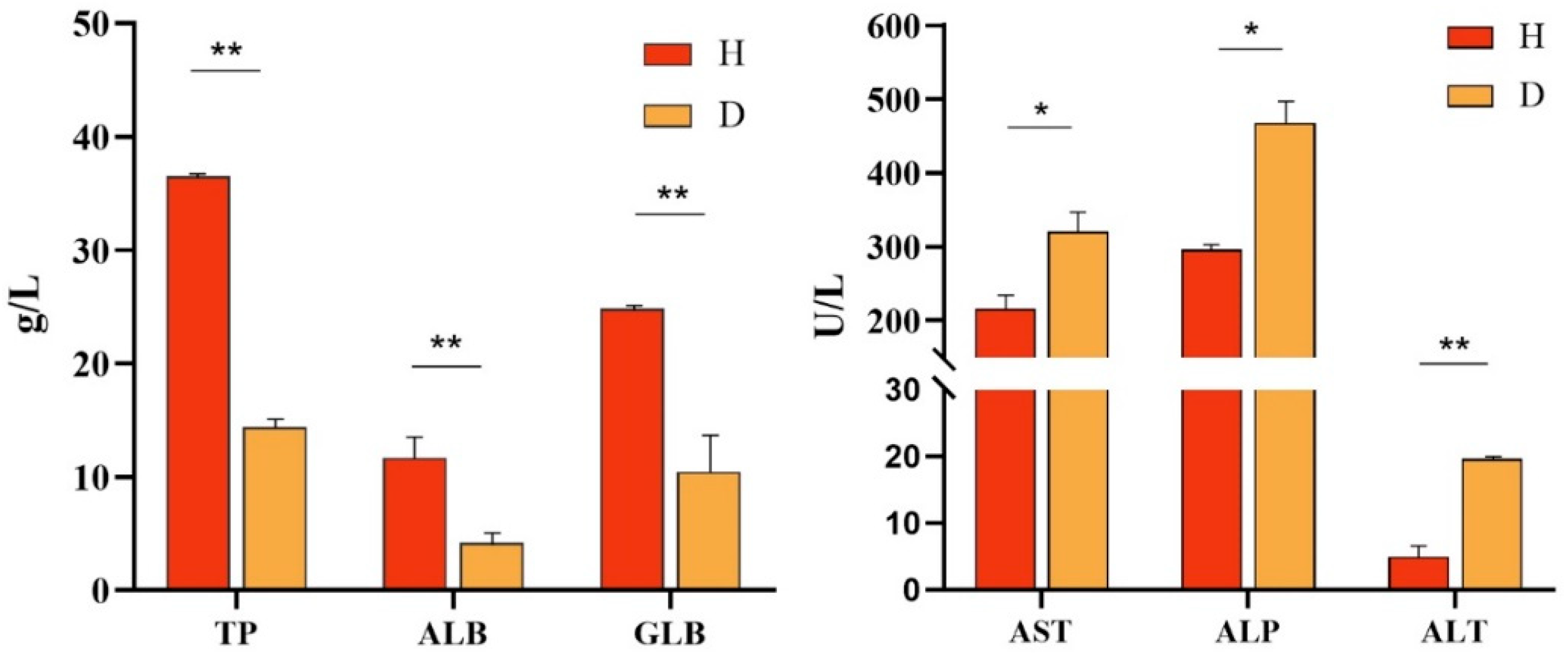
3.7. Serum Biochemistry Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, L.R.; Yu, Y.; Wen, X.X.; Xiao, H.W.; Liu, K.J.; Liu, Z.Y.; Liu, S.; Li, Q.; Wang, X.Q.; Deng, Z.F.; et al. Effects of dietary sodium butyrate on growth performance, immune function, and intestinal microflora of Chinese soft-shelled turtle (Pelodiscus sinensis). Front. Cell. Infect. Microbiol. 2023, 13, 1271912. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Hu, Y.Z.; Tan, J.; Wang, X.Q.; Liu, X.Y.; Zeng, C. Comparative Transcriptome Analysis Reveals the Molecular Immunopathogenesis of Chinese Soft-Shelled Turtle (Trionyx sinensis) Infected with Aeromonas hydrophila. Biology 2021, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lei, J.H.; Zhai, X.F.; Lu, N.N.; Shi, H.T.; Wang, J.C. Diversity of Underwater Vocalizations in Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Animals 2023, 13, 812. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.B.; Zhou, T.; Cao, J.Z.; Li, X.; Zhu, C.J.; Wang, L.; Zou, G.W.; Liang, H.W. Roles of estrogen receptors during sexual reversal in Pelodiscus sinensis. Mol. Biol. Rep. 2024, 51, 733. [Google Scholar] [CrossRef]
- Xiao, Z.D.; Cheng, M.M.; Hu, X.W.; Xue, M.Y.; Jiang, N.; Liu, W.; Fan, Y.D.; Meng, Y.; Xu, C.; Zhou, Y. Pathological changes of highly pathogenic Bacillus cereus on Pelodiscus sinensis. Vet. Q. 2023, 43, 1–10. [Google Scholar] [CrossRef]
- Xu, J.H.; Chen, H.X.; Zhang, W.R.; Xu, H.S. Evaluation of intraepithelial lymphocytes, goblet cells and immunoglobulin genes in the intestinal mucosal tissue of Pelodiscus sinensis after challenge with Aeromonas veronii bv. sobria and lipopolysaccharide. Fish. Sci. 2019, 85, 177–185. [Google Scholar] [CrossRef]
- Hu, X.W.; Xiao, Z.D.; Li, B.; Xue, M.Y.; Jiang, N.; Fan, Y.D.; Chen, P.; Qi, F.; Kong, X.H.; Zhou, Y. Isolation, Identification, and Characterization of Aeromonas veronii from Chinese Soft-Shelled Turtle (Trionyx sinensis). Microorganisms 2023, 11, 1304. [Google Scholar] [CrossRef]
- Liu, T.F.; Han, Y.W.; Chen, S.L.; Zhao, H.Y. Global characterization and expression analysis of interferon regulatory factors in response to Aeromonas hydrophila challenge in Chinese soft-shelled turtle (Pelodiscus sinensis). Fish Shellfish. Immunol. 2019, 92, 821–832. [Google Scholar] [CrossRef]
- Wang, R.H.; Yan, M.L.; Tan, Q.; Wang, J.L.; Luo, Y.S.; Chen, Z.Y. Isolation and genomic characterization of multi-drug resistant Morganella morganii strain from Chinese Soft-Shell Turtle Pelodiscus sinensis. Aquaculture 2025, 600, 742229. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Wang, W.W.; Zhang, J.Y. Antimicrobial drug resistance against Escherichia coli and its harmful effect on animal health. Vet. Med. Sci. 2022, 8, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Li, H.; Li, L.L.; Chi, Y.Y.; Tian, Q.W.; Zhou, T.T.; Han, C.H.; Zhu, Y.Q.; Zhou, Y.S. Development of a standardized Gram stain procedure for bacteria and inflammatory cells using an automated staining instrument. Microbiologyopen 2020, 9, e1099. [Google Scholar] [CrossRef]
- Arroyo, E.; Enríquez, L.; Sánchez, A.; Ovalle, M.; Olivas, A. Scanning electron microscopy of bacteria Tetrasphaera duodecadis. Scanning 2014, 36, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, C.; Fan, L.M.; Qiu, L.P.; Wu, W.; Meng, S.L.; Hu, G.D.; Kamira, B.; Chen, J.Z. Occurrence of antibiotics and their impacts to primary productivity in fishponds around Tai Lake, China. Chemosphere 2016, 161, 127–135. [Google Scholar] [CrossRef]
- Ben, Y.J.; Fu, C.X.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C.M. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Obimakinde, S.; Fatoki, O.; Opeolu, B.; Olatunji, O. Veterinary pharmaceuticals in aqueous systems and associated effects: An update. Environ. Sci. Pollut. Res. 2017, 24, 3274–3297. [Google Scholar] [CrossRef]
- Jindal, P.; Bedi, J.; Singh, R.; Aulakh, R.; Gill, J. Phenotypic and genotypic antimicrobial resistance patterns of Escherichia coli and Klebsiella isolated from dairy farm milk, farm slurry and water in Punjab, India. Environ. Sci. Pollut. Res. 2021, 28, 28556–28570. [Google Scholar] [CrossRef]
- Poole, T.L.; Callaway, T.R.; Norman, K.N.; Scott, H.M.; Loneragan, G.H.; Ison, S.A.; Beier, R.C.; Harhay, D.M.; Norby, B.; Nisbet, D.J. Transferability of antimicrobial resistance from multidrug-resistant Escherichia coli isolated from cattle in the USA to E. coli and Salmonella Newport recipients. J. Glob. Antimicrob. Resist. 2017, 11, 123–132. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, P.P.; Xie, W.J.; Liu, Y.; Lu, H.; Tang, Q.; Han, C.Q. Study on E. coli Activity and Sterilization Effect Based on Fluorescence Spectroscopy. Spectrosc. Spectr. Anal. 2019, 39, 3619–3623. [Google Scholar] [CrossRef]
- Patel, J.; Yin, H.B.; Bauchan, G.; Mowery, J. Inhibition of Escherichia coli O157:H7 and Salmonella enterica virulence factors by benzyl isothiocyanate. Food Microbiol. 2020, 86, 103303. [Google Scholar] [CrossRef] [PubMed]
- Morcinek-Orlowska, J.; Walter, B.; Forquet, R.; Cysewski, D.; Carlier, M.; Mozolewski, M.; Meyer, S.; Glinkowska, M. Interaction networks of Escherichia coli replication proteins under different bacterial growth conditions. Sci. Data 2023, 10, 788. [Google Scholar] [CrossRef]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Sarba, E.J.; Kelbesa, K.A.; Bayu, M.D.; Gebremedhin, E.Z.; Borena, B.M.; Teshale, A. Identification and antimicrobial susceptibility profile of Escherichia coli isolated from backyard chicken in and around ambo, Central Ethiopia. Bmc Vet. Res. 2019, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Liao, C.S.; Chang, S.K.; Ding, K.; Liu, Z.J.; Xue, Y. NDM-1-producing Escherichia coli isolated from pigs induces persistent infection with limited pathogenicity. Microb. Pathog. 2019, 135, 103620. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.X.; Ma, X.; Wu, Q.; Tao, Q.X.C.; Chai, Y.J.; Zhou, X.; Han, M.L.; Li, J.; Huang, X.; Wu, T.Z.; et al. Isolation, identification, molecular typing, and drug resistance of Escherichia coli from infected cattle and sheep in Xinjiang, China. Vet. Med. Sci. 2023, 9, 1359–1368. [Google Scholar] [CrossRef]
- Amézquita-López, B.A.; Soto-Beltrán, M.; Lee, B.G.; Yambao, J.C.; Quiñones, B. Isolation, genotyping and antimicrobial resistance of Shiga toxin-producing Escherichia coli. J. Microbiol. Immunol. Infect. 2018, 51, 425–434. [Google Scholar] [CrossRef]
- Xue, L.G.; Luo, X.; Xing, J.H.; Wang, D.; Zhang, D.X. Isolation and pathogenicity evaluation of Escherichia coli O157:H7 from common carp, Cyprinus carpio. Microb. Pathog. 2023, 182, 106250. [Google Scholar] [CrossRef]
- Cengiz, M.; Sahinturk, P.; Hepbostanci, G.; Akalin, H.; Sonal, S. The in vitro activity of danofloxacin plus ceftiofur combination: Implications for antimicrobial efficacy and resistance prevention. Vet. Res. Forum 2022, 13, 149–153. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, M.; Hu, X.; Jiang, N.; Liu, W.; Xiao, Z.; Zhang, C.; Wu, Y.; Liang, T.; Zhang, H.; Fan, Y.; et al. A Multidrug-Resistant Escherichia coli Caused the Death of the Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Vet. Sci. 2025, 12, 473. https://doi.org/10.3390/vetsci12050473
Xue M, Hu X, Jiang N, Liu W, Xiao Z, Zhang C, Wu Y, Liang T, Zhang H, Fan Y, et al. A Multidrug-Resistant Escherichia coli Caused the Death of the Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Veterinary Sciences. 2025; 12(5):473. https://doi.org/10.3390/vetsci12050473
Chicago/Turabian StyleXue, Mingyang, Xiaowei Hu, Nan Jiang, Wei Liu, Zidong Xiao, Chunjie Zhang, Yeying Wu, Tianwang Liang, Huixuan Zhang, Yuding Fan, and et al. 2025. "A Multidrug-Resistant Escherichia coli Caused the Death of the Chinese Soft-Shelled Turtle (Pelodiscus sinensis)" Veterinary Sciences 12, no. 5: 473. https://doi.org/10.3390/vetsci12050473
APA StyleXue, M., Hu, X., Jiang, N., Liu, W., Xiao, Z., Zhang, C., Wu, Y., Liang, T., Zhang, H., Fan, Y., Meng, Y., & Zhou, Y. (2025). A Multidrug-Resistant Escherichia coli Caused the Death of the Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Veterinary Sciences, 12(5), 473. https://doi.org/10.3390/vetsci12050473






