Investigating Stress and Coping Behaviors in African Green Monkeys (Chlorocebus aethiops sabaeus) Through Machine Learning and Multivariate Generalized Linear Mixed Models
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biological Sample Collection
2.2.1. Blood Collection
2.2.2. Saliva Collection
2.2.3. Hair Collection
2.3. Biological Sample Analysis
Hair Cortisol, β-Endorphin, and Lysozyme Analysis
2.4. Behavioral Observations
2.5. Statistical Analysis
2.5.1. Data Preparation
2.5.2. Assessing Effectiveness of Enrichment by Hair Cortisol Concentration
2.5.3. Assessing Effects of Enrichment and Behavior on Stress Biomarkers
3. Results
3.1. Descriptive Statistics
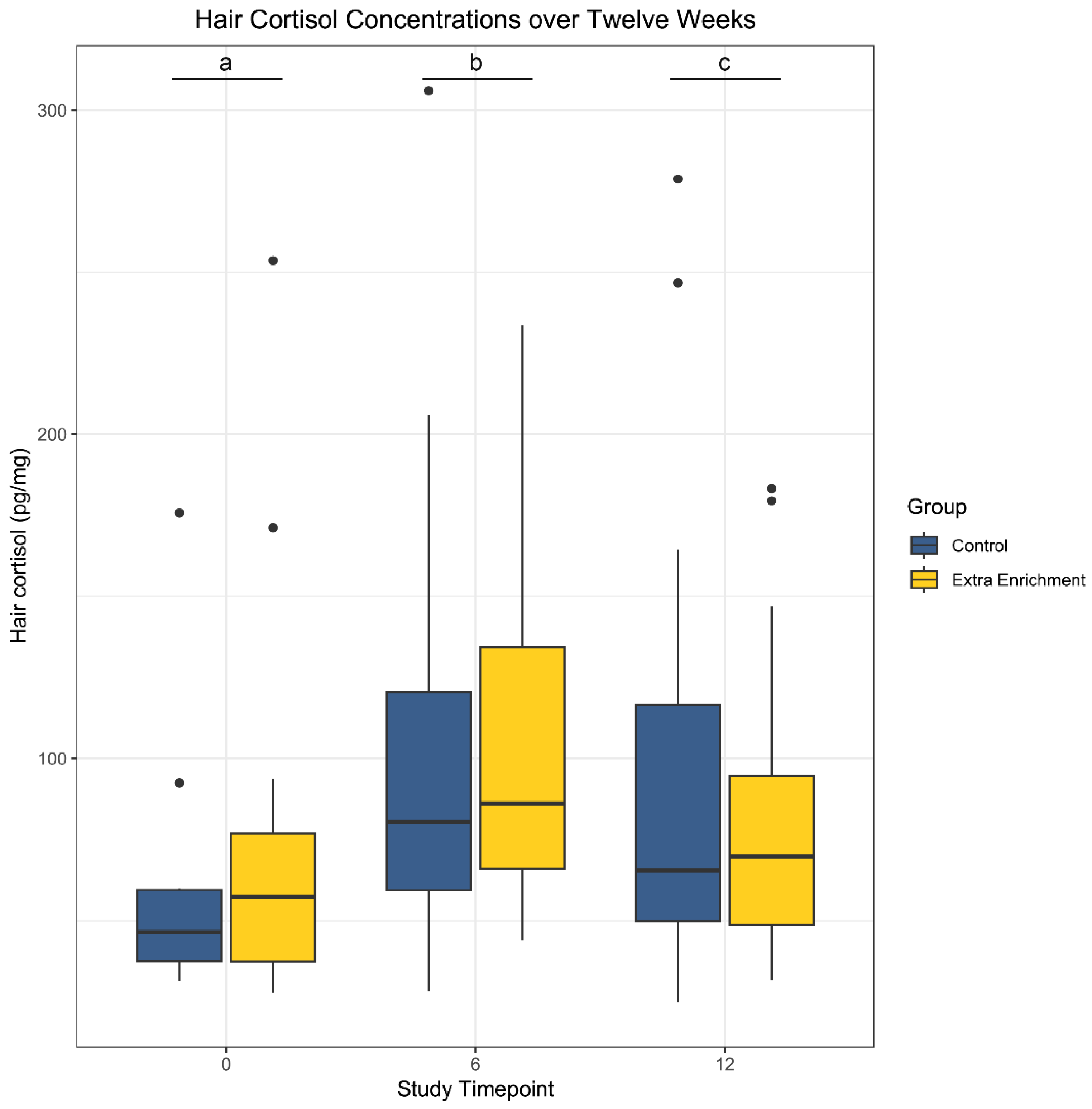
3.2. Hair Cortisol Concentrations
3.3. Principal Component Analysis of Behaviors
3.4. Behavioral Associations with Stress Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Ethogram
| Behavior | Description |
| Pace | Repeated locomotion, walking back and forth |
| Flip | Jumping and spinning forward/backward in midair |
| Drink | - |
| Eat | - |
| Sleep | - |
| Urination | - |
| Defecation | - |
| Vocalize—Chatter | Repetitive series of calls |
| Vocalize—Bark | Aggressive alarm call |
| Vocalize—Lip smack | Rapid, rhythmic movement of the lips with soft, popping sound |
| Reach for Neighbor | Extending past enclosure toward adjacent cohort |
| Stare at Observer | Extended eye contact with observer |
| Stare Ahead | Alert and watching but not at a specific object and with no movement |
| Look Around | Vigilantly examining surroundings with moving eyes/head rotation |
| Inspect Genitals | Looking closely at their own genital region |
| Groom | Cleaning/maintaining the body using the hands or mouth |
| Pick | Using fingers to investigate a spot |
| Scratch | Using hands or feet to rub/scrape skin |
| Enrichment Use | Interaction with the foraging board |
| Gape | Sustained wide-open mouth displaying teeth |
| Oral Exploration | Using mouth and tongue to manipulate/roam enclosure |
| Manual Exploration | Using hands to manipulate/roam enclosure |
| Fidget | Restless, repetitive movements (i.e., adjusting posture, tapping, shifting position) |
| Inactivity | Still, stationary, eyes downcast |
| Wiggle Ears | Rapid, small movement of the ears |
| Self-Play | Masturbation |
| Erect Penis | Displaying genitals in an aroused state |
| Cower | Submissive posture with lowered body, limbs close to the body, and/or hunched back |
| Pounce | Sudden quick movement where animal lunges forward |
| Grab | Attempting to seize an object |
| Yawn | Mouth opens widely with deep inhalation, followed by closing mouth |
| Head Twirl | Repetitive motion of the head sometimes referred to as head dance |
| Roll | Twisting body on bottom of the enclosure to perform a 360 degree rotation |
| Stretch | Extension of a limb for short period of time |
| Stare at Neighbor | Eye contact with adjacent cohort |
| Flee | Rapid, panicked movement away from observer |
| Eat Feces | Coprophagy |
| Sneeze | Sudden expulsion of air from the mouth and nostrils |
| Puff-Up Display | Expansion of the body, hair standing on end to appear larger |
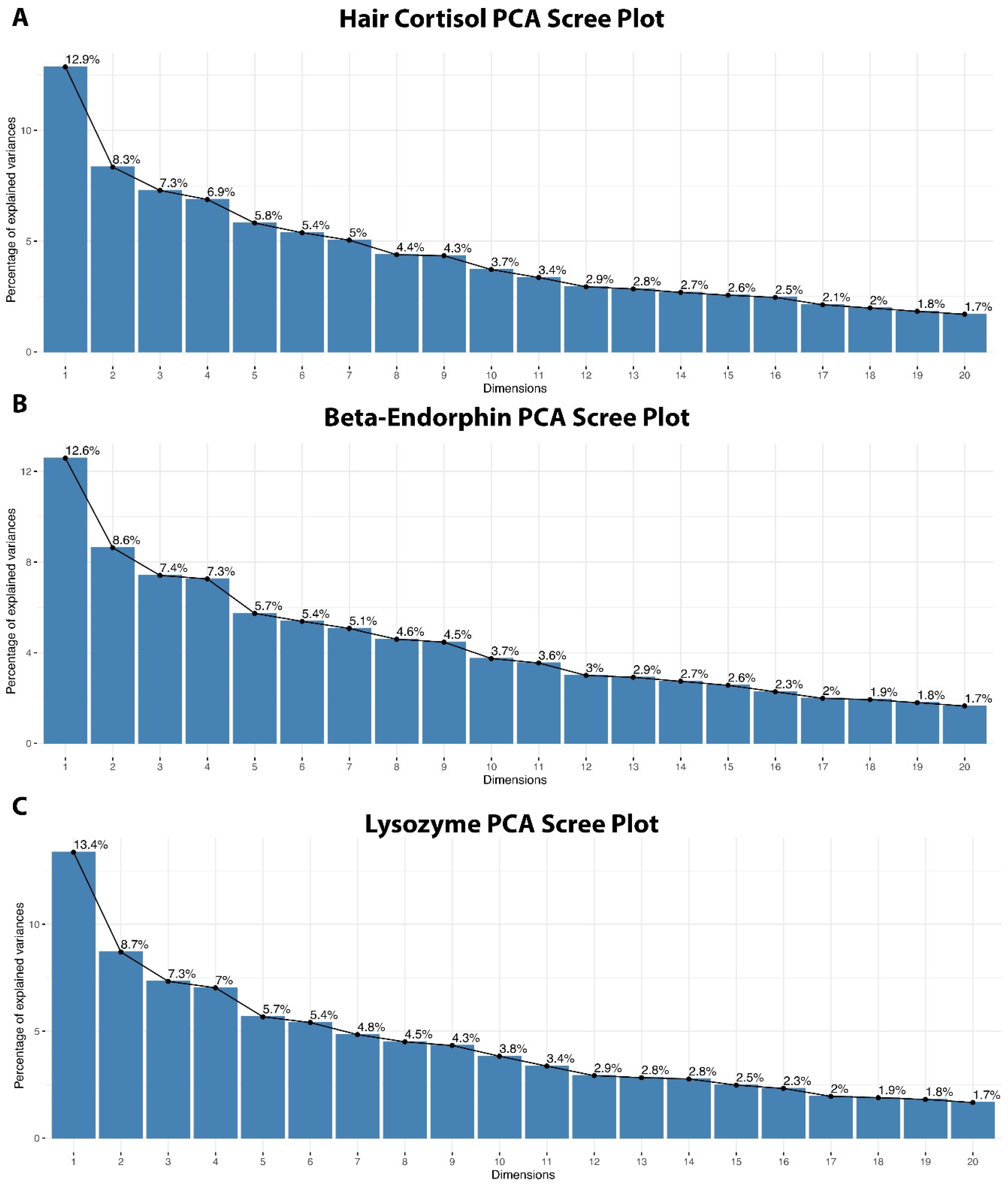
Appendix B. Eigenvalues, Percentage of Explained Variance for Each Principal Component, and the Cumulative Variance Explained for All Principal Components
| Hair Cortisol | |||
| eigenvalue | variance percent | cumulative variance | percent |
| Dim. 1 | 4.373180 × 100 | 1.286230 × 10¹ | 12.86230 |
| Dim. 2 | 2.836367 × 100 | 8.342257 × 100 | 21.20455 |
| Dim. 3 | 2.477107 × 100 | 7.285607 × 100 | 28.49016 |
| Dim. 4 | 2.338956 × 100 | 6.879282 × 100 | 35.36944 |
| Dim. 5 | 1.977292 × 100 | 5.815565 × 100 | 41.18501 |
| Dim. 6 | 1.828581 × 100 | 5.378179 × 100 | 46.56319 |
| Dim. 7 | 1.714059 × 100 | 5.041349 × 100 | 51.60453 |
| Dim. 8 | 1.490692 × 100 | 4.384389 × 100 | 55.98892 |
| Dim. 9 | 1.475160 × 100 | 4.338707 × 100 | 60.32763 |
| Dim. 10 | 1.262716 × 100 | 3.713870 × 100 | 64.04150 |
| Dim. 11 | 1.140611 × 100 | 3.354737 × 100 | 67.39624 |
| Dim. 12 | 9.985140 × 10−1 | 2.936806 × 100 | 70.33304 |
| Dim. 13 | 9.664956 × 10−1 | 2.842634 × 100 | 73.17568 |
| Dim. 14 | 9.126706 × 10−1 | 2.684325 × 100 | 75.86000 |
| Dim. 15 | 8.695998 × 10−1 | 2.557647 × 100 | 78.41765 |
| Dim. 16 | 8.360252 × 10−1 | 2.458898 × 100 | 80.87655 |
| Dim. 17 | 7.224518 × 10−1 | 2.124858 × 100 | 83.00141 |
| Dim. 18 | 6.737503 × 10−1 | 1.981618 × 100 | 84.98302 |
| Dim. 19 | 6.218685 × 10−1 | 1.829025 × 100 | 86.81205 |
| Dim. 20 | 5.772656 × 10−1 | 1.697840 × 100 | 88.50989 |
| Dim. 21 | 5.635752 × 10−1 | 1.657574 × 100 | 90.16746 |
| Dim. 22 | 5.368461 × 10−1 | 1.578959 × 100 | 91.74642 |
| Dim. 23 | 4.140269 × 10−1 | 1.217726 × 100 | 92.96415 |
| Dim. 24 | 3.785051 × 10−1 | 1.113250 × 100 | 94.07740 |
| Dim. 25 | 3.477195 × 10−1 | 1.022704 × 100 | 95.10010 |
| Dim. 26 | 3.229641 × 10−1 | 9.498943 × 10−1 | 96.05000 |
| Dim. 27 | 2.878527 × 10−1 | 8.466256 × 10−1 | 96.89662 |
| Dim. 28 | 2.520954 × 10−1 | 7.414571 × 10−1 | 97.63808 |
| Dim. 29 | 2.141251 × 10−1 | 6.297798 × 10−1 | 98.26786 |
| Dim. 30 | 1.870804 × 10−1 | 5.502364 × 10−1 | 98.81810 |
| Dim. 31 | 1.788896 × 10−1 | 5.261459 × 10−1 | 99.34424 |
| Dim. 32 | 1.264199 × 10−1 | 3.718233 × 10−1 | 99.71606 |
| Dim. 33 | 9.653793 × 10−2 | 2.839351 × 10−1 | 100.00000 |
| Dim. 34 | 1.421451 × 10−31 | 4.180737 × 10−31 | 100.00000 |
| β-endorphin | |||
| eigenvalue | variance percent | cumulative variance | percent |
| Dim. 1 | 4.279748 × 100 | 1.258749 × 10¹ | 12.58749 |
| Dim. 2 | 2.934928 × 100 | 8.632141 × 100 | 21.21963 |
| Dim. 3 | 2.517657 × 100 | 7.404874 × 100 | 28.62451 |
| Dim. 4 | 2.466634 × 100 | 7.254807 × 100 | 35.87932 |
| Dim. 5 | 1.947927 × 100 | 5.729197 × 100 | 41.60851 |
| Dim. 6 | 1.828326 × 100 | 5.377431 × 100 | 46.98594 |
| Dim. 7 | 1.722367 × 100 | 5.065785 × 100 | 52.05173 |
| Dim. 8 | 1.564152 × 100 | 4.600446 × 100 | 56.65217 |
| Dim. 9 | 1.520544 × 100 | 4.472189 × 100 | 61.12436 |
| Dim. 10 | 1.273914 × 100 | 3.746807 × 100 | 64.87117 |
| Dim. 11 | 1.207003 × 100 | 3.550009 × 100 | 68.42118 |
| Dim. 12 | 1.020432 × 100 | 3.001272 × 100 | 71.42245 |
| Dim. 13 | 9.925551 × 10−1 | 2.919280 × 100 | 74.34173 |
| Dim. 14 | 9.313611 × 10−1 | 2.739297 × 100 | 77.08103 |
| Dim. 15 | 8.719866 × 10−1 | 2.564666 × 100 | 79.64569 |
| Dim. 16 | 7.749164 × 10−1 | 2.279166 × 100 | 81.92486 |
| Dim. 17 | 6.764264 × 10−1 | 1.989489 × 100 | 83.91435 |
| Dim. 18 | 6.573791 × 10−1 | 1.933468 × 100 | 85.84782 |
| Dim. 19 | 6.113699 × 10−1 | 1.798147 × 100 | 87.64596 |
| Dim. 20 | 5.613037 × 10−1 | 1.650893 × 100 | 89.29686 |
| Dim. 21 | 5.176059 × 10−1 | 1.522370 × 100 | 90.81923 |
| Dim. 22 | 4.616193 × 10−1 | 1.357704 × 100 | 92.17693 |
| Dim. 23 | 4.087021 × 10−1 | 1.202065 × 100 | 93.37900 |
| Dim. 24 | 3.891853 × 10−1 | 1.144663 × 100 | 94.52366 |
| Dim. 25 | 3.355415 × 10−1 | 9.868868 × 10−1 | 95.51055 |
| Dim. 26 | 2.920089 × 10−1 | 8.588496 × 10−1 | 96.36940 |
| Dim. 27 | 2.606196 × 10−1 | 7.665282 × 10−1 | 97.13592 |
| Dim. 28 | 2.453875 × 10−1 | 7.217278 × 10−1 | 97.85765 |
| Dim. 29 | 2.010271 × 10−1 | 5.912563 × 10−1 | 98.44891 |
| Dim. 30 | 1.799797 × 10−1 | 5.293520 × 10−1 | 98.97826 |
| Dim. 31 | 1.637277 × 10−1 | 4.815522 × 10−1 | 99.45981 |
| Dim. 32 | 1.157071 × 10−1 | 3.403150 × 10−1 | 99.80013 |
| Dim. 33 | 6.795690 × 10−2 | 1.998732 × 10−1 | 100.00000 |
| Dim. 34 | 2.383994 × 10−31 | 7.011746 × 10−31 | 100.00000 |
| Lysozyme | |||
| eigenvalue | variance percent | cumulative variance | percent |
| Dim. 1 | 4.54613774 | 13.3709934 | 13.37099 |
| Dim. 2 | 2.95897179 | 8.7028582 | 22.07385 |
| Dim. 3 | 2.49274206 | 7.3315943 | 29.40545 |
| Dim. 4 | 2.39108524 | 7.0326036 | 36.43805 |
| Dim. 5 | 1.92960822 | 5.6753183 | 42.11337 |
| Dim. 6 | 1.83818528 | 5.4064273 | 47.51980 |
| Dim. 7 | 1.64612758 | 4.8415517 | 52.36135 |
| Dim. 8 | 1.53204624 | 4.5060183 | 56.86737 |
| Dim. 9 | 1.47361532 | 4.3341627 | 61.20153 |
| Dim. 10 | 1.30240830 | 3.8306127 | 65.03214 |
| Dim. 11 | 1.14546109 | 3.3690032 | 68.40114 |
| Dim. 12 | 0.99389777 | 2.9232287 | 71.32437 |
| Dim. 13 | 0.96212678 | 2.8297847 | 74.15416 |
| Dim. 14 | 0.94113320 | 2.7680388 | 76.92220 |
| Dim. 15 | 0.84308581 | 2.4796641 | 79.40186 |
| Dim. 16 | 0.79038943 | 2.3246748 | 81.72653 |
| Dim. 17 | 0.66477963 | 1.9552342 | 83.68177 |
| Dim. 18 | 0.64366601 | 1.8931353 | 85.57490 |
| Dim. 19 | 0.61563671 | 1.8106962 | 87.38560 |
| Dim. 20 | 0.56695915 | 1.6675269 | 89.05313 |
| Dim. 21 | 0.50938159 | 1.4981811 | 90.55131 |
| Dim. 22 | 0.45461576 | 1.3371052 | 91.88841 |
| Dim. 23 | 0.39410664 | 1.1591372 | 93.04755 |
| Dim. 24 | 0.37648575 | 1.1073110 | 94.15486 |
| Dim. 25 | 0.33654471 | 0.9898374 | 95.14470 |
| Dim. 26 | 0.30481138 | 0.8965041 | 96.04120 |
| Dim. 27 | 0.27165632 | 0.7989892 | 96.84019 |
| Dim. 28 | 0.23842921 | 0.7012624 | 97.54146 |
| Dim. 29 | 0.22271441 | 0.6550424 | 98.19650 |
| Dim. 30 | 0.18107016 | 0.5325593 | 98.72906 |
| Dim. 31 | 0.15716945 | 0.4622631 | 99.19132 |
| Dim. 32 | 0.11893874 | 0.3498198 | 99.54114 |
| Dim. 33 | 0.08444815 | 0.2483769 | 99.78952 |
| Dim. 34 | 0.07156437 | 0.2104835 | 100.00000 |
References
- Prescott, M.J.; Lidster, K. Improving quality of science through better animal welfare: The NC3Rs strategy. Lab Anim. 2017, 46, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Schapiro, S.J. Handbook of Primate Behavioral Management; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Shepherdson, D.J.; Mellen, J.D.; Hutchins, M. Second Nature: Environmental Enrichment for Captive Animals; Smithsonian Institution: Washington, DC, USA, 1999. [Google Scholar]
- Association of Zoos and Aquariums. The Accreditation Standards & Related Policies; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2022. [Google Scholar]
- Alligood, C.; Leighty, K. Putting the “E” in SPIDER: Evolving Trends in the Evaluation of Environmental Enrichment Efficacy in Zoological Settings. Anim. Behav. Cogn. 2015, 2, 200–217. [Google Scholar] [CrossRef]
- Mellen, J.; MacPhee, M. Philosophy of environmental enrichment: Past, present, and future. Zoo Biol. 2001, 20, 211–226. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral Diversity as a Potential Indicator of Positive Animal Welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Mason, G.; Latham, N.R. Can’t stop, won’t stop: Is stereotypy a reliable animal welfare indicator? Anim. Welf. 2004, 13, 57–69. [Google Scholar] [CrossRef]
- Jorgensen, M.J.; Lambert, K.R.; Breaux, S.D.; Baker, K.C.; Snively, B.M.; Weed, J.L. Pair housing of vervets/African green monkeys for biomedical research. Am. J. Primatol. 2017, 79, e22501. [Google Scholar] [CrossRef]
- Lutz, C.K.; Novak, M.A. Environmental Enrichment for Nonhuman Primates: Theory and Application. ILAR J. 2005, 46, 178–191. [Google Scholar] [CrossRef]
- Seier, J.; de Villiers, C.; van Heerden, J.; Laubscher, R. The effect of housing and environmental enrichment on stereotyped behavior of adult vervet monkeys (Chlorocebus aethiops). Lab Anim. 2011, 40, 218–224. [Google Scholar] [CrossRef]
- Winnicker, C.; Honess, P. Evaluating the Effectiveness of Environmental Enrichment. Lab. Anim. Sci. Prof. 2014, 2014, 16–20. [Google Scholar]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction. [Updated 7 May 2024]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541120/ (accessed on 23 October 2023).
- Singhal, G.; Baune, B.T. A Bibliometric Analysis of Studies on Environmental Enrichment Spanning 1967–2024: Patterns and Trends over the Years. Front. Behav. Neurosci. 2024, 18, 1501377. [Google Scholar] [CrossRef]
- Howell, C.P.; Cheyne, S.M. Complexities of Using Wild versus Captive Activity Budget Comparisons for Assessing Captive Primate Welfare. J. Appl. Anim. Welf. Sci. 2019, 22, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Browning, H. The Natural Behavior Debate: Two Conceptions of Animal Welfare. J. Appl. Anim. Welf. Sci. 2020, 23, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Lutz, C.K.; Coleman, K.; Hopper, L.M.; Novak, M.A.; Perlman, J.E.; Pomerantz, O. Nonhuman primate abnormal behavior: Etiology, assessment, and treatment. Am. J. Primatol. 2022, 84, e23380. [Google Scholar] [CrossRef]
- Špinka, M. How important is natural behaviour in animal farming systems? Appl. Anim. Behav. Sci. 2006, 100, 117–128. [Google Scholar] [CrossRef]
- Kim-McCormack, N.N.E.; Smith, C.L.; Behie, A.M. Is interactive technology a relevant and effective enrichment for captive great apes? Appl. Anim. Behav. Sci. 2016, 185, 1–8. [Google Scholar] [CrossRef]
- Chipangura, J.K.; Ganswindt, A.; Naidoo, V. A report on the housing vervet monkeys adjacent to domestic cats as a means of environmental enrichment. Onderstepoort J. Vet. Res. 2020, 87, e1–e6. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209. [Google Scholar] [CrossRef]
- Korte, S.M.; Olivier, B.; Koolhaas, J.M. A new animal welfare concept based on allostasis. Physiol. Behav. 2007, 92, 422–428. [Google Scholar] [CrossRef]
- Otovic, P.; Hutchinson, E. Limits to using HPA axis activity as an indication of animal welfare. ALTEX-Altern. Anim. Exp. 2015, 32, 41–50. [Google Scholar] [CrossRef]
- Rushen, J. Problems associated with the interpretation of physiological data in the assessment of animal welfare. Appl. Anim. Behav. Sci. 1991, 28, 381–386. [Google Scholar] [CrossRef]
- Van Reenen, C.G.; O’Connell, N.E.; Van der Werf, J.T.; Korte, S.M.; Hopster, H.; Jones, R.B.; Blokhuis, H.J. Responses of calves to acute stress: Individual consistency and relations between behavioral and physiological measures. Physiol. Behav. 2005, 85, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.M.; Fraser, A.F. Domestic Animal Behaviour and Welfare; CABI: Wallingford, UK, 2015. [Google Scholar]
- Ivković, N.; Božović, Đ.; Račić, M.; Popović, G.D.; Davidović, B. Biomarkers of stress in saliva. Acta Fac. Medicae Naissensis 2015, 32, 91–99. [Google Scholar] [CrossRef]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Sandman, C.A.; Touchette, P.; Lenjavi, M.; Marion, S.; Chicz-DeMet, A. β-Endorphin and ACTH are dissociated after self-injury in adults with developmental disabilities. Am. J. Ment. Retard. 2003, 108, 414–424. [Google Scholar] [CrossRef]
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-endorphin in stress, behavior, neuroinflammation, and brain energy metabolism. Int. J. Mol. Sci. 2020, 22, 338. [Google Scholar] [CrossRef]
- Veening, J.G.; Barendregt, H.P. The effects of beta-endorphin: State change modification. Fluids Barriers CNS 2015, 12, 3. [Google Scholar] [CrossRef]
- Lee, M.; Wardlaw, S.L. Beta-Endorphin. In Encyclopedia of Stress, 2nd ed.; Fink, G., Ed.; Academic Press: New York, NY, USA, 2007; pp. 332–335. [Google Scholar]
- Jackson, M.; Foret, B.L.; Fontenot, J.; Hasselschwert, D.; Smith, J.; Romero, E.; Smith, K.M. Molecular examination of the endogenous opioid system in rhesus macaque monkeys with self-injurious behavior. J. Neurosci. Res. 2023, 101, 70–85. [Google Scholar] [CrossRef]
- Crockett, C.M.; Sackett, G.P.; Sandman, C.A.; Chicz-DeMet, A.; Bentson, K.L. Beta-endorphin levels in longtailed and pigtailed macaques vary by abnormal behavior rating and sex. Peptides 2007, 28, 1987–1997. [Google Scholar] [CrossRef]
- Goodwin, G.; Austin, M.; Curran, S.; Ross, M.; Murray, C.; Prentice, N.; Ebmeier, K.; Bennie, J.; Carroll, S.; Dick, H. The elevation of plasma β-endorphin levels in major depression. J. Affect. Disord. 1993, 29, 281–289. [Google Scholar] [CrossRef]
- Roman, B.; Hooper, S.; Beierschmitt, A.; Gallagher, C.A. An Opportunistic Study to Assess African Green Monkey (Chlorocebus sabaeus aethiops) Welfare Using Novel and Classical Biomarkers. J. Am. Assoc. Lab. Anim. Sci. 2025, in press. [Google Scholar]
- Slominski, R.; Rovnaghi, C.R.; Anand, K.J. Methodological Considerations for Hair Cortisol Measurements in Children. Ther. Drug Monit. 2015, 37, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Plessow, F.; Rauh, M.; Gröschl, M.; Kirschbaum, C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology 2013, 38, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Holstein, C.A.; Griffin, M.; Hong, J.; Sampson, P.D. Statistical method for determining and comparing limits of detection of bioassays. Anal. Chem. 2015, 87, 9795–9801. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Paterson, J.D.; Vandenbeld, D. Primate Behavior: An Exercise Workbook, 2nd ed.; Waveland: Prospect Heights, IL, USA, 2001; pp. 144–147. [Google Scholar]
- Wirth, O.; Slaven, J.; Taylor, M.A. Interval sampling methods and measurement error: A computer simulation. J. Appl. Behav. Anal. 2014, 47, 83–100. [Google Scholar] [CrossRef]
- Delgado, R.R.; Delgado, J.M.R. An objective approach to measurement of behavior. Philos. Sci. 1962, 29, 253–268. [Google Scholar] [CrossRef]
- Gilliland-Lloyd, A.I. Variation in Stress Among Vervet Monkeys (Chlorocebus pygerythrus) at Different Stages of Rehabilitation in South Africa. Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation; R Package Version 1.1.4.; Posit PBC: Boston, MA, USA, 2023; Available online: https://github.com/tidyverse/dplyr (accessed on 23 October 2023).
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research; Northwestern University: Evanston, IL, USA, 2023. [Google Scholar]
- Seo, S. A Review and Comparison of Methods for Detecting Outliers in Univariate Data Sets. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2006. [Google Scholar]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Bates, D.M.M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.B.P.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 23 October 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.10.7. 2025. Available online: https://rvlenth.github.io/emmeans/ (accessed on 23 October 2023).
- Hadfield, J.D. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Houslay, T.M.; Wilson, A.J. Avoiding the misuse of BLUP in behavioural ecology. Behav. Ecol. 2017, 28, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Kluen, E.; Rönkä, K.; Thorogood, R. Prior experience of captivity affects behavioural responses to ‘novel’ environments. PeerJ 2022, 10, e13905. [Google Scholar] [CrossRef]
- MicroBatVet. MicroBatVet/AGMbehavior_biochem: Release for Zenodo, v1.0.1. 2024. [CrossRef]
- Melfi, V.; Feistner, A. A Comparison of the Activity Budgets of Wild and Captive Sulawesi Crested Black Macaques (Macaca nigra). Anim. Welf. 2002, 11, 213–222. [Google Scholar] [CrossRef]
- Budaev, S.V. Using principal components and factor analysis in animal behaviour research: Caveats and guidelines. Ethology 2010, 116, 472–480. [Google Scholar] [CrossRef]
- Gorsuch, R.L. Factor Analysis, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1983. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 3rd ed.; Harper & Row: New York, NY, USA, 1996. [Google Scholar]
- Bharadiya, J.P. A tutorial on principal component analysis for dimensionality reduction in machine learning. Int. J. Innov. Sci. Res. Technol. 2023, 8, 2028–2032. [Google Scholar]
- DeVries, A.C.; Glasper, E.R.; Detillion, C.E. Social modulation of stress responses. Physiol. Behav. 2003, 79, 399–407. [Google Scholar] [CrossRef]
- Kavanagh, E.; Kimock, C.; Whitehouse, J.; Micheletta, J.; Waller, B.M. Revisiting Darwin’s comparisons between human and non-human primate facial signals. Evol. Hum. Sci. 2022, 4, e27. [Google Scholar] [CrossRef]
- Poirier, C.; Bateson, M. Pacing stereotypies in laboratory rhesus macaques: Implications for animal welfare and the validity of neuroscientific findings. Neurosci. Biobehav. Rev. 2017, 83, 508–515. [Google Scholar] [CrossRef]
- Pomerantz, O.; Terkel, J.; Suomi, S.J.; Paukner, A. Stereotypic head twirls, but not pacing, are related to a ‘pessimistic’-like judgment bias among captive tufted capuchins (Cebus apella). Anim. Cogn. 2012, 15, 689–698. [Google Scholar] [CrossRef]
| Hair Cortisol | Week 0 (kg) | Week 6 (kg) | Week 12 (kg) |
|---|---|---|---|
| CG | 5.6 ± 1.1 | 5.3 ± 0.7 * | 5.3 ± 0.7 + |
| EG | 5.7 ± 0.8 | 5.2 ± 0.7 * | 5.2 ± 0.8 + |
| Hair Cortisol | Post. Mean | Lower 95% Confidence Interval | Upper 95% Confidence Interval | Eff. Samp | pMCMC |
|---|---|---|---|---|---|
| (Intercept) | 1.677882 | 1.549845 | 1.795571 | 10,000 | <1 × 10−4 * |
| PC4 | −0.027003 | −0.057058 | 0.004783 | 10,000 | 0.0898 |
| PC7 | −0.037074 | −0.073222 | −0.002166 | 10,000 | 0.0404 * |
| β-Endorphin | Post. Mean | Lower 95% Confidence Interval | Upper 95% Confidence Interval | Eff. Samp | pMCMC |
|---|---|---|---|---|---|
| (Intercept) | 2069.874 | 1636.65 | 2467.752 | 2000 | <5 × 10−4 * |
| PC4 | 60.3249 | −0.6442 | 123.799 | 2000 | 0.052 |
| PC6 | 70.1278 | −0.4766 | 147.6484 | 2000 | 0.058 |
| PC11 | −104.818 | −201.881 | −2.7427 | 2000 | 0.035 * |
| PC13 | −122.691 | −232.863 | −18.7865 | 2606 | 0.025 * |
| PC26 | −174.213 | −363.973 | 22.1145 | 2000 | 0.078 |
| PC31 | 322.7265 | 68.517 | 581.9805 | 2122 | 0.02 * |
| Lysozyme | Post. Mean | Lower 95% Confidence Interval | Upper 95% Confidence Interval | Eff. Samp | pMCMC |
|---|---|---|---|---|---|
| (Intercept) | 1.169086 | 0.970994 | 1.380056 | 2000 | <5 × 10−4 * |
| PC11 | 0.058574 | −0.00726 | 0.128076 | 2000 | 0.091 |
| PC15 | 0.083078 | 0.013917 | 0.17204 | 1744 | 0.041 * |
| (a) | |||||
| Hair Cortisol | |||||
| PC7 | |||||
| Post. Mean | −0.037074 | ||||
| Inspect Genitals | 0.3445 | ||||
| Groom | 0.2506 | ||||
| Flee | 0.2439 | ||||
| Manual Exploration | 0.2348 | ||||
| Oral Exploration | −0.2113 | ||||
| Vocalize: Chatter | −0.2119 | ||||
| Eat Feces | −0.2999 | ||||
| Enrichment Use | −0.3175 | ||||
| (b) | |||||
| β-Endorphin | |||||
| PC13 | PC31 | PC 11 | |||
| Post. Mean | −122.691 | Post. Mean | 322.7265 | Post. Mean | −104.818 |
| Vocalize: Bark | 0.4112 | Drink | 0.4322 | Self-Play | 0.3823 |
| Stare Ahead | 0.2516 | Scratch | 0.4263 | Scratch | 0.3530 |
| Scratch | 0.2420 | Pounce | 0.2155 | Wiggle Ears | 0.3250 |
| Drink | 0.2249 | Pick | 0.2138 | Cower | 0.3076 |
| Wiggle Ears | −0.1689 | Flee | −0.1968 | Stare at Neighbor | −0.2033 |
| Stare at Observer | −0.2144 | Inspect Genitals | −0.2171 | Inspect Genitals | −0.1950 |
| Roll | −0.2422 | Defecation | −0.2586 | Urination | −0.2719 |
| Reach for Neighbor | −0.5376 | Pace | −0.3407 | Vocalize: Bark | −0.3739 |
| (c) | |||||
| Lysozyme | |||||
| PC15 | |||||
| Post. Mean | 0.083078 | ||||
| Inactivity | 0.3911 | ||||
| Vocalize: Bark | 0.3707 | ||||
| Self-Play | 0.3056 | ||||
| Roll | 0.3052 | ||||
| Stare Ahead | −0.1615 | ||||
| Gape | −0.1796 | ||||
| Cower | −0.2174 | ||||
| Flee | −0.3265 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roman, B.; Gallagher, C.; Beierschmitt, A.; Hooper, S. Investigating Stress and Coping Behaviors in African Green Monkeys (Chlorocebus aethiops sabaeus) Through Machine Learning and Multivariate Generalized Linear Mixed Models. Vet. Sci. 2025, 12, 209. https://doi.org/10.3390/vetsci12030209
Roman B, Gallagher C, Beierschmitt A, Hooper S. Investigating Stress and Coping Behaviors in African Green Monkeys (Chlorocebus aethiops sabaeus) Through Machine Learning and Multivariate Generalized Linear Mixed Models. Veterinary Sciences. 2025; 12(3):209. https://doi.org/10.3390/vetsci12030209
Chicago/Turabian StyleRoman, Brittany, Christa Gallagher, Amy Beierschmitt, and Sarah Hooper. 2025. "Investigating Stress and Coping Behaviors in African Green Monkeys (Chlorocebus aethiops sabaeus) Through Machine Learning and Multivariate Generalized Linear Mixed Models" Veterinary Sciences 12, no. 3: 209. https://doi.org/10.3390/vetsci12030209
APA StyleRoman, B., Gallagher, C., Beierschmitt, A., & Hooper, S. (2025). Investigating Stress and Coping Behaviors in African Green Monkeys (Chlorocebus aethiops sabaeus) Through Machine Learning and Multivariate Generalized Linear Mixed Models. Veterinary Sciences, 12(3), 209. https://doi.org/10.3390/vetsci12030209






