Seroprevalence of West Nile Fever and Associated Risk Factors in Livestock of Afar Region, Northeast Ethiopia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
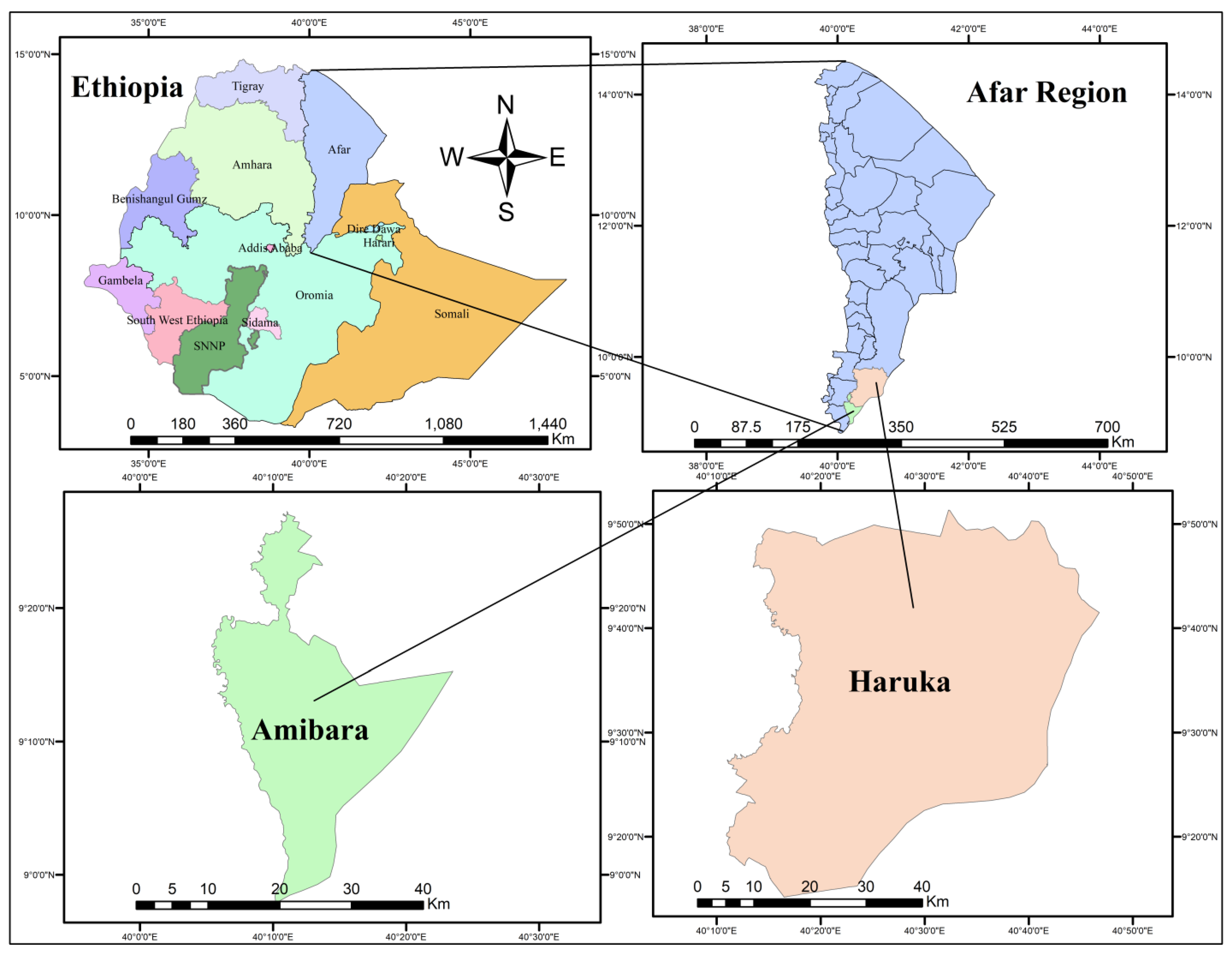
2.1. Description of the Study Area
2.2. Study Design
2.3. Study Animals
2.4. Sample Size Determination and Sampling Method
2.5. Sampling Procedure and Data Collection
2.6. Serological Analysis
2.7. Ethical Considerations
2.8. Data Analysis
3. Results
3.1. Description of Study Participants and Animals
3.2. Seroprevalence of WNFV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faulde, M.K.; Rueda, L.M.; Khaireh, B.A. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 2014, 139, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. Hyg. 1940, 20, 471–472. [Google Scholar] [CrossRef]
- MacIntyre, C.; Lourens, C.; Mendes, A.; de Villiers, M.; Avenant, T.; du Plessis, N.M.; Leendertz, F.H.; Venter, M. West Nile Virus, an Underdiagnosed Cause of Acute Fever of Unknown Origin and Neurological Disease among Hospitalized Patients in South Africa. Viruses 2023, 15, 2207. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.N.; Yasmin, A.R.; Ramanoon, S.Z.; Noraniza, M.A.; Ooi, P.T.; Ain-Najwa, M.Y.; Natasha, J.A.; Nur-Fazila, S.H.; Arshad, S.S.; Mohammed, H.O. Serological and molecular surveillance of West Nile virus in domesticated mammals of peninsular Malaysia. Front. Vet. Sci. 2023, 10, 1126199. [Google Scholar] [CrossRef] [PubMed]
- Sule, W.F.; Oluwayelu, D.O.; Hernández-Triana, L.M.; Fooks, A.R.; Venter, M.; Johnson, N. Epidemiology and ecology of West Nile virus in sub-Saharan Africa. Parasites Vectors 2018, 11, 414. [Google Scholar] [CrossRef]
- Abdullahi, I.N.; Emeribe, A.U.; Ghamba, P.E.; Omosigho, P.O.; Bello, Z.M.; Oderinde, B.S.; Fasogbon, S.A.; Olayemi, L.; Daneji, I.M.; Musa, M.H.; et al. Distribution pattern and prevalence of West Nile virus infection in Nigeria from 1950 to 2020: A systematic review. Epidemiol Health 2020, 42, e2020071. [Google Scholar] [CrossRef]
- Alzuheir, I.; Fayyad, A.; Jalboush, N.; Abdallah, R.; Abutarbush, S.; Gharaibeh, M.; Bdarneh, M.; Khraim, N.; Helal, M.A.; Helal, B.A. Seroprevalence and risk factors of West Nile virus infection in veterinarians and horses in Northern Palestine. Vet. World 2021, 14, 1241. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Conway, M.J.; Montgomery, R.R.; Fikrig, E. West Nile Virus: Biology, transmission, and human infection. Clin. Microbiol. Rev. 2012, 25, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Thompson, E.A.; Vig, P.J.S.; Leis, A.A. Current Understanding of West Nile Virus Clinical Manifestations, Immune Responses, Neuroinvasion, and Immunotherapeutic Implications. Pathogens 2019, 8, 193. [Google Scholar] [CrossRef]
- Seid, M.; Aklilu, E.; Animut, A. Spatio-temporal occurrence and habitat characteristics of Aedes aegypti (Diptera: Culicidae) larvae in Southern Afar region, Ethiopia. Trop. Med. Health 2024, 52, 51. [Google Scholar] [CrossRef]
- Asebe, G.; Mamo, G.; Michlmayr, D.; Abegaz, W.E.; Endale, A.; Medhin, G.; Larrick, J.W.; Legesse, M. Seroprevalence of Rift Valley Fever and West Nile Fever in Cattle in Gambella Region, South West Ethiopia. Vet. Med. 2020, 11, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Endale, A.; Michlmayr, D.; Abegaz, W.E.; Geda, B.; Asebe, G.; Medhin, G.; Larrick, J.W.; Legesse, M. Seroprevalence of West Nile virus and Rift Valley fever virus infections among cattle under extensive production systems in South Omo area, Southern Ethiopia. Trop. Anim. Health Prod. 2021, 53, 92. [Google Scholar] [CrossRef] [PubMed]
- Zerfu, B.; Medhin, G.; Mamo, G.; Getahun, G.; Tschopp, R.; Legesse, M. Community-based prevalence of typhoid fever, typhus, brucellosis, and malaria among symptomatic individuals in Afar Region, Ethiopia. PLoS Negl. Trop. Dis. 2018, 12, e0006749. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMJ Open Sci. 2020, 4, e100115. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.-J.J. Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 2005, 18, 608–637. [Google Scholar] [CrossRef]
- Llorente, F.; Gutierrez-Lopez, R.; Perez-Ramirez, E.; Sanchez-Seco, M.P.; Herrero, L.; Jimenez-Clavero, M.A.; Vazquez, A. Experimental infections in red-legged partridges reveal differences in host competence between West Nile and Usutu virus strains from Southern Spain. Front. Cell Infect. Microbiol. 2023, 13, 1163467. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Abdelhady, A. The first detection of anti-West Nile virus antibodies in domestic ruminants in Egypt. Trop. Anim. Health Prod. 2020, 52, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, A.; Ferguson, H.H.; Mendez-Sanchez, J.D.; Danis-Lozano, R.; Casas-Martinez, M.; Bond, J.G.; Garcia-Zebadua, J.C.; Orozco-Bonilla, A.; Juarez-Ordaz, J.A.; Farfan-Ale, J.A.; et al. West Nile virus activity in mosquitoes and domestic animals in Chiapas, Mexico. Vector Borne Zoonotic Dis. 2009, 9, 555–560. [Google Scholar] [CrossRef]
- Idoko, I.S.; Schvartz, G.; Tirosh-Levy, S.; Erster, O.; Jibril, J.Y.; Adamu, A.M.; Enem, S.I.; Omeje, J.N.; Nafarnda, W.D.; Steinman, A. West Nile virus neutralizing antibody prevalence in donkeys from northern Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 566–568. [Google Scholar] [CrossRef]
- Molini, U.; Franzo, G.; Nel, H.; Khaiseb, S.; Ntahonshikira, C.; Chiwome, B.; Baines, I.; Madzingira, O.; Monaco, F.; Savini, G.; et al. West Nile virus seroprevalence in a selected donkey population of Namibia. Front. Vet. Sci. 2021, 8, 681354. [Google Scholar] [CrossRef]
- Hassanien, R.T.; Hussein, H.A.; Abdelmegeed, H.K.; Abdelwahed, D.A.; Khattab, O.M.; Ali, M.H.; Habashi, A.R.; Ibraheem, E.M.; Shahein, M.A.; Abohatab, E.M. West Nile virus: The current situation in Egypt. Vet. World 2023, 16, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Azmi, K.; Tirosh-Levy, S.; Manasrah, M.; Mizrahi, R.; Nasereddin, A.; Al-Jawabreh, A.; Ereqat, S.; Abdeen, Z.; Lustig, Y.; Gelman, B.; et al. West Nile Virus: Seroprevalence in animals in Palestine and Israel. Vector Borne Zoonotic Dis. 2017, 17, 558–566. [Google Scholar] [CrossRef]
- Mehmet, K.; Sibel, G.; Orhan, Y.; Nuri, M.; Sibel, Y.; Sibel, H.; Oya, B.; Metin, G. Serological investigation of West Nile Virus infection in domestic horses and donkeys in Turkey. J. Anim. Vet. Adv. 2017, 16, 51–54. [Google Scholar]
- Gangoso, L.; Aragones, D.; Martinez-de la Puente, J.; Lucientes, J.; Delacour-Estrella, S.; Estrada Pena, R.; Montalvo, T.; Bueno-Mari, R.; Bravo-Barriga, D.; Frontera, E.; et al. Determinants of the current and future distribution of the West Nile virus mosquito vector Culex pipiens in Spain. Environ. Res. 2020, 188, 109837. [Google Scholar] [CrossRef]
- Wegdan, H.A.; Rayan, M.A.; Mohamed, S.I.; Mutwakil, S.M.; Alsarraj, S.M.A. Seroprevalence of West Nile Virus in Equine and Chickens in Sudan. J. Vet. Sci. Dairy. Poult. Res. 2022, 1, 1–6. [Google Scholar]
- Davoust, B.; Maquart, M.; Roqueplo, C.; Gravier, P.; Sambou, M.; Mediannikov, O.; Leparc-Goffart, I. Serological survey of West Nile virus in domestic animals from Northwest Senegal. Vector Borne Zoonotic Dis. 2016, 16, 359–361. [Google Scholar] [CrossRef]
- Baba, S.S.; NNnadi, O.D.; Hamman, K.D.; Saidu, A.; El Yuguda, A.; Oderinde, B.S. Preliminary study on the prevalence of West Nile virus antibody among horses, donkeys, and camels in Borno State, Nigeria. J. Appl. Virol. 2014, 3, 39–45. [Google Scholar] [CrossRef]
- Erol, N.; Gürçay, M.; Kırdar, S.; Ertuğrul, B.; Gür, S.; Koç, B.; Tan, M. A serological investigation of West Nile virus infections in various animal species and humans in Western Turkey. Isr. J. Vet. Med. 2016, 71, 42–46. [Google Scholar]
- Olaleye, O.; Omilabu, S.; Ilomechina, E.; Fagbami, A. A survey for haemagglutination-inhibiting antibody to West Nile virus in human and animal sera in Nigeria. Comp. Immunol. Microbiol. Infect. Dis. 1990, 13, 35–39. [Google Scholar] [CrossRef]
- Paz, S. Climate change impacts on West Nile virus transmission in a global context. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20130561. [Google Scholar] [CrossRef]
| Study Animals | Frequency | Proportion (%) |
|---|---|---|
| Species | ||
| Camel | 155 | 21.1 |
| Cattle | 224 | 30.4 |
| Donkey | 92 | 12.5 |
| Goat | 121 | 16.4 |
| Sheep | 144 | 19.6 |
| Sex | ||
| Female | 582 | 79.1 |
| Male | 154 | 20.9 |
| District | ||
| Amibara | 421 | 57.2 |
| Haruka | 315 | 42.8 |
| Variable | Category | No. of Animals Tested | No. Positive | Prevalence (%) | 95% CI | χ2 | p Value |
|---|---|---|---|---|---|---|---|
| Camel | 155 | 107 | 69.3 | 60.2–75.4 | |||
| Cattle | 224 | 117 | 52.2 | 45.7–59.2 | |||
| Species | Donkey | 92 | 70 | 76.1 | 65.7–84.2 | 93.171 | 0.000 |
| Goat | 121 | 42 | 34.7 | 23.5–40.8 | |||
| Sheep | 144 | 37 | 25.7 | 20.2–35.3 | |||
| Sex | Female | 582 | 298 | 51.2 | 46.6–57.0 | 0.305 | 0.322 |
| Male | 154 | 75 | 48.7 | 39.5–55.3 | |||
| District | Amibara | 421 | 216 | 51.3 | 46.7–57.2 | 0.155 | 0.694 |
| Haruka | 315 | 157 | 49.8 | 37.5–53.5 | |||
| <2 years | 74 | 41 | 55.4 | 51.3–60.3 | |||
| Age | 2 to <5 years | 208 | 114 | 54.8 | 50.9–57.1 | 3.629 | 0.092 |
| 5 to <10 years | 332 | 157 | 47.3 | 44.2–52.4 | |||
| >10 years | 122 | 61 | 50.0 | 47.8–55.1 | |||
| Overall | 736 | 373 | 50.7 | 47–54.4 |
| Variable | Category | No. of Sera Tested | No. of Positive Sera | Prevalence (%) | COR (95% CI) | AOR (95% CI) |
|---|---|---|---|---|---|---|
| Species | Camel | 155 | 107 | 69.3 | 4.193 (2.528–6.955) | 3.162 (1.973–5.067) |
| Cattle | 224 | 117 | 52.2 | 2.039 (1.327–3.133) | 1.515 (1.038–2.212) | |
| Donkey | 92 | 70 | 76.1 | 6.447 (3.888–10.688) | 4.937 (3.076–7.925) | |
| Goat | 121 | 42 | 34.7 | 0.701 (0.389–1.261) | 0.477 (0.275–0.828) | |
| Sheep | 144 | 37 | 25.7 | Ref. | Ref. | |
| Sex | Female | 582 | 298 | 51.2 | 1.105 (0.775–1.577) | 1.174 (0.802–1.718) |
| Male | 154 | 75 | 48.7 | Ref. | Ref. | |
| Age | <2 years | 74 | 41 | 55.4 | 1.024 (0.601–1.747) | 0.475 (0.318–0.707) |
| 2 to <5 years | 208 | 114 | 54.8 | 1.385 (0.835–2.298) | 0.576 (0.401–0.828) | |
| 5 to <10 years | 332 | 157 | 47.3 | 1.242 (0.656–2.218) | 0.504 (0.314–0.811) | |
| >10 years | 122 | 61 | 50.0 | Ref. | Ref. | |
| District | Amibara | 421 | 216 | 51.3 | 1.006 (0.807–1.255) | 1.086 (0.793–1.486) |
| Haruka | 315 | 157 | 49.8 | Ref. | Ref. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megenas, J.A.; Dadi, M.L.; Mekonnen, T.K.; Larrick, J.W.; Kassa, G.M. Seroprevalence of West Nile Fever and Associated Risk Factors in Livestock of Afar Region, Northeast Ethiopia. Vet. Sci. 2025, 12, 141. https://doi.org/10.3390/vetsci12020141
Megenas JA, Dadi ML, Mekonnen TK, Larrick JW, Kassa GM. Seroprevalence of West Nile Fever and Associated Risk Factors in Livestock of Afar Region, Northeast Ethiopia. Veterinary Sciences. 2025; 12(2):141. https://doi.org/10.3390/vetsci12020141
Chicago/Turabian StyleMegenas, Jemberu Alemu, Mengistu Legesse Dadi, Tesfu Kassa Mekonnen, James W. Larrick, and Gezahegne Mamo Kassa. 2025. "Seroprevalence of West Nile Fever and Associated Risk Factors in Livestock of Afar Region, Northeast Ethiopia" Veterinary Sciences 12, no. 2: 141. https://doi.org/10.3390/vetsci12020141
APA StyleMegenas, J. A., Dadi, M. L., Mekonnen, T. K., Larrick, J. W., & Kassa, G. M. (2025). Seroprevalence of West Nile Fever and Associated Risk Factors in Livestock of Afar Region, Northeast Ethiopia. Veterinary Sciences, 12(2), 141. https://doi.org/10.3390/vetsci12020141






