Immunogenicity and Tolerance of BNT162b2 mRNA Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethic Statement
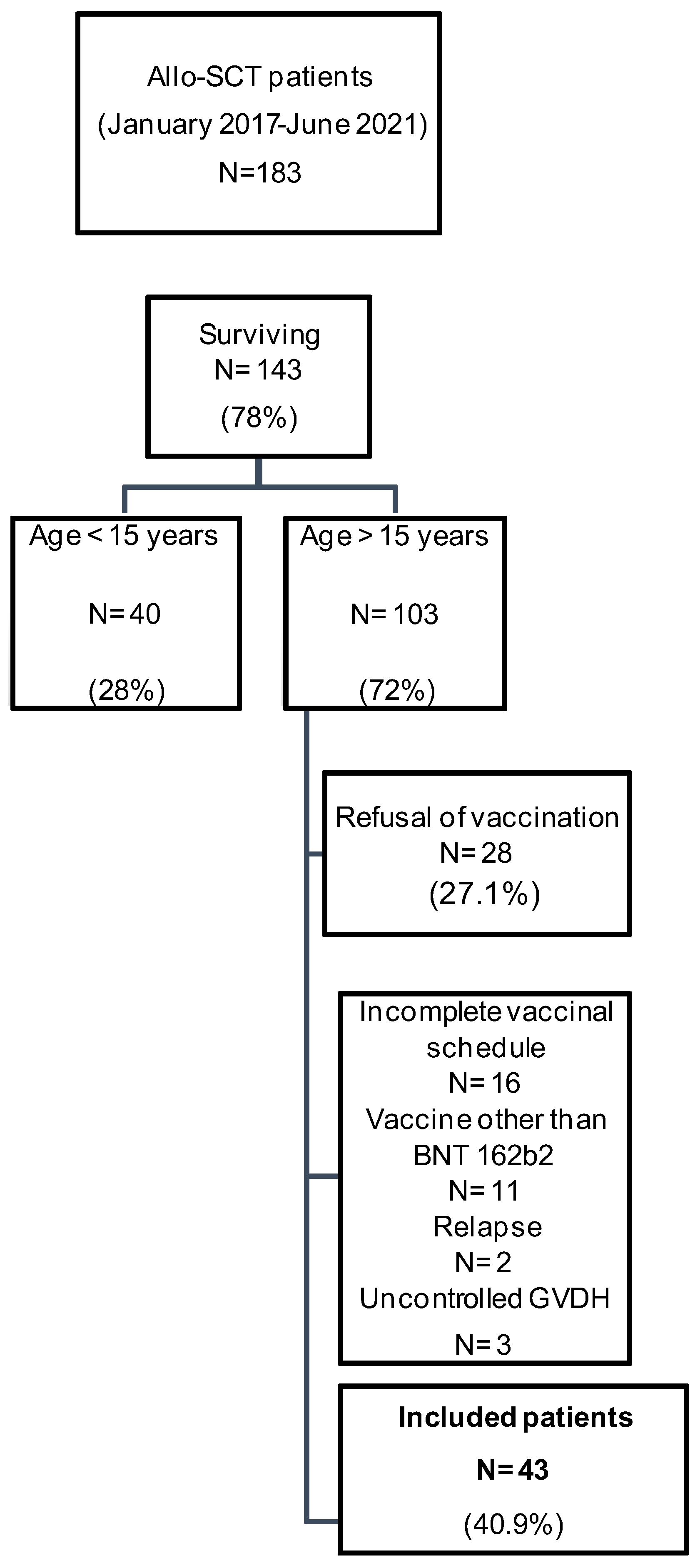
2.2. Study Population
- Eligibility for vaccination: aged over 15 years and at least 3 months post-ASCT without uncontrolled GVHD*. *Chronic GVHD is considered active when there is an evolving symptomatology that varies depending on the affected organ (for example, wheezing dyspnea in pulmonary GVHD, cytolytic and cholestatic hepatopathy in hepatic GVHD, etc.). It is classified as uncontrolled if clinical and paraclinical manifestations do not improve or worsen despite an optimal immunosuppressive treatment.
- Having received two doses of anti-SARS-CoV-2 BNT162b2 mRNA vaccine.
- Providing their written, free, and informed consent to participate in the study.
2.3. Sampling
2.4. Peripheral Anti-N and Anti-S Antibodies Measurement
2.5. Cellular Immunity Analyses
2.6. Statistical Analysis
3. Results
3.1. Study Population Description
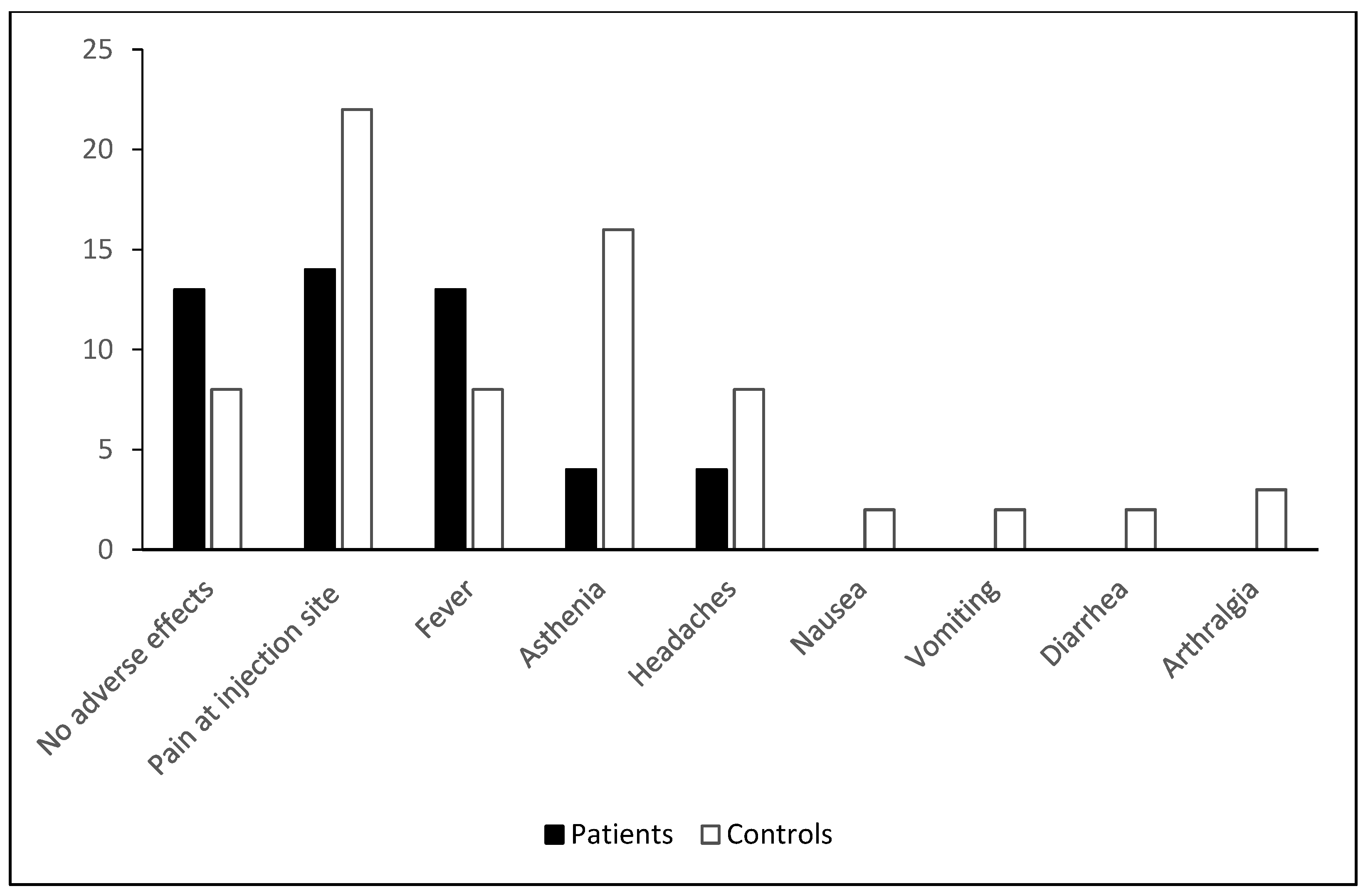
3.2. Clinical Tolerance of Vaccination
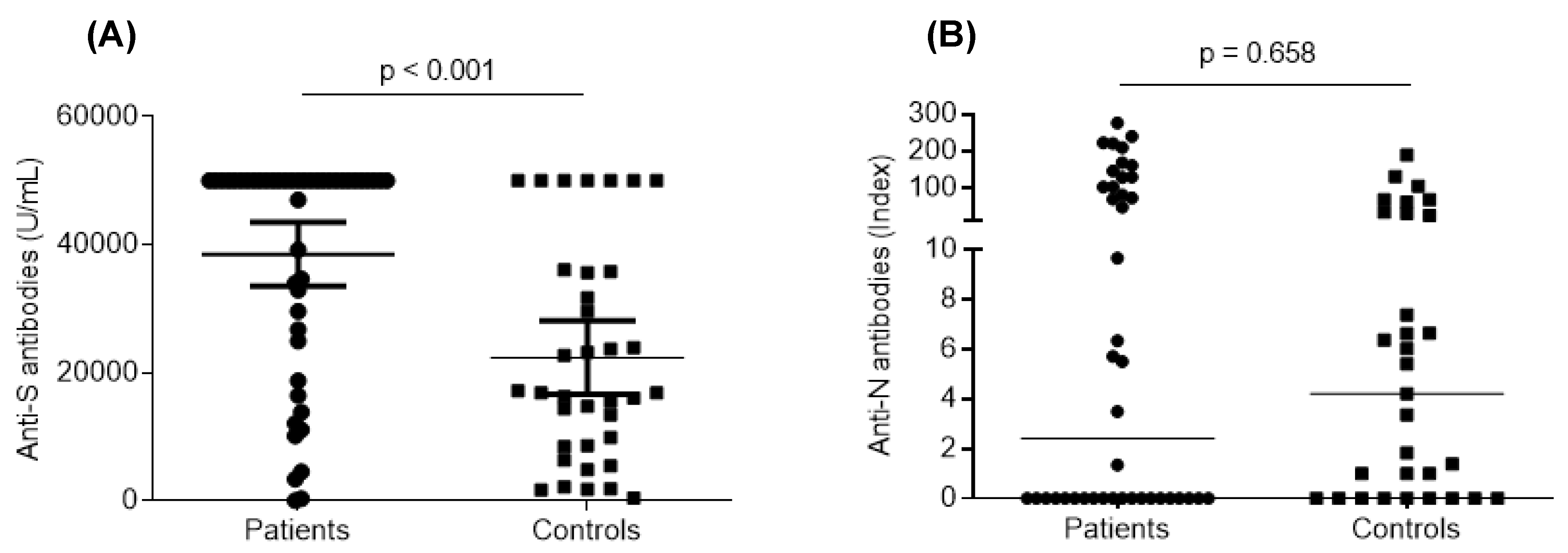
3.3. Humoral Response after Vaccination
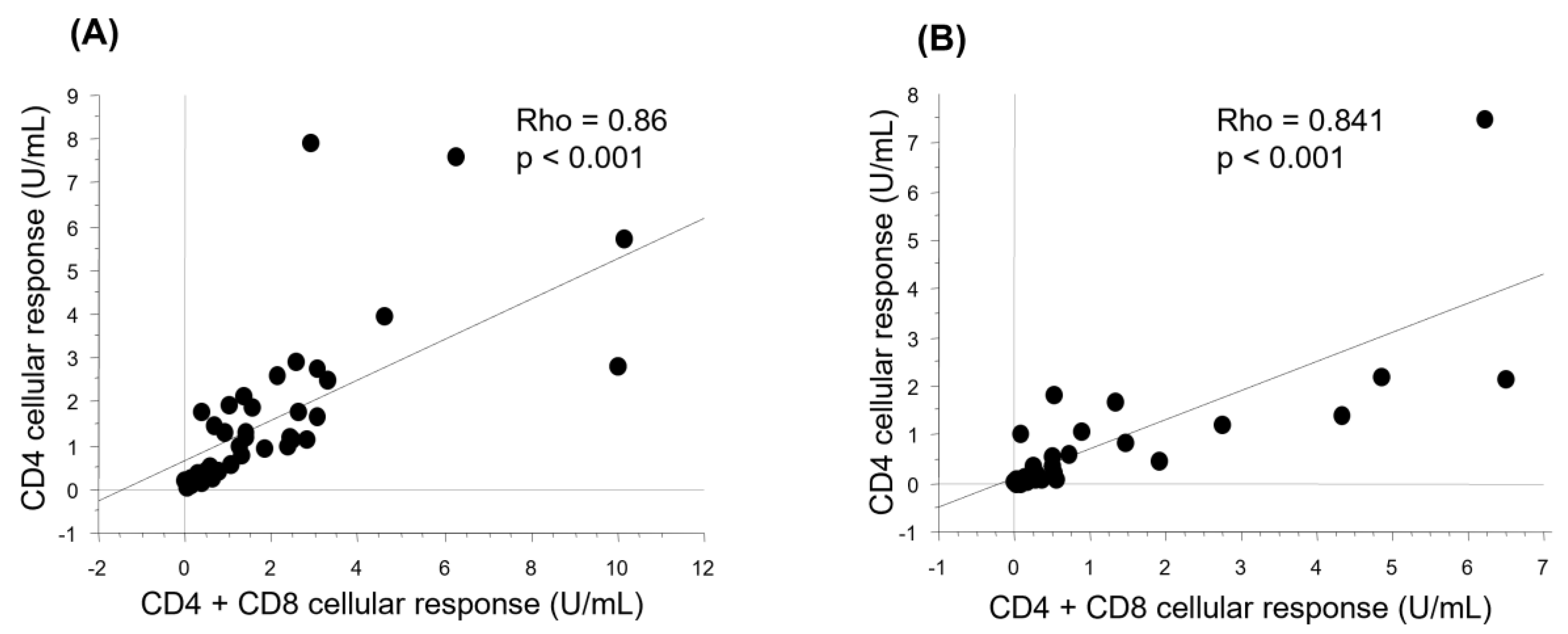
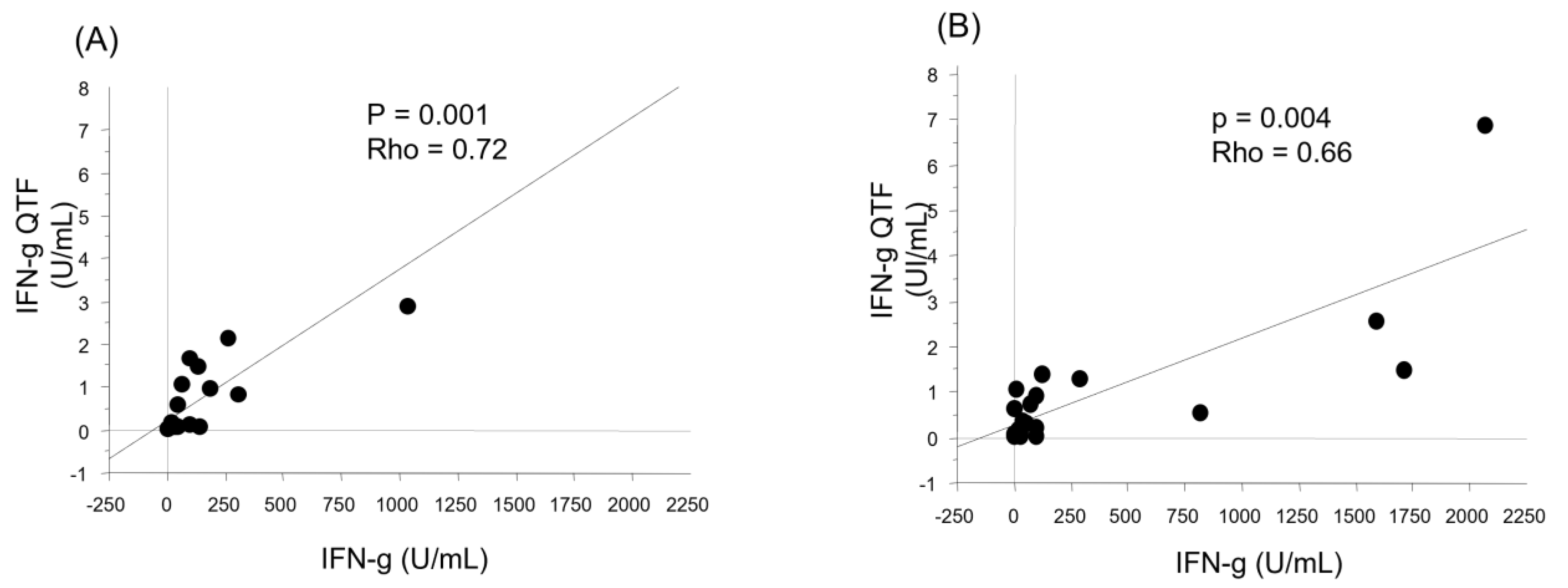
3.4. Cellular Response after Vaccination
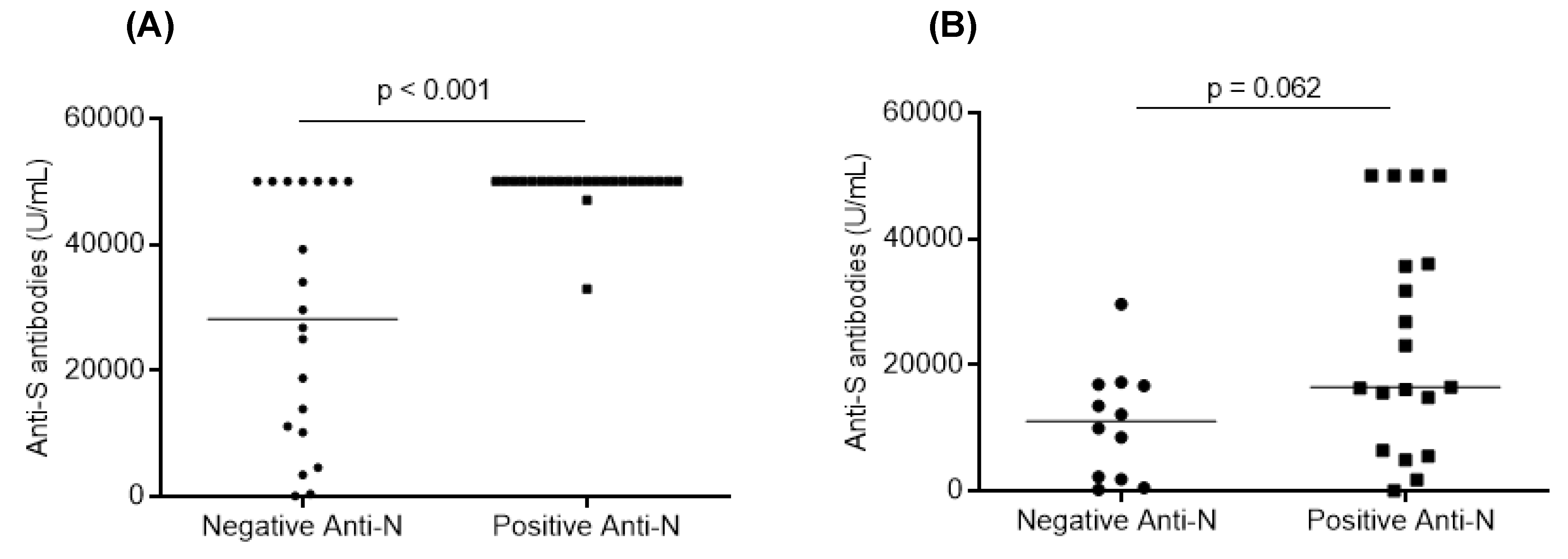
3.5. Factors Associated with the Humoral and Cellular Responses after Vaccination
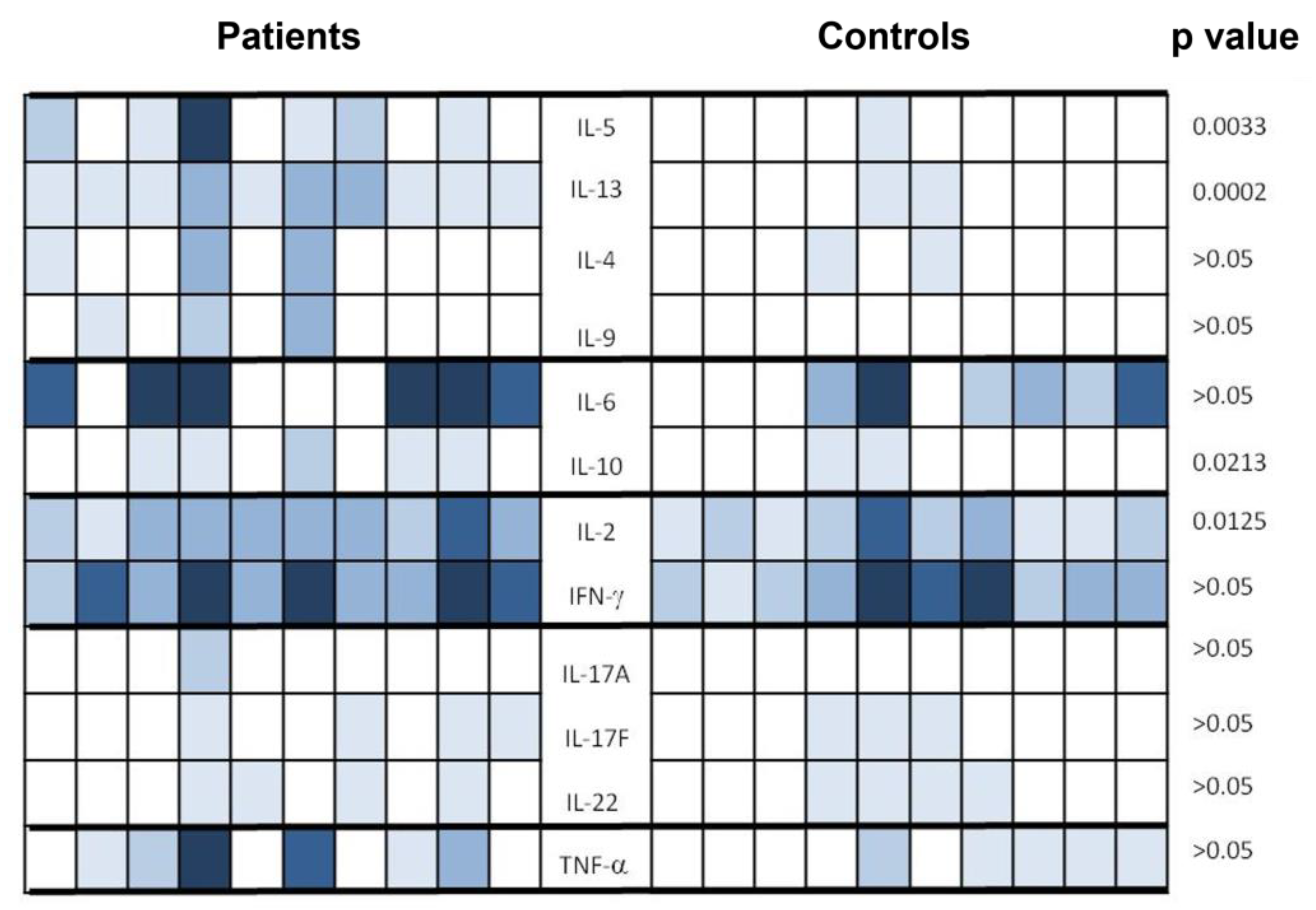
3.6. Cytokine Profile after Vaccination on a Sample of Patients and Controls
3.7. Clinical and Immunological Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19)—World Health Organization. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 12 October 2022).
- Levy, I.; Sharf, G.; Norman, S.; Tadmor, T. The Impact of COVID-19 on Patients with Hematological Malignancies: The Mixed-Method Analysis of an Israeli National Survey. Support. Care Cancer 2021, 29, 7591–7599. [Google Scholar] [CrossRef] [PubMed]
- Avetisyan, G.; Aschan, J.; Hassan, M.; Ljungman, P. Evaluation of Immune Responses to Seasonal Influenza Vaccination in Healthy Volunteers and in Patients After Stem Cell Transplantation. Transplantation 2008, 86, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, A.C.; van Aalst, M.; Meek, B.; van Leeuwen, E.M.M.; Zeerleder, S.; Meijer, E.; Hazenberg, M.D.; Grobusch, M.P.; Goorhuis, A. Long-Term Pneumococcal Vaccine Immunogenicity Following Allogeneic Hematopoietic Stem Cell Transplantation. Vaccine 2019, 37, 510–515. [Google Scholar] [CrossRef]
- Janssen, M.; Bruns, A.; Kuball, J.; Raijmakers, R.; van Baarle, D. Vaccine Responses in Adult Hematopoietic Stem Cell Transplant Recipients: A Comprehensive Review. Cancers 2021, 13, 6140. [Google Scholar] [CrossRef] [PubMed]
- Baudoux, E.; Bay, J.O.; Beguin, Y.; Chalandon, Y.; Colledani, F.; Louis, S.; Daguindau, E.; Dalle, J.H.; Debré, R.; Devillier, R.; et al. Recommandations de la SFGM-TC Stratégie de Vaccination Pour les Patients Recevant une Allogreffe de Cellules Souches Hématopoïétiques. 2021. Vesrion 2. Available online: https://www.sfgm-tc.com/images/remboursements/reco_vaccin_sfgmtc_V2.pdf (accessed on 1 February 2024).
- Lee, A.R.Y.B.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of COVID-19 Vaccines in Immunocompromised Patients: Systematic Review and Meta-Analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef]
- Dimeglio, C.; Herin, F.; Martin-Blondel, G.; Miedougé, M.; Izopet, J. Antibody Titers and Protection against a SARS-CoV-2 Infection. J. Infect. 2022, 84, 248–288. [Google Scholar] [CrossRef]
- Ram, R.; Hagin, D.; Kikozashvilli, N.; Freund, T.; Amit, O.; Bar-On, Y.; Beyar-Katz, O.; Shefer, G.; Moshiashvili, M.M.; Karni, C.; et al. Safety and Immunogenicity of the BNT162b2 MRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-Based CART Therapy-A Single-Center Prospective Cohort Study. Transplant. Cell. Ther. 2021, 27, 788–794. [Google Scholar] [CrossRef]
- Ali, H.; Ngo, D.; Aribi, A.; Arslan, S.; Dadwal, S.; Marcucci, G.; Nakamura, R.; Forman, S.J.; Chen, J.; Al Malki, M.M. Safety and Tolerability of SARS-CoV2 Emergency-Use Authorized Vaccines for Allogeneic Hematopoietic Stem Cell Transplant Recipients. Transplant. Cell. Ther. 2021, 27, 938.e1–938.e6. [Google Scholar] [CrossRef]
- Le Bourgeois, A.; Coste-Burel, M.; Guillaume, T.; Peterlin, P.; Garnier, A.; Béné, M.C.; Chevallier, P. Safety and Antibody Response After 1 and 2 Doses of BNT162b2 MRNA Vaccine in Recipients of Allogeneic Hematopoietic Stem Cell Transplant. JAMA Netw. Open 2021, 4, e2126344. [Google Scholar] [CrossRef]
- Redjoul, R.; Le Bouter, A.; Beckerich, F.; Fourati, S.; Maury, S. Antibody Response after Second BNT162b2 Dose in Allogeneic HSCT Recipients. Lancet 2021, 398, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yakushijin, K.; Funakoshi, Y.; Ohji, G.; Hojo, W.; Sakai, H.; Saeki, M.; Hirakawa, Y.; Matsumoto, S.; Sakai, R.; et al. The Safety and Immunogenicity of the BNT162b2 MRNA COVID-19 Vaccine in Japanese Patients after Allogeneic Stem Cell Transplantation. Vaccines 2022, 10, 158. [Google Scholar] [CrossRef]
- Henig, I.; Isenberg, J.; Yehudai-Ofir, D.; Leiba, R.; Ringelstein-Harlev, S.; Ram, R.; Avni, B.; Amit, O.; Grisariu, S.; Azoulay, T.; et al. Third BNT162b2 mRNA SARS-CoV-2 Vaccine Dose Significantly Enhances Immunogenicity in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Vaccines 2023, 11, 775. [Google Scholar] [CrossRef]
- Chiarucci, M.; Paolasini, S.; Isidori, A.; Guiducci, B.; Loscocco, F.; Capalbo, M.; Visani, G. Immunological Response Against SARS-COV-2 After BNT162b2 Vaccine Administration Is Impaired in Allogeneic but Not in Autologous Stem Cell Transplant Recipients. Front. Oncol. 2021, 11, 737300. [Google Scholar] [CrossRef]
- Chevallier, P.; Coste-Burel, M.; Le Bourgeois, A.; Peterlin, P.; Garnier, A.; Béné, M.C.; Imbert, B.-M.; Drumel, T.; Le Gouill, S.; Moreau, P.; et al. Safety and Immunogenicity of a First Dose of SARS-CoV-2 MRNA Vaccine in Allogeneic Hematopoietic Stem-Cells Recipients. EJHaem 2021, 2, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Mamez, A.-C.; Pradier, A.; Giannotti, F.; Petitpas, A.; Urdiola, M.F.; Vu, D.-L.; Masouridi-Levrat, S.; Morin, S.; Dantin, C.; Clerc-Renaud, D.; et al. Antibody Responses to SARS-CoV2 Vaccination in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Bone Marrow Transplant. 2021, 56, 3094–3096. [Google Scholar] [CrossRef]
- Huang, A.; Cicin-Sain, C.; Pasin, C.; Epp, S.; Audigé, A.; Müller, N.J.; Nilsson, J.; Bankova, A.; Wolfensberger, N.; Vilinovszki, O.; et al. Antibody Response to SARS-CoV-2 Vaccination in Patients Following Allogeneic Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2022, 28, 214.e1–214.e11. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion Rates Following COVID-19 Vaccination among Patients with Cancer. Cancer Cell. 2021, 39, 1081–1090.e2. [Google Scholar] [CrossRef] [PubMed]
- Easdale, S.; Shea, R.; Ellis, L.; Bazin, J.; Davis, K.; Dallas, F.; Thistlethwayte, E.; Ethell, M.; Potter, M.; Arias, C.; et al. Serologic Responses Following a Single Dose of SARS-CoV-2 Vaccination in Allogeneic Stem Cell Transplantation Recipients. Transplant. Cell. Ther. 2021, 27, 880.e1–880.e4. [Google Scholar] [CrossRef]
- Kronbichler, A.; Anders, H.-J.; Fernandez-Juárez, G.M.; Floege, J.; Goumenos, D.; Segelmark, M.; Tesar, V.; Turkmen, K.; van Kooten, C.; Bruchfeld, A.; et al. Recommendations for the Use of COVID-19 Vaccines in Patients with Immune-Mediated Kidney Diseases. Nephrol. Dial. Transplant. 2021, 2021, gfab064. [Google Scholar] [CrossRef]
- Kang, C.K.; Kim, M.; Lee, S.; Kim, G.; Choe, P.G.; Park, W.B.; Kim, N.J.; Lee, C.-H.; Kim, I.S.; Jung, K.; et al. Longitudinal Analysis of Human Memory T-Cell Response According to the Severity of Illness up to 8 Months After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 224, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Laing, E.D.; Pena DaMata, J.; Pohida, K.; Tso, M.S.; Samuels, E.C.; Epsi, N.J.; Dorjbal, B.; Lake, C.; Richard, S.A.; et al. Durability of SARS-CoV-2-Specific T-Cell Responses at 12 Months Postinfection. J. Infect. Dis. 2021, 224, 2010–2019. [Google Scholar] [CrossRef]
- Jung, J.H.; Rha, M.-S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.-H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-Specific T Cell Memory Is Sustained in COVID-19 Convalescent Patients for 10 Months with Successful Development of Stem Cell-like Memory T Cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Zhong, J.; Zhou, Y.; Tang, Z.; Zhou, H.; He, J.; Mei, X.; Tang, Y.; Lin, B.; et al. Exposure to SARS-CoV-2 Generates T-Cell Memory in the Absence of a Detectable Viral Infection. Nat. Commun. 2021, 12, 1724. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; da Silva Antunes, R.; Frazier, A.; et al. Negligible Impact of SARS-CoV-2 Variants on CD4 + and CD8 + T Cell Reactivity in COVID-19 Exposed Donors and Vaccinees. bioRxiv 2021. bioRxiv: 2021.02.27.433180. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Garliss, C.C.; Blankson, J.N. SARS-CoV-2 MRNA Vaccines Induce Broad CD4+ T Cell Responses That Recognize SARS-CoV-2 Variants and HCoV-NL63. J. Clin. Investig. 2021, 131, 149335. [Google Scholar] [CrossRef]
- Murugesan, K.; Jagannathan, P.; Altamirano, J.; Maldonado, Y.A.; Bonilla, H.F.; Jacobson, K.B.; Parsonnet, J.; Andrews, J.R.; Shi, R.Z.; Boyd, S.; et al. Long-Term Accuracy of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Interferon-γ Release Assay and Its Application in Household Investigation. Clin. Infect. Dis. 2022, 75, e314–e321. [Google Scholar] [CrossRef]
- Krüttgen, A.; Klingel, H.; Haase, G.; Haefner, H.; Imöhl, M.; Kleines, M. Evaluation of the QuantiFERON SARS-CoV-2 Interferon-ɣ Release Assay in MRNA-1273 Vaccinated Health Care Workers. J. Virol. Methods 2021, 298, 114295. [Google Scholar] [CrossRef]
- Goletti, D.; Petrone, L.; Manissero, D.; Bertoletti, A.; Rao, S.; Ndunda, N.; Sette, A.; Nikolayevskyy, V. The Potential Clinical Utility of Measuring Severe Acute Respiratory Syndrome Coronavirus 2-Specific T-Cell Responses. Clin. Microbiol. Infect. 2021, 27, 1784–1789. [Google Scholar] [CrossRef]
- Jaganathan, S.; Stieber, F.; Rao, S.N.; Nikolayevskyy, V.; Manissero, D.; Allen, N.; Boyle, J.; Howard, J. Preliminary Evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 Total Test in Recently Vaccinated Individuals. Infect. Dis. Ther. 2021, 10, 2765–2776. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Papadopoulou, A.; Touloumenidou, T.; Stavridou, F.; Koravou, E.-E.; Giannaki, M.; Papalexandri, A.; Karavalakis, G.; Batsis, I.; Kourelis, A.; et al. Neutralizing Antibody and T Cell Responses to SARS-CoV-2 Vaccination in Hematopoietic Cell Transplant Recipients. Bone Marrow Transplant. 2022, 57, 1183–1186. [Google Scholar] [CrossRef]
- Clémenceau, B.; Guillaume, T.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Le Bourgeois, A.; Jullien, M.; Ollier, J.; Grain, A.; Béné, M.C.; et al. SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients Following Two Doses of BNT162b2 MRNA Vaccine. Vaccines 2022, 10, 448. [Google Scholar] [CrossRef]
- Ljungman, P.; de la Camara, R.; Mikulska, M.; Tridello, G.; Aguado, B.; Al Zahrani, M.; Apperley, J.; Berceanu, A.; Bofarul, R.M.; Calbacho, M.; et al. COVID-19 and Stem Cell Transplantation; Results from an EBMT and GETH Multicenter Prospective Survey. Leukemia 2021, 35, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Cherif, I.; Kharroubi, G.; Chaabane, S.; Yazidi, R.; Dellagi, M.; Snoussi, M.A.; Salem, S.; Marzouki, S.; Kammoun Rebai, W.; Rourou, S.; et al. Séroprévalence du SARS-CoV-2 parmi la population générale de Tunis, mars-avril 2021. Rev. D’épidémiologie St. Publique 2022, 70, S228. [Google Scholar] [CrossRef]
- Zyskind, I.; Rosenberg, A.Z.; Zimmerman, J.; Naiditch, H.; Glatt, A.E.; Pinter, A.; Theel, E.S.; Joyner, M.J.; Hill, D.A.; Lieberman, M.R.; et al. SARS-CoV-2 Seroprevalence and Symptom Onset in Culturally Linked Orthodox Jewish Communities Across Multiple Regions in the United States. JAMA Netw. Open 2021, 4, e212816. [Google Scholar] [CrossRef] [PubMed]
- Stringhini, S.; Zaballa, M.-E.; Pullen, N.; Perez-Saez, J.; de Mestral, C.; Loizeau, A.J.; Lamour, J.; Pennacchio, F.; Wisniak, A.; Dumont, R.; et al. Seroprevalence of Anti-SARS-CoV-2 Antibodies 6 Months into the Vaccination Campaign in Geneva, Switzerland, 1 June to 7 July 2021. Eurosurveillance 2021, 26, 2100830. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 Vaccine BNT162b1 Elicits Human Antibody and TH1 T Cell Responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Knulst, A.C.; Tibbe, G.J.; Bril-Bazuin, C.; Breedland, E.G.; van Oudenaren, A.; Benner, R.; Savelkoul, H.F. Cytokine Detection and Modulation in Acute Graft vs. Host Disease in Mice. Mediat. Inflamm. 1994, 3, 33–40. [Google Scholar] [CrossRef]
- Pawelec, G. Age and Immunity: What Is “Immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Rasquinha, M.T.; Sur, M.; Lasrado, N.; Reddy, J. IL-10 as a Th2 Cytokine: Differences Between Mice and Humans. J. Immunol. 2021, 207, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Mingomataj, E.Ç.; Bakiri, A.H. Regulator Versus Effector Paradigm: Interleukin-10 as Indicator of the Switching Response. Clin. Rev. Allergy Immunol. 2016, 50, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-T.; Yao, X.-T.; Peng, Q.; Chen, D.-K. The Protective and Pathogenic Roles of IL-17 in Viral Infections: Friend or Foe? Open Biol. 2019, 9, 190109. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.F.Y.; Wong, C.K.; Lam, C.W.K. Molecular Mechanisms of Cytokine and Chemokine Release from Eosinophils Activated by IL-17A, IL-17F, and IL-23: Implication for Th17 Lymphocytes-Mediated Allergic Inflammation. J. Immunol. 2008, 180, 5625–5635. [Google Scholar] [CrossRef]
- Dias, P.M.; Banerjee, G. The Role of Th17/IL-17 on Eosinophilic Inflammation. J. Autoimmun. 2013, 40, 9–20. [Google Scholar] [CrossRef]


| Patients (n = 43) | Healthy Controls (n = 31) | |
|---|---|---|
| Age (Range) | 31 (17–47) | 50 (40–53) |
| Male | 24 | 14 |
| Female | 19 | 17 |
| History of COVID infection | 4 | 8 |
| Median duration from transplant to vaccination (months) (Range) | 28 (3–54) | NA |
| Underlying disease | 31 AL 6 AA 2 MDS 2 HL 2 CML | NA |
| Peripheral stem cells graft | 21 | NA |
| Bone marrow graft | 22 | NA |
| Lymphopenia | 6 | NA |
| Hypogammaglobulinemia | 10 | NA |
| History of acute GVHD | 15 | NA |
| Active controlled chronic GVHD | 21 | NA |
| Ongoing immunosuppressive treatment | 17 | NA |
| Long term corticosteroids | 6 | NA |
| Variable | Correlation/Association with Side Effects in Patients (p=) | Correlation/Association with Side Effects in Controls (p=) |
|---|---|---|
| Age | 0.345 | 0.651 |
| Gender | 0.748 | 0.007 |
| Duration from ASCT to vaccination | 0.252 | NA |
| AA versus MH | 0.466 | NA |
| History of acute GVHD | 0.429 | NA |
| Active chronic GVHD | 0.675 | NA |
| IS within 3 months | 0.729 | NA |
| Long-term steroid therapy | 1.000 | NA |
| Variable | Correlation/Association with Humoral Response (p=) | Correlation/Association with CD4+ Cellular Response (p=) | Correlation/Association with CD4+ + CD8+ Cellular Response (p=) |
|---|---|---|---|
| Age | 0.560 (Rho = −0.090) | 0.807 (Rho = 0.039) | 0.738 (Rho = 0.053) |
| Gender | 0.405 | 0.870 | 0.536 |
| Side effects after BNT162b2 vaccination | 0.619 | 0.686 | 0.523 |
| Duration from ASCT to vaccination | 0.004 (Rho = 0.434) | 0.581 (Rho = 0.088) | 0.368 (Rho = 0.142) |
| AA versus MH | 0.774 | 0.652 | 0.631 |
| Absolute lymphocyte count (<1 × 109/L) | 0.388 (Rho = 0.137) | 0.356 (Rho = −0.183) | 0.252 (Rho = −0.170) |
| Hypogammaglobulinemia | 0.175 | 0.566 | 0.501 |
| History of acute GVHD | 0.683 | 0.157 | 0.467 |
| Active chronic GVHD | 0.926 | 0.003 | 0.053 |
| IS within 3 months | 0.072 | 0.812 | 0.944 |
| Long-term steroid therapy | 0.107 | 0.102 | 0.115 |
| Anti-N antibodies (+) | <0.001 | 0.467 | 0.476 |
| Variable | Correlation/Association with Humoral Response (p=) | Correlation/Association with CD4+ Cellular Response (p=) | Correlation/Association with CD4+ + CD8+ Cellular Response (p=) |
|---|---|---|---|
| Age | 0.070 (Rho = −0.330) | 0.096 (Rho = 0.315) | 0.710 (Rho = 0.072) |
| Sex | 0.068 | 0.121 | 0.597 |
| Anti-N antibodies (+) | 0.062 | 0.185 | 0.157 |
| Variable | Correlation/Association with Humoral Response (p=) | Correlation/Association with CD4+ Cellular Response (p=) | Correlation/Association with CD4+ + CD8+ Cellular Response (p=) |
|---|---|---|---|
| Age | 0.005 (Rho = −0.425) | 0.241 (Rho = −0.141) | 0.344 (Rho = −0.114) |
| Sex | 0.960 | 0.545 | 0.792 |
| Anti-N antibodies (+) | <0.001 | 0.355 | 0.457 |
| Vaccine Immune Response at the First Sampling * | Vaccine Immune Response at the Second Sampling | Follow up Duration (Months) | 3rd Dose 1 = Yes/0 = No | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Humoral Response | Cellular Response | Humoral Response | Cellular Response | |||||||||
| anti-N | anti-S | CD4 | CD4 CD8 | QFN Result | anti-N | anti-S | CD4 | CD4 CD8 | QFN Result | |||
| P1 | 145.3 | 50,000 | 0.970 | 1.278 | 1 | 182.7 | 12,500 | 2.682 | 2.799 | 1 | 6 | 1 |
| P2 | 72.4 | 50,000 | 1.125 | 2.462 | 1 | 32.2 | 13,100 | 0.230 | 0.860 | 1 | 11 | 0 |
| P3 | 0 | 50,000 | 2.813 | 10.024 | 1 | 67.5 | 43,580 | 0.084 | 0.058 | 0 | 11 | 0 |
| P4 | 220.6 | 50,000 | 1.764 | 2.636 | 1 | 247.4 | 50,000 | 3.095 | 3.245 | 1 | 8 | 0 |
| P5 | 0 | 3434 | 5.698 | 10.171 | 1 | 202.8 | 40,480 | 6.755 | 3.585 | 1 | 5 | 1 |
| P6 | 0 | 50,000 | 0.087 | 0.132 | 0 | 14.59 | 50,000 | 0.144 | 0.302 | 1 | 10 | 0 |
| P7 | 68.86 | 50,000 | 1.925 | 1.029 | 1 | 126.9 | 9675 | 0 | 0 | 0 | 10 | 0 |
| P8 | 0 | 60.97 | 0.230 | 0.150 | 1 | 173.2 | 50,000 | 4.523 | 1.659 | 1 | 10 | 0 |
| P9 | 209 | 50,000 | 1.112 | 2.804 | 1 | 25.2 | 50,000 | 3.262 | 3.443 | 1 | 10 | 0 |
| P10 | 5.5 | 50,000 | 0.385 | 0.747 | 1 | 5.95 | 17,772 | 0.872 | 0.537 | 1 | 10 | 0 |
| P11 | 0 | 50,000 | 3.938 | 4.612 | 1 | 78.44 | 50,000 | 1.275 | 1.309 | 1 | 11 | 0 |
| P12 | 0 | 10,170 | 0.346 | 0.270 | 1 | 18.15 | 10,315 | 0.295 | 0.237 | 1 | 10 | 0 |
| P13 | 0 | 50,000 | 1.865 | 1.528 | 1 | 0 | 12,500 | 0.440 | 0.390 | 1 | 4 | 1 |
| P14 | 0 | 18,792 | 0.082 | 0.075 | 0 | 0 | 2500 | 0.010 | 0.013 | 0 | 5 | 1 |
| P15 | 0 | 39,200 | 2.813 | 10.021 | 1 | 0 | 12,500 | 1.511 | 5.295 | 1 | 6 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Khlil, A.A.; Zamali, I.; Belloumi, D.; Gdoura, M.; Kharroubi, G.; Marzouki, S.; Dachraoui, R.; Ben Yaiche, I.; Bchiri, S.; Hamdi, W.; et al. Immunogenicity and Tolerance of BNT162b2 mRNA Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Patients. Vaccines 2024, 12, 174. https://doi.org/10.3390/vaccines12020174
Ben Khlil AA, Zamali I, Belloumi D, Gdoura M, Kharroubi G, Marzouki S, Dachraoui R, Ben Yaiche I, Bchiri S, Hamdi W, et al. Immunogenicity and Tolerance of BNT162b2 mRNA Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Patients. Vaccines. 2024; 12(2):174. https://doi.org/10.3390/vaccines12020174
Chicago/Turabian StyleBen Khlil, Ahmed Amine, Imen Zamali, Dorra Belloumi, Mariem Gdoura, Ghassen Kharroubi, Soumaya Marzouki, Rym Dachraoui, Insaf Ben Yaiche, Soumaya Bchiri, Walid Hamdi, and et al. 2024. "Immunogenicity and Tolerance of BNT162b2 mRNA Vaccine in Allogeneic Hematopoietic Stem Cell Transplant Patients" Vaccines 12, no. 2: 174. https://doi.org/10.3390/vaccines12020174





