Homologous COVID-19 BNT162b2 mRNA Vaccination at a German Tertiary Care University Hospital: A Survey-Based Analysis of Reactogenicity, Safety, and Inability to Work among Healthcare Workers
Abstract
:1. Introduction
2. Methods
2.1. Setting
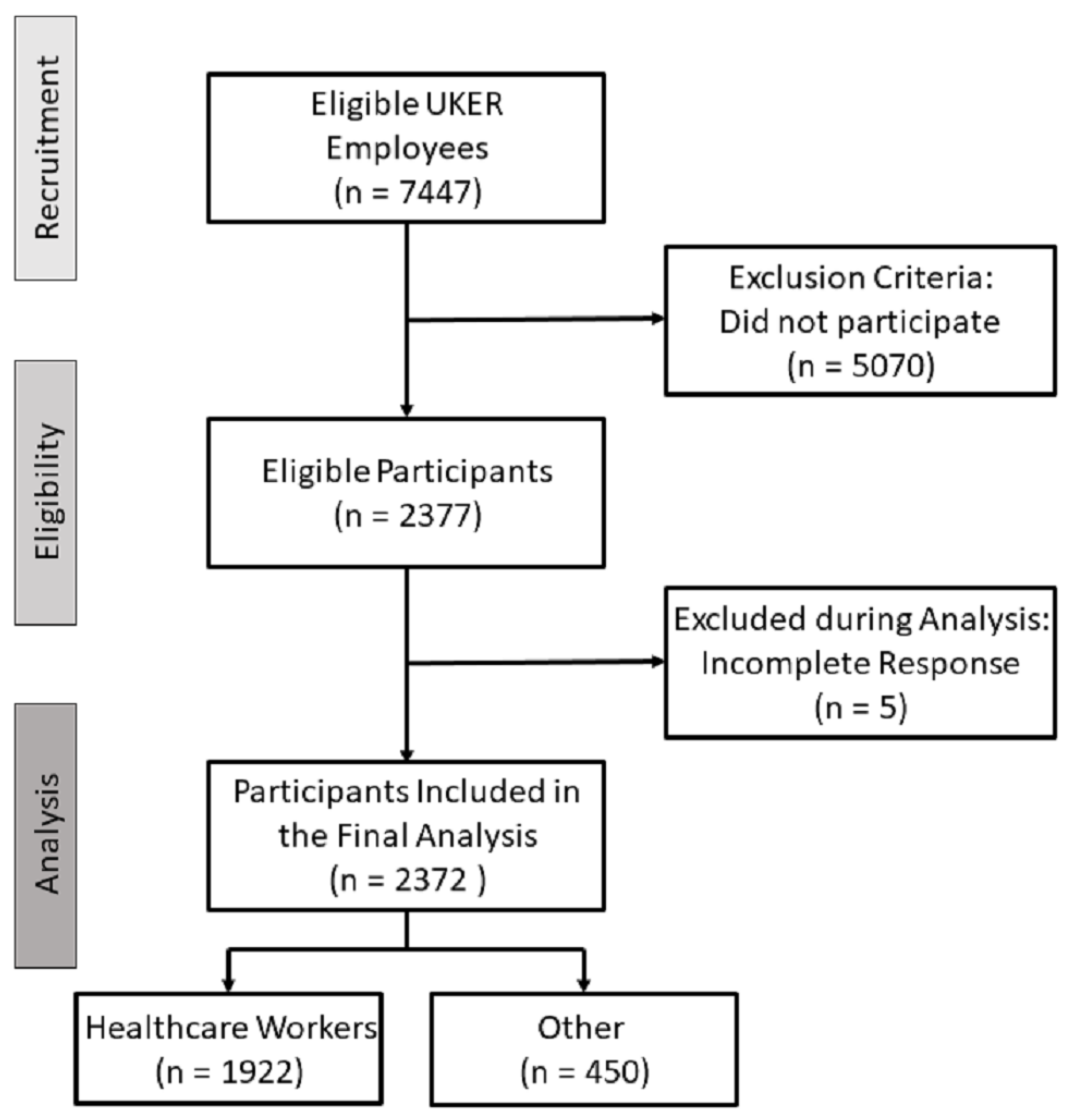
2.2. Study Design and Participants
2.3. Instrument
2.4. Analyses
3. Results
3.1. Characteristics of the Survey Participants
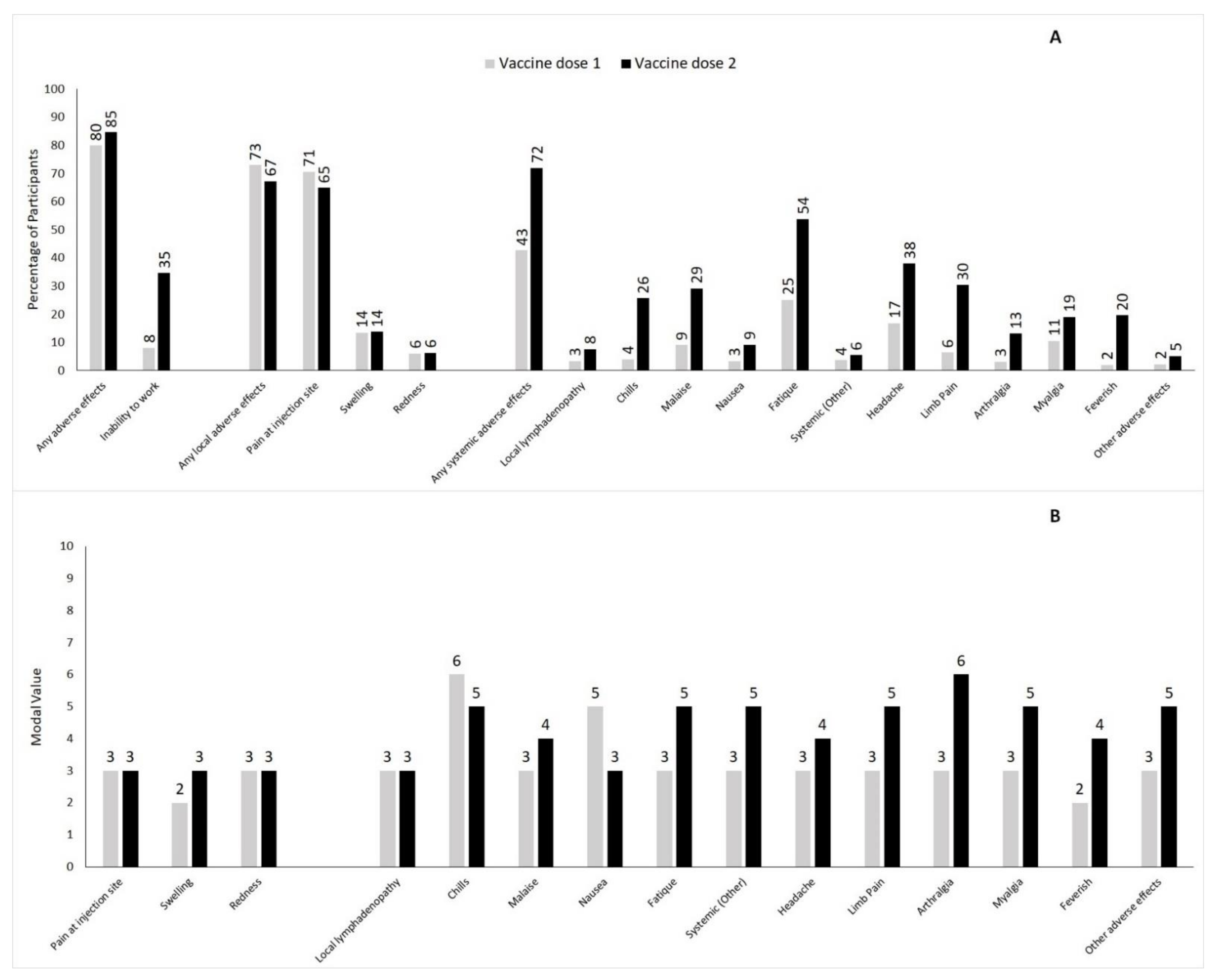
3.2. Local and Systemic Adverse Effects
3.3. Impact of Age
3.4. Impact of Sex
3.5. Intercorrelation Analysis
3.6. Participants with Allergies
3.7. Severe Adverse Events
3.8. Inability to Work
3.9. Estimated Preservation of Working Time Due to COVID-19 Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 18 February 2022).
- Batty, C.J.; Heise, M.T.; Bachelder, E.M.; Ainslie, K.M. Vaccine formulations in clinical development for the prevention of severe acute respiratory syndrome coronavirus 2 infection. Adv. Drug Deliv. Rev. 2021, 169, 168–189. [Google Scholar] [CrossRef] [PubMed]
- Paul-Ehrlich-Institut. Coronavirus and COVID-19. Available online: https://www.pei.de/EN/newsroom/dossier/coronavirus/coronavirus-content.html;jsessionid=6559302DB7720509E57E22B66E0788B0.intranet212?cms_pos=2 (accessed on 18 February 2022).
- Vygen-Bonnet, S.; Koch, J.; Bogdan, C.; Harder, T.; Heininger, U.; Kling, K.; Littmann, M.; Meerpohl, J.; Meyer, H.; Mertens, T.; et al. Decision and Scientific Justification of the Standing Commission on Vaccination (STIKO) for the COVID-19 Vaccination Recommendation. Epid. Bull. 2021, 3–63. (In German) [Google Scholar] [CrossRef]
- Robert-Koch-Institute. COVID-19 Daily Situation Report (Status of 18 February 2022). Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Dez_2021/2021-12-17-en.pdf?__blob=publicationFile (accessed on 18 February 2022).
- Robert-Koch-Institite and German-Federal-Ministry-of-Health. Current Vaccination Status. Available online: https://impfdashboard.de/en/ (accessed on 18 February 2022).
- Coppeta, L.; Ferrari, C.; Mazza, A.; Trabucco Aurilio, M.; Rizza, S. Factors Associated with Pre-Vaccination SARS-CoV-2 Infection Risk among Hospital Nurses Facing COVID-19 Outbreak. Int. J. Environ. Res. Public. Health 2021, 18, 13053. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- European-Medicines-Agency. Assessment Report on Comirnaty by the Committee for Medicinal Products for Human Use. Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf (accessed on 18 February 2022).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, J.; Esse, J.; Tascilar, K.; Steininger, P.; Schober, K.; Irrgang, P.; Alsalameh, R.; Tenbusch, M.; Seggewies, C.; Bogdan, C. Reactogenicity Correlates Only Weakly with Humoral Immunogenicity after COVID-19 Vaccination with BNT162b2 mRNA (Comirnaty®). Vaccines 2021, 9, 1063. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Klimek, L.; Novak, N.; Hamelmann, E.; Werfel, T.; Wagenmann, M.; Taube, C.; Bauer, A.; Merk, H.; Rabe, U.; Jung, K.; et al. Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA: Position statement of the German Allergy Societies: Medical Association of German Allergologists (AeDA), German Society for Allergology and Clinical Immunology (DGAKI) and Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J. Int. 2021, 30, 51–55. [Google Scholar] [CrossRef]
- Phillips, S.P.; Wei, X.; Kwong, J.C.; Gubbay, J.; Schwartz, K.L.; Majury, A.; Groome, P.A. Duration of SARS-CoV-2 shedding: A population-based, Canadian study. PLoS ONE 2021, 16, e0252217. [Google Scholar] [CrossRef]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef]
- D’Arminio Monforte, A.; Tavelli, A.; Perrone, P.M.; Za, A.; Razzini, K.; Tomasoni, D.; Bordoni, V.; Romano, L.; Orfeo, N.; Marchetti, G.; et al. Association between previous infection with SARS-CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: Data from 3078 health care workers. EClinicalMedicine 2021, 36, 100914. [Google Scholar] [CrossRef]
- Ziemann, M.; Görg, S. Inability to Work After Corona Vaccination in Medical Staff. Dtsch. Arztebl. Int. 2021, 118, 298–299. [Google Scholar] [CrossRef]
- Riad, A.; Pokorna, A.; Attia, S.; Klugarova, J.; Koscik, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef]
- Jeskowiak, I.; Wiatrak, B.; Grosman-Dziewiszek, P.; Szelag, A. The Incidence and Severity of Post-Vaccination Reactions after Vaccination against COVID-19. Vaccines 2021, 9, 502. [Google Scholar] [CrossRef]
- Amodio, E.; Minutolo, G.; Casuccio, A.; Costantino, C.; Graziano, G.; Mazzucco, W.; Pieri, A.; Vitale, F.; Zarcone, M.; Restivo, V. Adverse Reactions to Anti-SARS-CoV-2 Vaccine: A Prospective Cohort Study Based on an Active Surveillance System. Vaccines 2022, 10, 345. [Google Scholar] [CrossRef]
- Harris, T.; Nair, J.; Fediurek, J.; Deeks, S.L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–2015. Vaccine 2017, 35, 2600–2604. [Google Scholar] [CrossRef]
- Klein, S.L.; Marriott, I.; Fish, E.N. Sex-based differences in immune function and responses to vaccination. Trans. R Soc. Trop. Med. Hyg. 2015, 109, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.P.; Desai, A.P.; Loomis, G.J. Relationship between pre-existing allergies and anaphylactic reactions post mRNA COVID-19 vaccine administration. Vaccine 2021, 39, 4407–4409. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A., Jr.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA 2021, 325, 1562–1565. [Google Scholar] [CrossRef] [PubMed]
- Paul-Ehrlich-Institute. Safety Assessment: Suspected Cases of Adverse Effects and Vaccine Complications after Vaccination against COVID-19 since the Start of Vaccination Compaign on December 27, 2020 until August 31, 2021. Paul Ehrlich Institute 2021, 20 September 2021. Available online: https://www.pei.de/SharedDocs/Downloads/EN/newsroom-en/dossiers/safety-reports/safety-report-27-december-31-august-2021.pdf?__blob=publicationFile&v=5 (accessed on 18 February 2022).
- Renoud, L.; Khouri, C.; Revol, B.; Lepelley, M.; Perez, J.; Roustit, M.; Cracowski, J.L. Association of Facial Paralysis with mRNA COVID-19 Vaccines: A Disproportionality Analysis Using the World Health Organization Pharmacovigilance Database. JAMA Intern. Med. 2021, 181, 1243–1245. [Google Scholar] [CrossRef] [PubMed]
- Standing-Committee-on-Vaccination-at-the-Robert-Koch-Institute. 14th Update of the STIKO Recommendation on COVID-19 vaccination. Epid. Bull 2021, 48, 3–14. [Google Scholar] [CrossRef]
- Robert-Koch-Institute. Serological Analysis of Blood Donors for Antibodies against SARS-CoV-2 (SeBluCo Study)—An Interims Report. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Projekte_RKI/SeBluCo_Zwischenbericht.html (accessed on 18 February 2022).
- Wagner, R.; Peterhoff, D.; Beileke, S.; Gunther, F.; Berr, M.; Einhauser, S.; Schutz, A.; Niller, H.H.; Steininger, P.; Knoll, A.; et al. Estimates and Determinants of SARS-Cov-2 Seroprevalence and Infection Fatality Ratio Using Latent Class Analysis: The Population-Based Tirschenreuth Study in the Hardest-Hit German County in Spring 2020. Viruses 2021, 13, 1118. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, Y.W.; Kim, S.R.; Kim, J.H.; Min, T.K.; Park, H.S.; Shin, M.; Ye, Y.M.; Lee, S.; Lee, J.; et al. COVID-19 Vaccine-associated Anaphylaxis and Allergic Reactions: Consensus Statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working Group. Allergy Asthma. Immunol. Res. 2021, 13, 526–544. [Google Scholar] [CrossRef]
- FDA Briefing Document. Pfizer-BioNTech COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee Meeting 10 December 2020. Available online: https://www.fda.gov/media/144245/download (accessed on 18 February 2022).
| Adverse Effects | Odds Ratio (95% CI) after Dose 1 | Odds Ratio (95% CI) after Dose 2 |
|---|---|---|
| Pain at injection site | 2.12 (1.68–2.67) *** | 2.20 (1.75–2.77) *** |
| Swelling | 0.96 (0.69–1.33) | 0.98 (0.71–1.37) |
| Redness | 1.09 (0.67–1.76) | 0.85 (0.55–1.33) |
| Itching | 1.20 (0.57–2.55) | 1.32 (0.56–3.12) |
| Any local reaction | 1.96 (1.55–2.49) *** | 2.01 (1.60–2.53) *** |
| Swelling of lymph nodes | 3.40 (1.24–9.35) * | 2.53 (1.40–4.60) ** |
| Chills | 1.52 (0.78–2.95) | 2.50 (1.81–3.44) *** |
| Malaise | 1.50 (0.96–2.35) | 3.11 (2.25–4.30) *** |
| Nausea | 1.61 (0.77–3.37) | 1.90 (1.17–3.10) ** |
| Fatigue | 1.10 (0.84–1.43) | 1.79 (1.42–2.25) *** |
| Headache | 1.63 (1.15–2.30) ** | 2.00 (1.55–2.59) *** |
| Limb pain | 1.14 (0.70–1.85) | 1.79 (1.36–2.36) *** |
| Arthralgia | 0.84 (0.46–1.54) | 1.60 (1.09–2.36) * |
| Myalgia | 1.22 (0.82–1.79) | 1.38 (1.01–1.89) * |
| Feverish | 1.25 (0.53–2.97) | 2.02 (1.43–2.84) *** |
| Any systemic reaction | 1.35 (1.07–1.70) * | 2.08 (1.64–2.63) *** |
| Inability to work | 1.19 (0.76–1.86) | 2.20 (1.67–2.88) *** |
| Odds Ratio (95% CI) after Dose 1 | Odds Ratio (95% CI) after Dose 2 | |
|---|---|---|
| Pain | 1.21 (1.00–1.46) | 1.31 (1.09–1.57) ** |
| Swelling | 1.37 (1.05–1.80) * | 1.28 (0.98–1.66) |
| Redness | 1.30 (0.88–1.92) | 1.17 (0.80–1.70) |
| Itching | 2.08 (1.08–4.00) * | 10.65 (2.58–43.95) *** |
| Any local reaction | 1.22 (1.01–1.49) * | 1.39 (1.16–1.68) *** |
| Swelling of lymph nodes | 1.20 (0.72–2.00) | 2.05 (1.38–3.04) *** |
| Chills | 1.47 (0.91–2.39) | 1.45 (1.17–1.79) *** |
| Malaise | 1.05 (0.77–1.42) | 1.38 (1.13–1.69) ** |
| Nausea | 2.78 (1.47–5.29) ** | 1.76 (1.25–2.48) ** |
| Fatigue | 1.35 (1.10–1.67) ** | 1.40 (1.17–1.67) *** |
| Headache | 1.89 (1.45–2.46) *** | 1.35 (1.12–1.62) ** |
| Limb pain | 1.40 (0.95–2.05) | 1.70 (1.39–2.08) *** |
| Arthralgia | 1.14 (0.68–1.89) | 1.60 (1.20–2.12) ** |
| Myalgia | 0.95 (0.72–1.27) | 1.60 (1.25–2.03) *** |
| Feverish | 3.15 (1.34–7.44) ** | 1.66 (1.31–2.11) *** |
| Any systemic reaction | 1.48 (1.24–1.77) *** | 1.49 (1.23–1.81) *** |
| Inability to work | 1.63 (1.14–2.34) ** | 1.85 (1.52–2.25) *** |
| Any Local Reaction | Any Systemic Reaction | Inability to Work | |||||
|---|---|---|---|---|---|---|---|
| cOR (95% CI) | aOR (95% CI) | cOR (95% CI) | aOR (95% CI) | cOR (95% CI) | aOR (95% CI) | ||
| First dose | Female vs. Male | 1.22 (1.01–1.49) | 1.22 (1.00–1.48) | 1.48 (1.24–2.34) | 1.47 (1.23–1.76) | 1.63 (1.14–2.34) | 1.62 (1.23–2.32) |
| Age ≤ 55 vs. Age ≥ 56 | 1.96 (1.55–1.49) | 1.96 (1.54–2.48) | 1.35 (1.07–1.70) | 1.33 (1.05–1.69) | 1.19 (0.76–1.86) | 1.17 (0.75–1.82) | |
| Second dose | Female vs. Male | 1.39 (1.16–1.68) | 1.39 (1.16–1.68) | 1.49 (1.23–1.81) | 1.49 (1.23–1.80) | 1.85 (1.52–2.25) | 1.85 (1.52–2.26) |
| Age ≤ 55 vs. Age ≥ 56 | 2.01 (1.60–2.53) | 2.00 (1.59–2.52) | 2.08 (1.64–2.63) | 2.07 (1.64–2.62) | 2.20 (1.67–2.88) | 2.19 (1.66–2.89) | |
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age group | 30–39 | <30 | 30–39 | >59 | 30–39 |
| Sex | Female | Female | Female | Female | Female |
| Vaccination | Dose 1 | Dose 1 | Dose 2 | Dose 2 | Dose 2 |
| If dose 2: reaction to first vaccination | - | - | Generalized skin rash after 4 h | Headache, numbness of the tongue | N/A |
| Known allergies | Antibiotics, analgesics, tetanus toxoid vaccine | Six previous allergic events of unknown origin that required treatment | Penicillin | - | One allergic reaction of unknown origin that required treatment |
| Other medical history | - | Allergic Asthma | - | Rheumatism | - |
| Prior medication | Cetirizine (long-term medication) | - | 4 mg clemastine-fumarate i.v. | - | - |
| Symptoms | Tingling sensation on entire body, constricted airways, tachycardia, hypotension | Erythema of the neck, malaise, breathlessness, globus sensation | Generalized itch | Nausea, headache, heat sensation, “furry sensation” in mouth | Light-headedness, strong nausea |
| Male | Female | |||
|---|---|---|---|---|
| Years of Age | Dose 1 | Dose 2 | Dose 1 | Dose 2 |
| <30 | 7.3% | 30.5% | 10.9% | 45.9% |
| 30–39 | 6.0% | 27.2% | 9.1% | 41.1% |
| 40–49 | 3.1% | 23.3% | 6.9% | 39% |
| 50–59 | 6.8% | 23.8% | 8.2% | 31.2% |
| >59 | 3.3% | 13.3% | 5.5% | 17.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niekrens, V.; Esse, J.; Held, J.; Knobloch, C.S.; Steininger, P.; Kunz, B.; Seggewies, C.; Bogdan, C. Homologous COVID-19 BNT162b2 mRNA Vaccination at a German Tertiary Care University Hospital: A Survey-Based Analysis of Reactogenicity, Safety, and Inability to Work among Healthcare Workers. Vaccines 2022, 10, 650. https://doi.org/10.3390/vaccines10050650
Niekrens V, Esse J, Held J, Knobloch CS, Steininger P, Kunz B, Seggewies C, Bogdan C. Homologous COVID-19 BNT162b2 mRNA Vaccination at a German Tertiary Care University Hospital: A Survey-Based Analysis of Reactogenicity, Safety, and Inability to Work among Healthcare Workers. Vaccines. 2022; 10(5):650. https://doi.org/10.3390/vaccines10050650
Chicago/Turabian StyleNiekrens, Valentin, Jan Esse, Jürgen Held, Carina Sophia Knobloch, Philipp Steininger, Bernd Kunz, Christof Seggewies, and Christian Bogdan. 2022. "Homologous COVID-19 BNT162b2 mRNA Vaccination at a German Tertiary Care University Hospital: A Survey-Based Analysis of Reactogenicity, Safety, and Inability to Work among Healthcare Workers" Vaccines 10, no. 5: 650. https://doi.org/10.3390/vaccines10050650
APA StyleNiekrens, V., Esse, J., Held, J., Knobloch, C. S., Steininger, P., Kunz, B., Seggewies, C., & Bogdan, C. (2022). Homologous COVID-19 BNT162b2 mRNA Vaccination at a German Tertiary Care University Hospital: A Survey-Based Analysis of Reactogenicity, Safety, and Inability to Work among Healthcare Workers. Vaccines, 10(5), 650. https://doi.org/10.3390/vaccines10050650






