Differences in Immunogenicity of Three Different Homo- and Heterologous Vaccination Regimens against SARS-CoV-2
Abstract
:1. Introduction
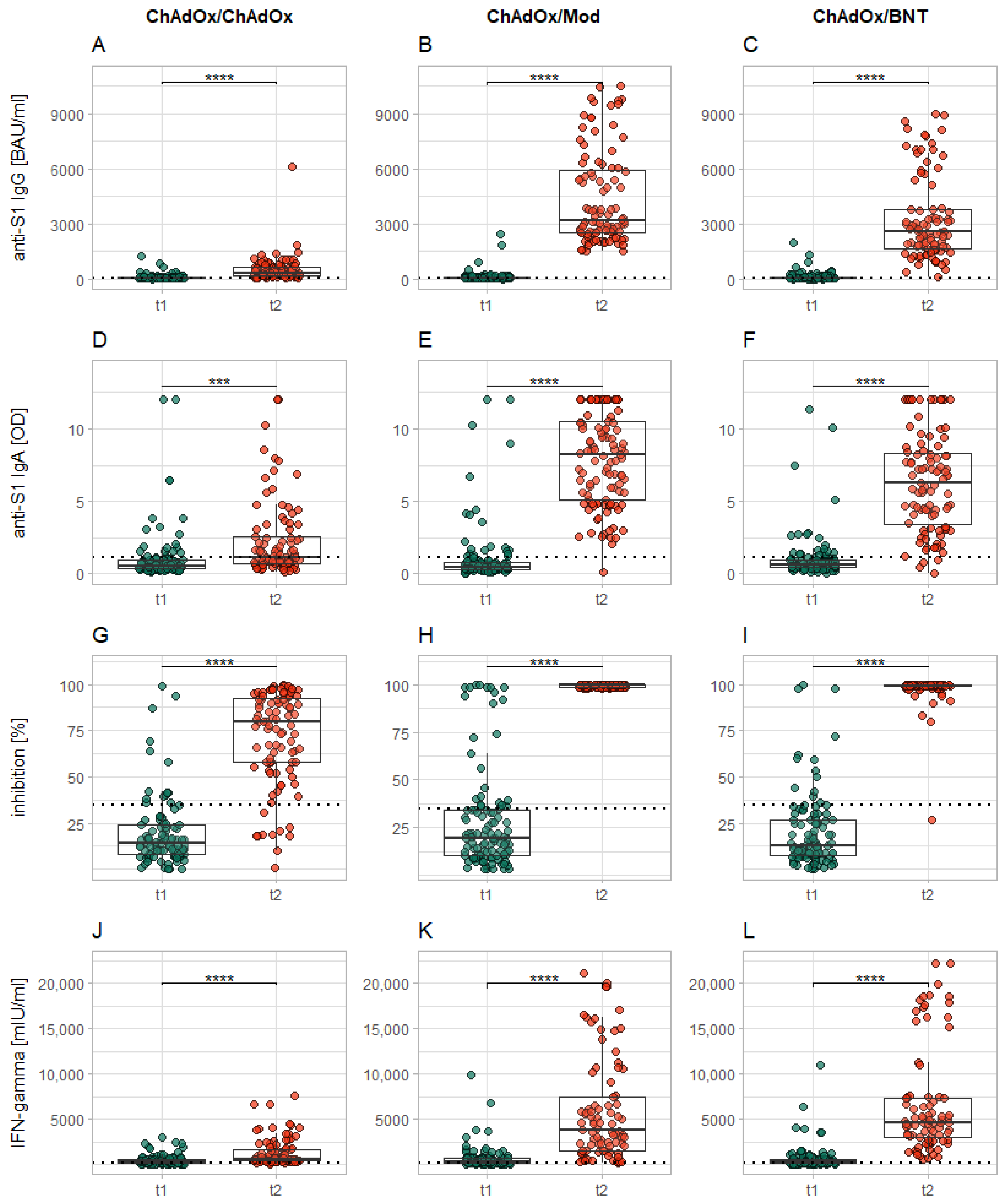
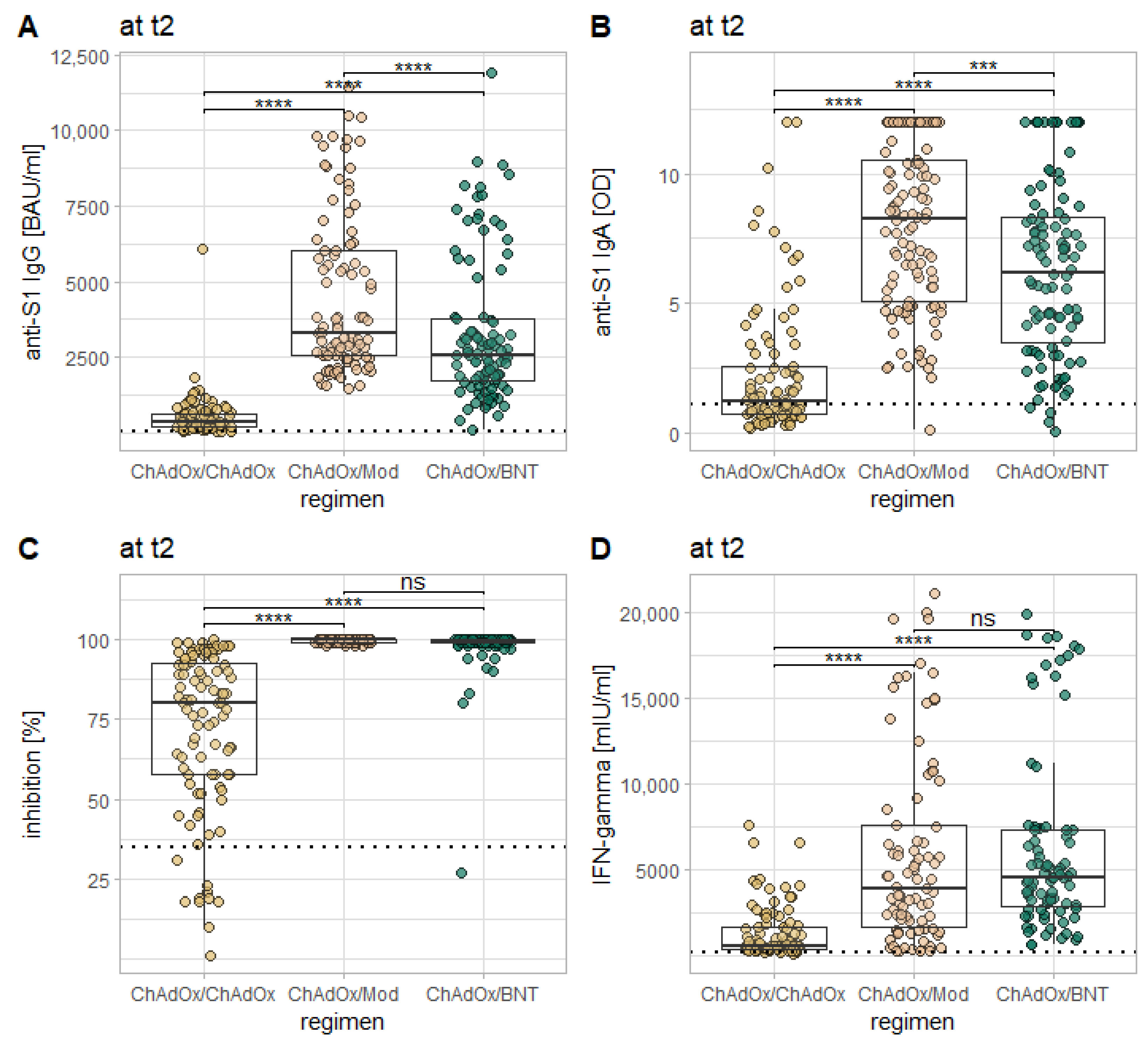
- In continuation of the trend already seen within the first seven days after the second vaccination, heterologous vaccinations induce an immune response comparable or superior in size to that induced by homologous vaccination with ChAdOx.
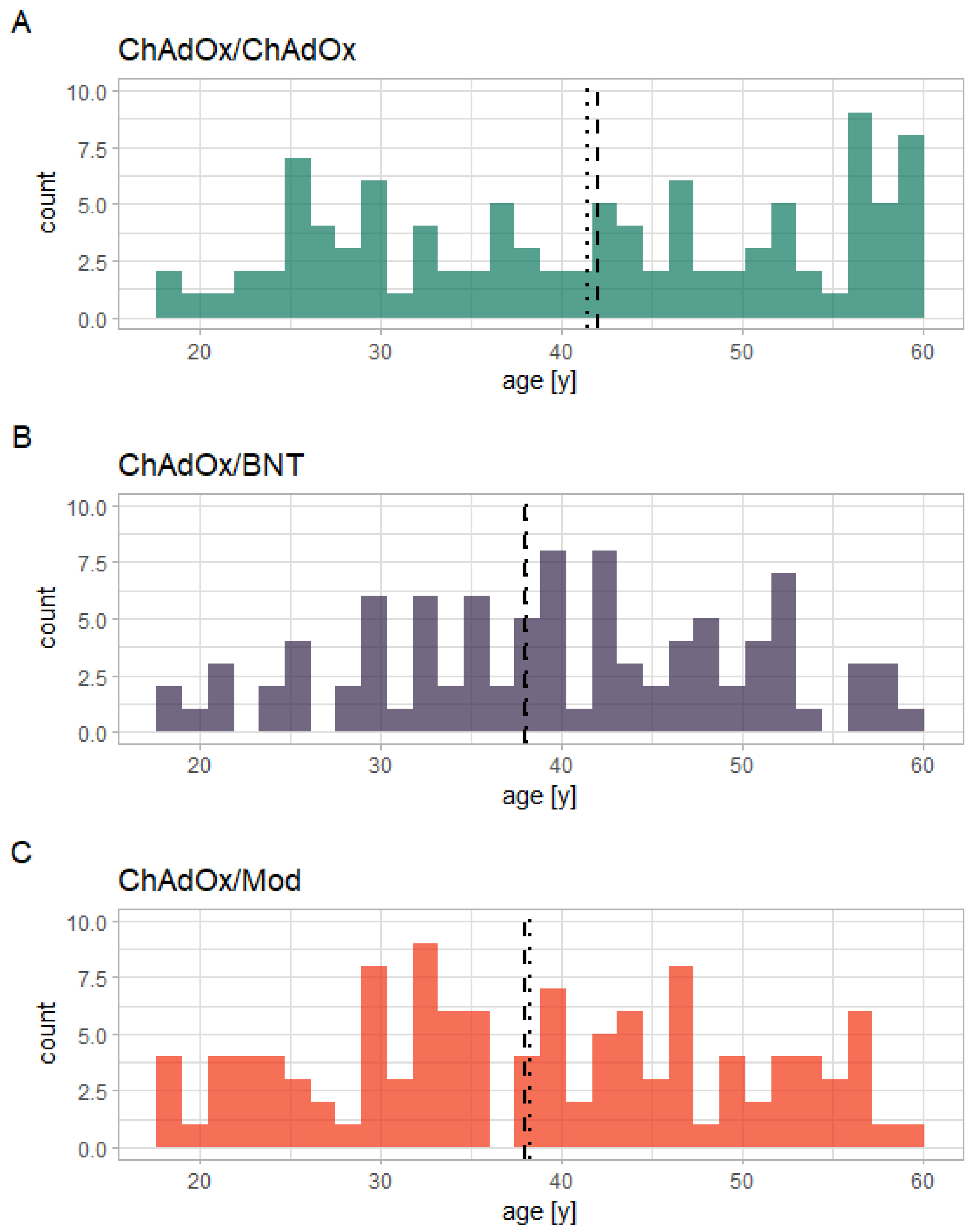
- Age might be a general influencing factor, with older age possibly being associated with weaker immune responses.
2. Methods
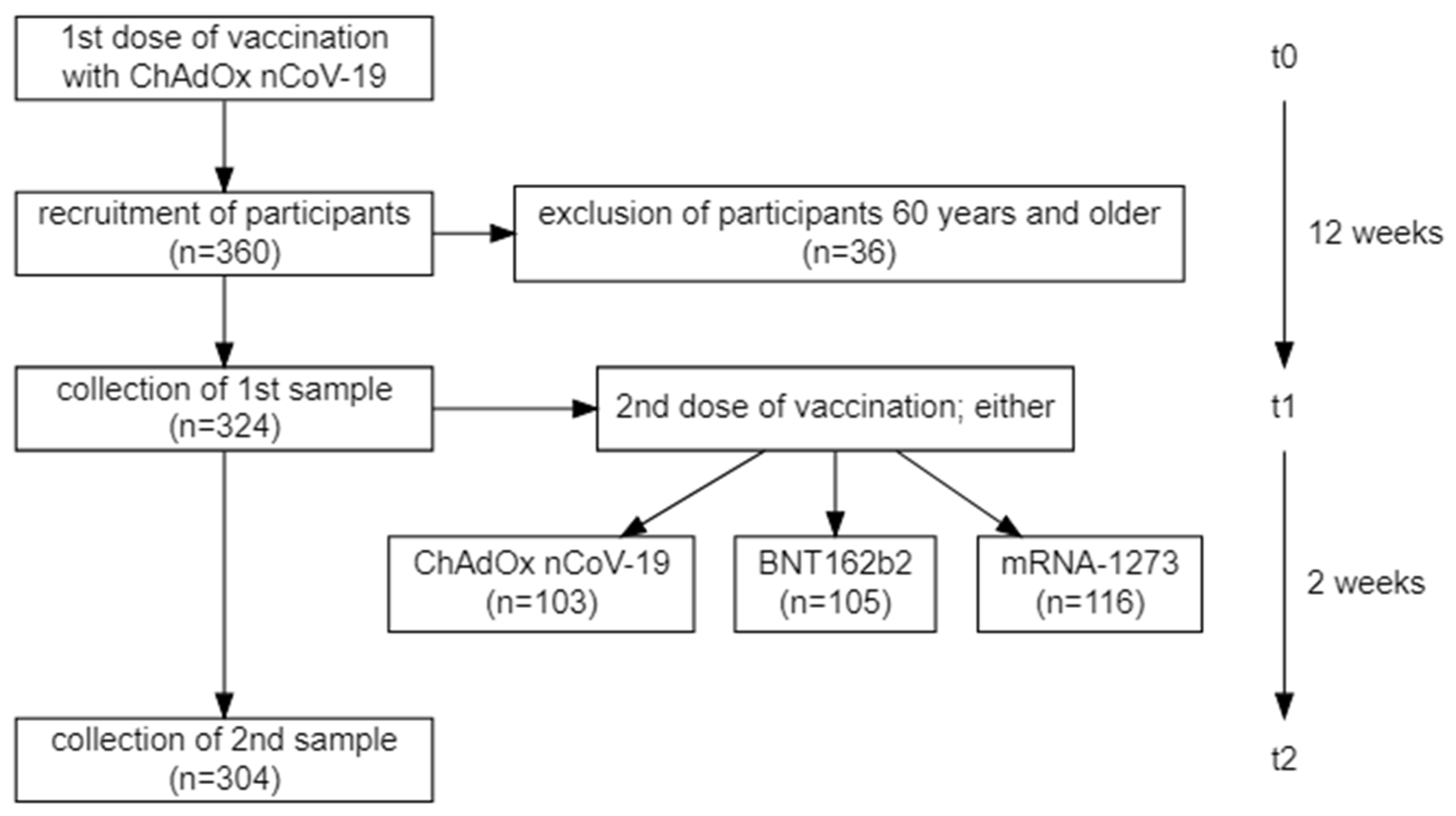
2.1. Study Population
2.2. Sample Characteristics
2.3. Anti-SARS-CoV-2 Antibodies
2.4. Surrogate Neutralization Assay
2.5. Interferon-γ Release Assay
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Effect of the Second Dose
3.3. Influence of Sex on the Immune Response
3.4. Influence of the Vaccination Regimen on the Immune Response
3.5. Influence of Age on the Immune Response
3.6. Influence of Prior Infection on the Immune Response
3.7. Borderline or Non-Reactive Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 MRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and MRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef]
- Daniel, W.; Nivet, M.; Warner, J.; Podolsky, D.K. Early Evidence of the Effect of SARS-CoV-2 Vaccine at One Medical Center. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 NCov-19 Vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, Reactogenicity, and Immunogenicity of Homologous and Heterologous Prime-Boost Immunisation with ChAdOx1 NCoV-19 and BNT162b2: A Prospective Cohort Study. Lancet Respir. Med. 2021. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and Reactogenicity of Heterologous ChAdOx1 NCoV-19/MRNA Vaccination. Nat. Med. 2021, 1–6. [Google Scholar] [CrossRef]
- Rose, R.; Neumann, F.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Humoral Immune Response after Different SARS-CoV-2 Vaccination Regimens. BMC Med. 2022, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-García, J.; et al. Heterologous Prime-Boost Vaccination with ChAdOx1 NCoV-19 and BNT162b2. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Markewitz, R.; Pauli, D.; Dargvainiene, J.; Steinhagen, K.; Engel, S.; Herbst, V.; Zapf, D.; Krüger, C.; Sharifzadeh, S.; Schomburg, B.; et al. The Temporal Course of T- and B-Cell-Responses to Vaccination with BNT162b2 and MRNA-1273. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Markewitz, R.; Juhl, D.; Pauli, D.; Görg, S.; Junker, R.; Rupp, J.; Engel, S.; Steinhagen, K.; Herbst, V.; Zapf, D.; et al. Kinetics of the Antibody Response to Boostering with Three Different Vaccines Against SARS-CoV-2. Front. Immunol. 2022, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-Reactive T Cells in Healthy Donors and Patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef]
- Giménez, E.; Albert, E.; Torres, I.; Remigia, M.J.; Alcaraz, M.J.; Galindo, M.J.; Blasco, M.L.; Solano, C.; Forner, M.J.; Redón, J.; et al. SARS-CoV-2-Reactive Interferon-γ-Producing CD8+ T Cells in Patients Hospitalized with Coronavirus Disease 2019. J. Med. Virol. 2021, 93, 375–382. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The Control of the False Discovery Rate in Multiple Testing under Dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Liu, X.; Shaw, R.H.; Stuart, A.S.; Greenland, M.; Dinesh, T.; Provstgaard-Morys, S.; Clutterbuck, E.; Ramasamy, M.N.; Aley, P.K.; Farooq Mujadidi, Y.; et al. Safety and Immunogenicity Report from the Com-COV Study—A Single-Blind Randomised Non-Inferiority Trial Comparing Heterologous And Homologous Prime-Boost Schedules with An Adenoviral Vectored and MRNA COVID-19 Vaccine; Social Science Research Network: Rochester, NY, USA, 2021. [Google Scholar]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; Morales, M.T.G.; et al. Immunogenicity and Reactogenicity of BNT162b2 Booster in ChAdOx1-S-Primed Participants (CombiVacS): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Normark, J.; Vikström, L.; Gwon, Y.-D.; Persson, I.-L.; Edin, A.; Björsell, T.; Dernstedt, A.; Christ, W.; Tevell, S.; Evander, M.; et al. Heterologous ChAdOx1 NCoV-19 and MRNA-1273 Vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Groß, R.; Zanoni, M.; Seidel, A.; Conzelmann, C.; Gilg, A.; Krnavek, D.; Erdemci-Evin, S.; Mayer, B.; Hoffmann, M.; Pöhlmann, S.; et al. Heterologous ChAdOx1 NCoV-19 and BNT162b2 Prime-Boost Vaccination Elicits Potent Neutralizing Antibody Responses and T Cell Reactivity against Prevalent SARS-CoV-2 Variants. eBioMedicine 2022, 75. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.-B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and Efficacy of Heterologous ChadOx1/BNT162b2 Vaccination. Nature 2021. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 1–7. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Gal Levin, E.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Wyllie, D.; Jones, H.E.; Mulchandani, R.; Trickey, A.; Taylor-Phillips, S.; Brooks, T.; Charlett, A.; Ades, A.E.; Investigators, E.-H.; Moore, P.; et al. SARS-CoV-2 Responsive T Cell Numbers and Anti-Spike IgG Levels Are Both Associated with Protection from COVID-19: A Prospective Cohort Study in Keyworkers. medRxiv 2021. [Google Scholar] [CrossRef]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody Response to SARS-CoV-2 Infection in Humans: A Systematic Review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021. [Google Scholar] [CrossRef]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.-X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of Immune Responses to SARS-CoV-2 in Mild-Moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 MRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody Response to First BNT162b2 Dose in Previously SARS-CoV-2-Infected Individuals. Lancet Lond. Engl. 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Bradley, T.; Grundberg, E.; Selvarangan, R.; LeMaster, C.; Fraley, E.; Banerjee, D.; Belden, B.; Louiselle, D.; Nolte, N.; Biswell, R.; et al. Antibody Responses after a Single Dose of SARS-CoV-2 MRNA Vaccine. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Saadat, S.; Tehrani, Z.R.; Logue, J.; Newman, M.; Frieman, M.B.; Harris, A.D.; Sajadi, M.M. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA 2021. [Google Scholar] [CrossRef] [PubMed]
- Green, M.S.; Shohat, T.; Lerman, Y.; Cohen, D.; Slepon, R.; Duvdevani, P.; Varsano, N.; Dagan, R.; Mendelson, E. Sex Differences in the Humoral Antibody Response to Live Measles Vaccine in Young Adults. Int. J. Epidemiol. 1994, 23, 1078–1081. [Google Scholar] [CrossRef]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex Differences in Vaccine-Induced Humoral Immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoica, G.; Macarie, E.; Michiu, V.; Stoica, R.C. Biologic Variation of Human Immunoglobulin Concentration. I. Sex-Age Specific Effects on Serum Levels of IgG, IgA, IgM and IgD. Med. Interne 1980, 18, 323–332. [Google Scholar]
- Metzger, D.W. IgA and Respiratory Immunity. In Mucosal Immune Defense: Immunoglobulin A; Kaetzel, C.S., Ed.; Springer: Boston, MA, USA, 2007; pp. 269–290. ISBN 978-0-387-72232-0. [Google Scholar]
- Brewer, R.C.; Ramadoss, N.S.; Lahey, L.J.; Jahanbani, S.; Robinson, W.H.; Lanz, T.V. BNT162b2 Vaccine Induces Divergent B Cell Responses to SARS-CoV-2 S1 and S2. Nat. Immunol. 2021, 1–7. [Google Scholar] [CrossRef]

| Subgroup | Age (years) | 12 Weeks after First Dose | 14 Days after Second Dose | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-S1 IgG (BAU/mL) | Anti-S1 IgA (OD Ratio) | Neutralizing Antibodies (%) | IGRA (mIU/mL) | Anti-S1 IgG (BAU/mL) | Anti-S1 IgA (OD Ratio) | Neutralizing Antibodies (%) | IGRA (mIU/mL) | ||
| Whole cohort | 39 ± 13.3 | 63.9 ± 51 | 0.57 ± 0.36 | 16 ± 13.3 | 258 ± 262 | 2314 ± 2365 | 5.1 ± 4.9 | 99 ± 1.5 | 3289 ± 4028 |
| females | 39 ± 13.3 | 65.2 ± 53 | 0.5 ± 0.33 | 16 ± 13.3 | 280 ± 270 | 2557 ± 2961 | 4.7 ± 4.7 | 99 ± 1.5 | 3698 ± 4151 |
| males | 39.5 ± 15.6 | 63.0 ± 49.4 | 0.68 ± 0.44 | 14 ± 10.4 | 235 ± 251 | 2066 ± 2126 | 6.5 ± 5.0 | 99 ± 1.5 | 3025 ± 3747 |
| ChAdOx/ChAdOx | 42 ± 17.8 | 60.5 ± 49.6 | 0.56 ± 0.34 | 13 ± 8.9 | 307 ± 302 | 358 ± 289 | 1.3 ± 1 | 80 ± 23.7 | 564 ± 465 |
| ChAdOx/Mod | 38 ± 12.6 | 69.1 ± 48.6 | 0.49 ± 0.31 | 19 ± 16.3 | 286 ± 289 | 3747 ± 2492 | 8.2 ± 4.1 | 100 ± 0 | 5324 ± 6073 |
| ChAdOx/BNT | 38 ± 13.3 | 66.4 ± 58.6 | 0.66 ± 0.37 | 13 ± 11.9 | 235 ± 222 | 2595 ± 1548 | 6.3 ± 3.4 | 99 ± 1.5 | 4872 ± 3639 |
| Anti-NCP IgG pos. | 34 ± 8.9 | 841 ± 953 | 3.9 ± 4.6 | 94 ± 7.4 | 978 ± 1119 | 2771 ± 2540 | 8.3 ± 5.6 | 99 ± 1.5 | 7313 ± 8597 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markewitz, R.D.H.; Juhl, D.; Pauli, D.; Görg, S.; Junker, R.; Rupp, J.; Engel, S.; Steinhagen, K.; Herbst, V.; Zapf, D.; et al. Differences in Immunogenicity of Three Different Homo- and Heterologous Vaccination Regimens against SARS-CoV-2. Vaccines 2022, 10, 649. https://doi.org/10.3390/vaccines10050649
Markewitz RDH, Juhl D, Pauli D, Görg S, Junker R, Rupp J, Engel S, Steinhagen K, Herbst V, Zapf D, et al. Differences in Immunogenicity of Three Different Homo- and Heterologous Vaccination Regimens against SARS-CoV-2. Vaccines. 2022; 10(5):649. https://doi.org/10.3390/vaccines10050649
Chicago/Turabian StyleMarkewitz, Robert Daniel Heinrich, David Juhl, Daniela Pauli, Siegfried Görg, Ralf Junker, Jan Rupp, Sarah Engel, Katja Steinhagen, Victor Herbst, Dorinja Zapf, and et al. 2022. "Differences in Immunogenicity of Three Different Homo- and Heterologous Vaccination Regimens against SARS-CoV-2" Vaccines 10, no. 5: 649. https://doi.org/10.3390/vaccines10050649
APA StyleMarkewitz, R. D. H., Juhl, D., Pauli, D., Görg, S., Junker, R., Rupp, J., Engel, S., Steinhagen, K., Herbst, V., Zapf, D., Krüger, C., Brockmann, C., Leypoldt, F., Dargvainiene, J., Schomburg, B., Sharifzadeh, S. R., Salek Nejad, L., Wandinger, K.-P., & Ziemann, M. (2022). Differences in Immunogenicity of Three Different Homo- and Heterologous Vaccination Regimens against SARS-CoV-2. Vaccines, 10(5), 649. https://doi.org/10.3390/vaccines10050649






