Safety and Immunogenicity of Standard and Double Doses of Hepatitis B Vaccine in Children after Liver Transplantation: An Open-Label, Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.1.1. Randomisation and Masking
2.1.2. Vaccine and Administration
2.1.3. Study Procedures
2.1.4. Definition
2.2. Outcomes
2.2.1. Safety Assessments
2.2.2. Immunogenicity Assessments
Humoral Response
Cellular Response
2.3. Statistical Analysis
3. Results
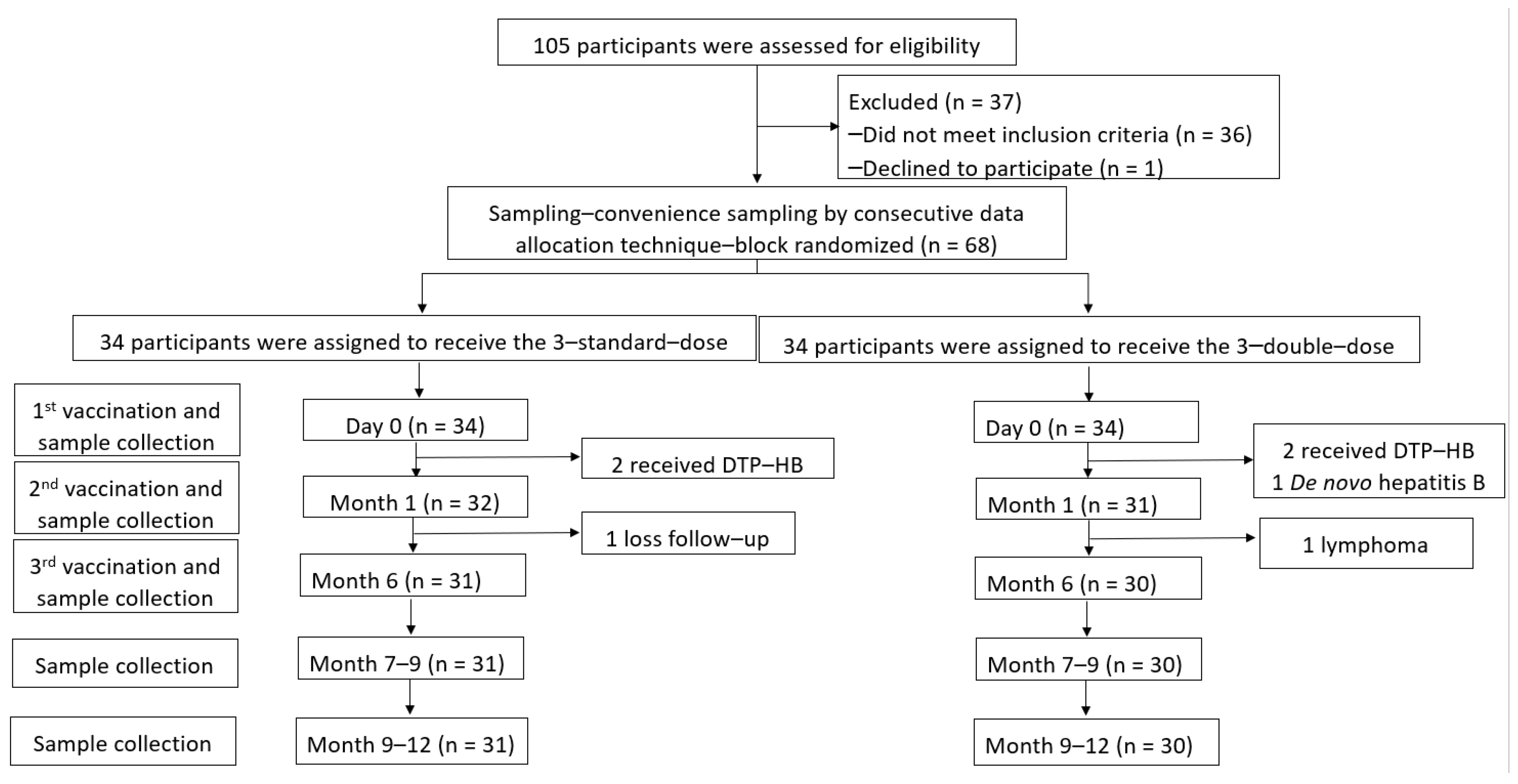
3.1. Study Populations
3.2. Safety of HepB Revaccination
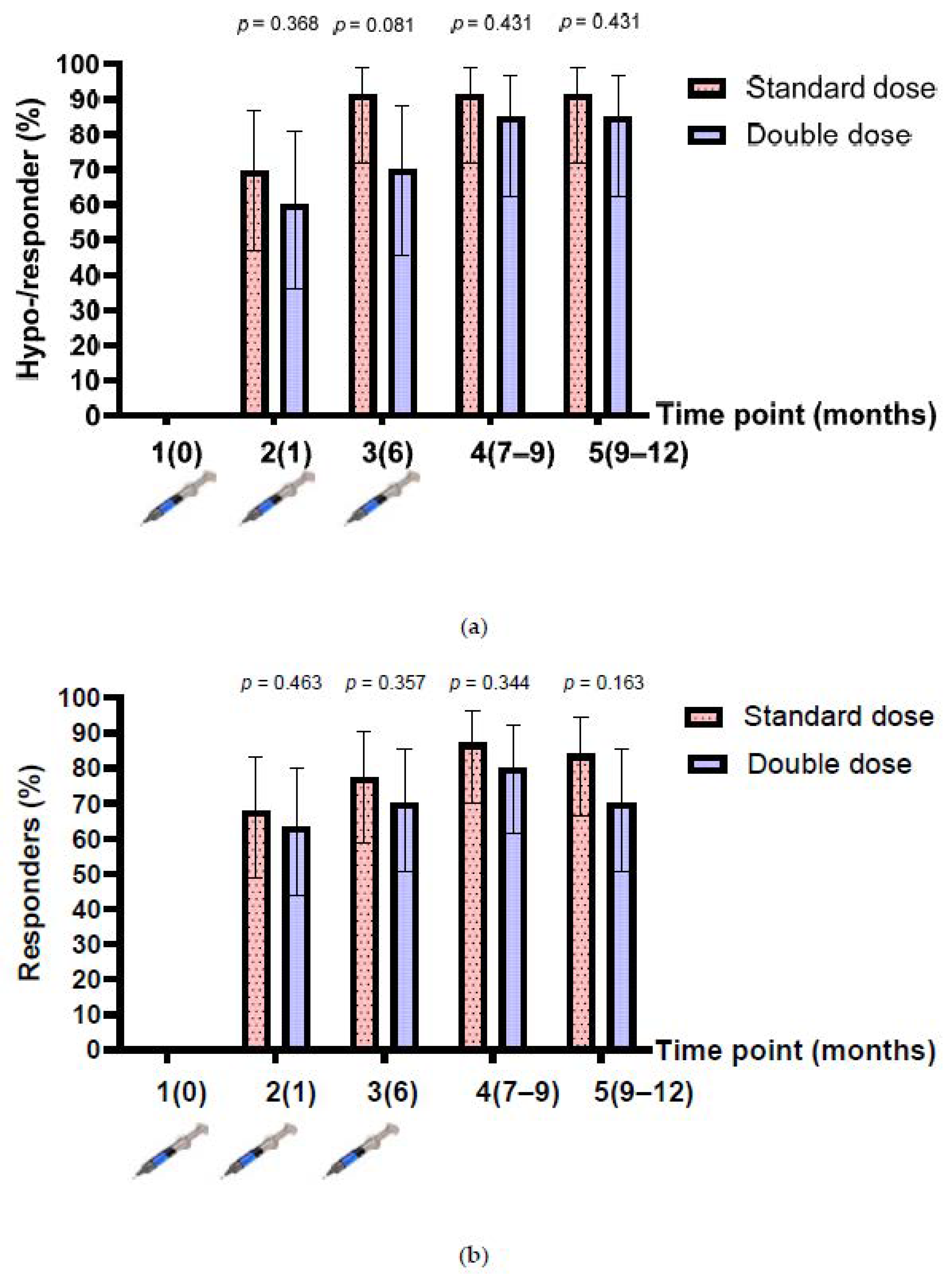
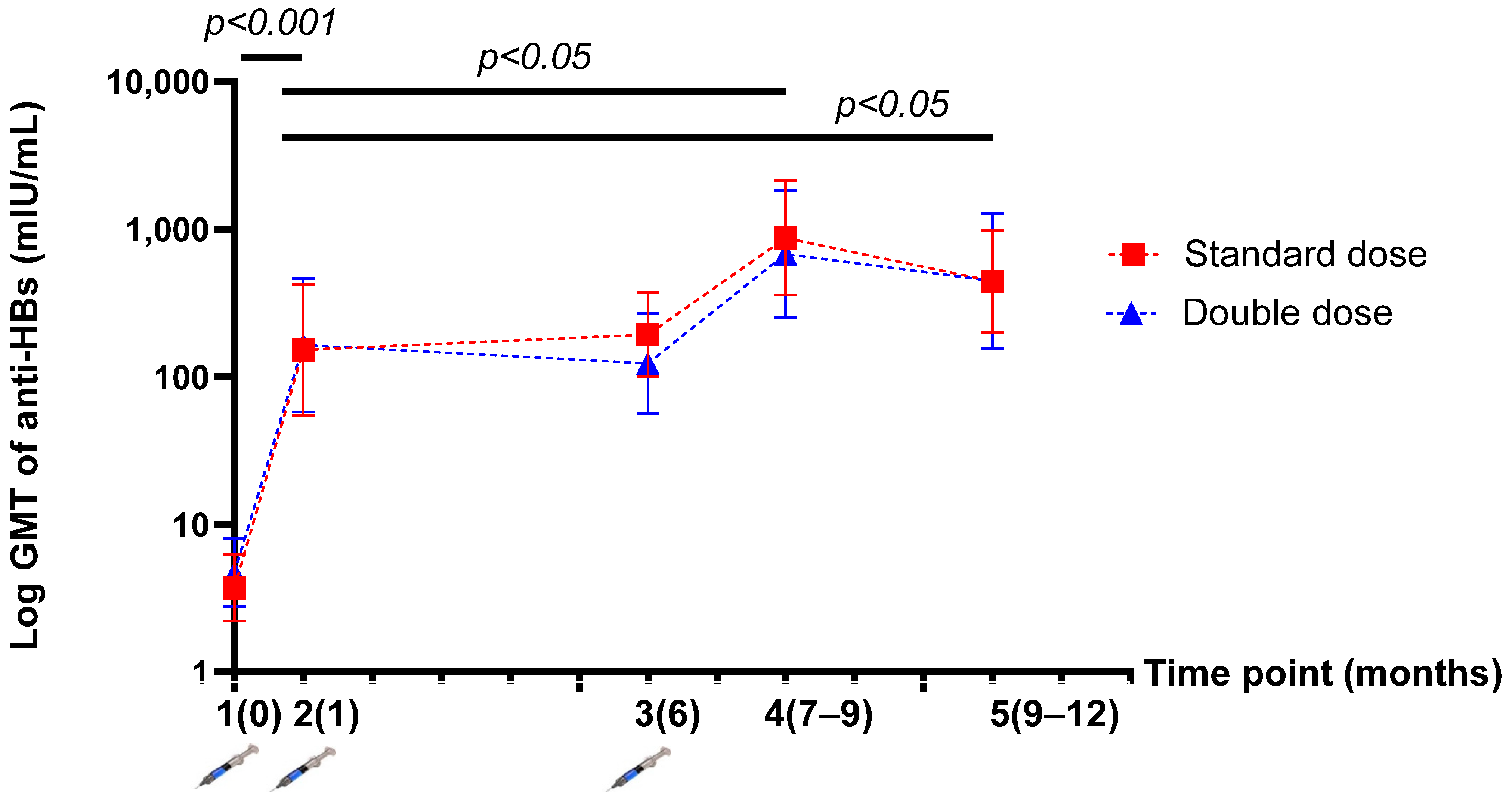
3.3. Humoral Response after HepB Revaccination
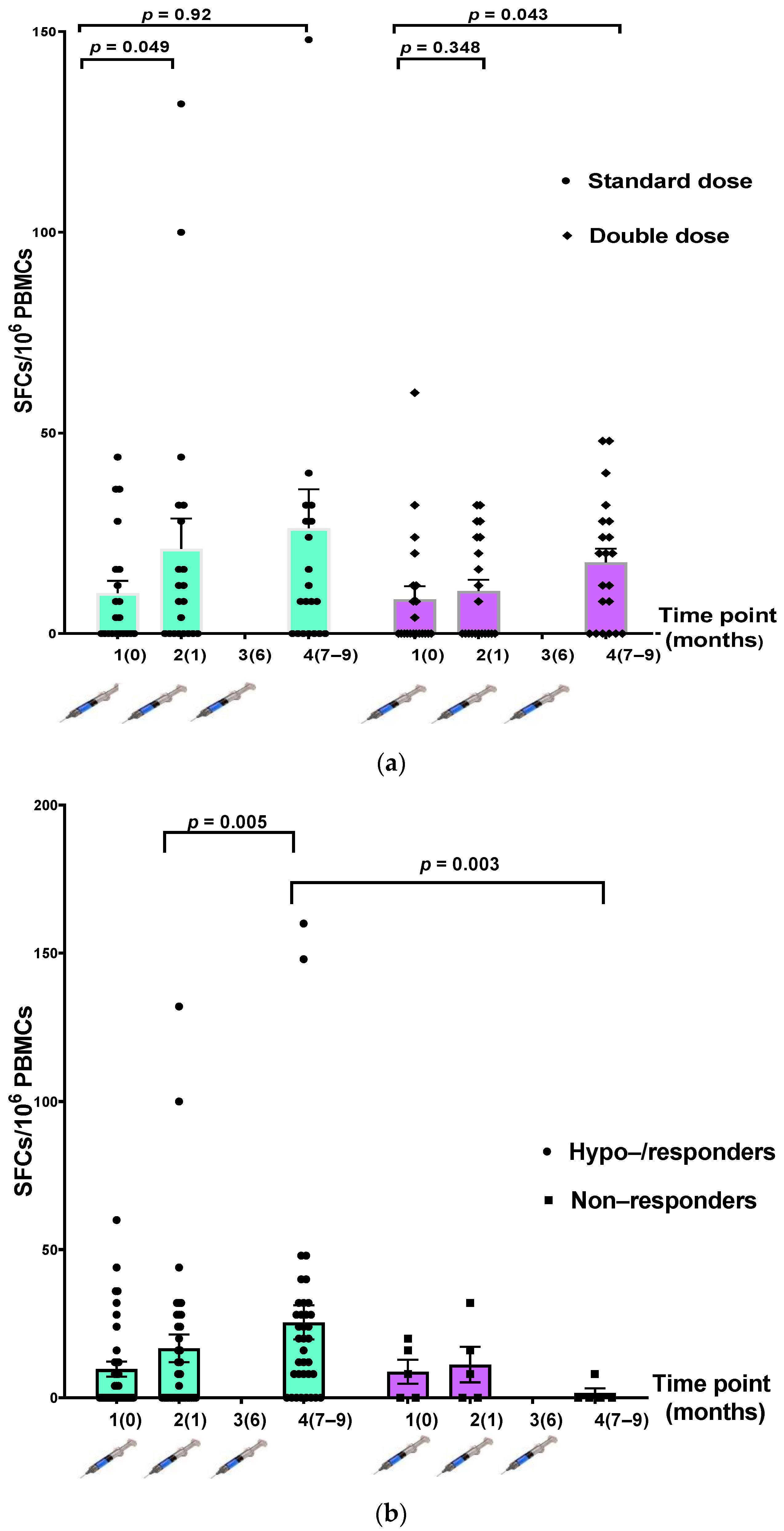
3.4. Cellular Response after HepB Vaccination
3.5. Factors Related to Humoral Immune Response after Three-Dose Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zampino, R.; Boemio, A.; Sagnelli, C.; Alessio, L.; Adinolfi, L.E.; Sagnelli, E.; Coppola, N. Hepatitis B virus burden in developing countries. World J. Gastroenterol. 2015, 21, 11941–11953. [Google Scholar] [CrossRef]
- WHO Global Hepatitis Report Hawaii. 2017. Available online: bitstream/handle/10665/255016/9789241565455-eng.pdf?sequence51 (accessed on 18 June 2021).
- Posuwan, N.; Wanlapakorn, N.; Sa-Nguanmoo, P.; Wasitthankasem, R.; Vichaiwattana, P.; Klinfueng, S.; Vuthitanachot, V.; Sae-Lao, S.; Foonoi, M.; Fakthongyoo, A.; et al. The success of a universal hepatitis B immunization program as part of Thailand’s EPI after 22 years’ implementation. PLoS ONE 2016, 11, e0150499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitz Simons, D.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B vaccination: A completed schedule enough to control HBV lifelong? Vaccine 2013, 31, 584–590. [Google Scholar] [CrossRef]
- Sintusek, P.; Posuwan, N.; Wanawongsawad, P.; Jitraruch, S.; Poovorawan, Y.; Chongsrisawat, V. High prevalence of hepatitis B-antibody loss and a case report of de novo hepatitis B virus infection in a child after living-donor liver transplantation. World J. Gastroenterol. 2018, 24, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chen, C.L.; Concejero, A.; Wang, C.-C.; Wang, S.-H.; Liu, Y.-W.; Yang, C.-H.; Yong, C.-C.; Lin, T.-S.; Jawan, B.; et al. Active immunization to prevent de novo hepatitis B virus infection in pediatric live donor liver recipients. Am. J. Transplant. 2007, 7, 195–200. [Google Scholar] [CrossRef]
- Song, Z.; Dong, C.; Meng, X.; Sun, C.; Wang, K.; Qin, H.; Han, C.; Yang, Y.; Zhang, F.; Zheng, W.; et al. Prophylactic strategy against de novo hepatitis B virus infection for pediatric recipients who receive hepatitis B core antibody–Positive liver grafts. Liver Transplant. 2021, 27, 96–105. [Google Scholar] [CrossRef]
- Chazouillères, O.; Mamish, D.; Kim, M.; Carey, K.; Wright, T.; Ferrell, L.; Roberts, J.; Ascher, N. “Occult” hepatitis B virus as source of infection in liver transplant recipients. Lancet 1994, 343, 142–146. [Google Scholar] [CrossRef]
- Douglas, D.D.; Rakela, J.; Taswell, H.F.; Krom, R.A.; Wiesner, R.H. Hepatitis B virus replication patterns after orthotopic liver transplantation: De novo versus recurrent infection. Transplant. Proc. 1993, 25, 1755–1757. [Google Scholar]
- Fabia, R.; Levy, M.F.; Crippin, J.; Tillery, W.; Netto, G.J.; Aguanno, J.; Dysert, P.; Goldstein, R.M.; Husberg, B.S.; Gonwa, T.A.; et al. De novo hepatitis B infection after liver transplantation: Source of disease, incidence, and impact. Liver Transplant. Surg. 1998, 4, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Y.-H.; Ho, M.-C.; Wu, J.-F.; Chen, H.-L.; Wu, Y.-M.; Hu, R.-H.; Lee, P.-H.; Chang, M.-H. Response to booster hepatitis B vaccines in liver-transplanted children primarily vaccinated in infancy. Transplantation 2008, 86, 1531–1535. [Google Scholar] [CrossRef]
- Lin, C.C.; Yong, C.C.; Chen, C.L. Active vaccination to prevent de novo hepatitis B virus infection in liver transplantation. World J. Gastroenterol. 2015, 21, 11112–11117. [Google Scholar] [CrossRef]
- Duca, P.; Del Pont, J.M.; D’Agostino, D. Successful immune response to a recombinant hepatitis B vaccine in children after liver transplantation. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 168–170. [Google Scholar] [CrossRef] [PubMed]
- David, M.C.; Ha, S.H.; Paynter, S.; Lau, C. A systematic review and meta-analysis of management options for adults who respond poorly to hepatitis B vaccination. Vaccine 2015, 33, 6564–6569. [Google Scholar] [CrossRef]
- Lee, J.H.; Hong, S.; Im, J.H.; Lee, J.S.; Baek, J.H.; Kwon, H.Y. Systematic review and meta-analysis of immune response of double dose of hepatitis B vaccination in HIV-infected patients. Vaccine 2020, 38, 3995–4000. [Google Scholar] [CrossRef]
- Bartholdy, B.; Matthias, P. Transcriptional control of B cell development and function. Gene 2004, 327, 1–23. [Google Scholar] [CrossRef]
- Arvilommi, H. ELISPOT for detecting antibody-secreting cells in response to infections and vaccination. APMIS 1996, 104, 401–410. [Google Scholar] [CrossRef]
- Leung, D.H.; Ton-That, M.; Economides, J.M.; Healy, C.M. High prevalence of hepatitis B nonimmunity in vaccinated pediatric liver transplant recipients. Am. J. Transplant. 2015, 15, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Cruciani, M.; Mengoli, C.; Serpelloni, G.; Lanza, A.; Gomma, M.; Nardi, S.; Rimondo, C.; Bricolo, F.; Consolaro, S.; Trevisan, M.; et al. Serologic response to hepatitis B vaccine with high dose and increasing number of injections in HIV infected adult patients. Vaccine 2009, 27, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Pettit, N.N.; De Pestel, D.D.; Malani, P.N.; Riddell, J., IV. Factors associated with seroconversion after standard dose hepatitis B vaccination and high-dose revaccination among HIV-infected patients. HIV Clin. Trials 2010, 11, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Rowley, K.; Payne, B.A.; Schmid, M.L. Determinants of response to repeat hepatitis B vaccination in HIV-infected prior non-responders. J. Infect. 2014, 69, 98–99. [Google Scholar] [CrossRef]
- De Vries-Sluijs, T.E.; Hansen, B.E.; van Doornum, G.J.; Springeling, T.; Evertsz, N.M.; De Man, R.A.; van der Ende, M.E. A prospective open study of the efficacy of high-dose recombinant hepatitis B rechallenge vaccination in HIV-infected patients. J. Infect. Dis. 2008, 197, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Psevdos, G.; Kim, J.H.; Groce, V.; Sharp, V. Efficacy of double-dose hepatitis B rescue vaccination in HIV-infected patients. AIDS Patient Care STDS 2010, 24, 403–407. [Google Scholar] [CrossRef]
- Rey, D.; Piroth, L.; Wendling, M.-J.; Miailhes, P.; Michel, M.-L.; Dufour, C.; Haour, G.; Sogni, P.; Rohel, A.; Ajana, F.; et al. Safety and immunogenicity of double-dose versus standard-dose hepatitis B revaccination in non-responding adults with HIV-1 (ANRS HB04 B-BOOST): A multicentre, open-label, randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1283–1291. [Google Scholar] [CrossRef]
- Siberry, G.K.; Abzug, M.J.; Nachman, S.; on behalf of the Panel on guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Exposed and HIV-infected Children–a working group of the Office of AIDS Research (OAR) Advisory Council. Executive summary: Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: Recommendations from the National Institutes of Health, the Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. J. Pediatric. Infect. Dis. Soc. 2013, 2, 293–308. [Google Scholar]
- Wilkins, E.; Nelson, M.; Agarwal, K.; Awoyemi, D.; Barnes, E.; Bhagani, S.; Brook, G.; Brown, A.; Castelino, S.; Cooke, G.; et al. British HIV Association guidelines for the management of hepatitis viruses in adults infected with HIV 2013. HIV Med. 2013, 14 (Suppl. S4), 1–71. [Google Scholar]
- Doi, H.; Kanto, T. Factors influencing the durability of hepatitis B vaccine responses. Vaccine 2021, 39, 5224–5230. [Google Scholar] [CrossRef]
- Ou, G.; Liu, X.; Jiang, Y. HLA-DPB1 alleles in hepatitis B vaccine response: A meta-analysis. Medicine 2021, 14, e24904. [Google Scholar] [CrossRef]
- Carollo, M.; Palazzo, R.; Bianco, M.; Pandolfi, E.; Chionne, P.; Fedele, G.; Tozzi, A.E.; Carsetti, R.; Romano, L.; Ausiello, C.M. Hepatitis B specific T cell immunity induced by primary vaccination persists independently of the protective serum antibody level. Vaccine 2013, 31, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Bauer, T.; Gunther, M.; Bienzle, U.; Neuhaus, R.; Jilg, W. Vaccination against hepatitis B in liver transplant recipients: Pilot analysis of cellular immune response shows evidence of HBsAg-specific regulatory T cells. Liver Transpl. 2007, 13, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Bolther, M.; Andersen, K.L.D.; Tolstrup, M.; Visvanathan, K.; Woolley, I.; Skinner, N.; Warner, N.; Ostergaard, L.; Jensen-Fangel, S. Levels of regulatory B cells do not predict serological responses to hepatitis B vaccine. Hum. Vaccin. Immunother. 2018, 14, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Leung, D.H. Hepatitis B immunity in the pediatric liver transplant population. Curr. Opin. Pediatr. 2016, 28, 653–658. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Standard Dose (n = 34) | Double Dose (n = 34) |
|---|---|---|
| Age at transplantation (years) | 1.06 (0.84, 2.87) | 1.72 (1.19, 5.74) |
| Male (n, % male) | 15 (44.2) | 18 (52.9) |
| Time at hepatitis B revaccination (years) | 1.44 (0.66, 3.59) | 1.69 (0.67, 4.97) |
| Number of immunosuppressants administered (n, %) | ||
| None | 1 (2.9) | 1 (2.9) |
| One | 15 (44.1) | 15 (44.1) |
| Two | 13 (38.2) | 14 (41.2) |
| Three | 5 (14.7) | 4 (11.7) |
| Type of immunosuppressants administered (n, %) prevaccination | ||
| Tacrolimus | 15 (44.1) | 18 (52.9) |
| Cyclosporin | 5 (14.7) | 4 (11.8) |
| More than one | 14 (40.2) | 12 (35.2) |
| Level of immunosuppression (ng/mL) | ||
| Tacrolimus | 3.4 (2.7, 5.4) | 3.7 (3.0, 4.8) |
| Cyclosporin | 217 (123, 629) | 386 (106, 937.5) |
| Pretransplant disease; BA (n, %) | 26 (76.5) | 24 (70.5) |
| Complications (PTLD, graft rejection, and surgical conditions) (n, %) | 18 (52.9) | 14 (41.2) |
| Anti-HBs level (mIU/mL) prevaccination | 1.75 (0.40, 13.8) | 2.7 (0.8, 14) |
| Anti-HBs level prevaccination (n, %) | ||
| <10 mIU/mL | 24 (70.6) | 22 (64.7) |
| 10–99 mIU/mL | 8 (23.5) | 10 (29.4) |
| ≥100 mIU/mL | 2 (5.9) | 2 (5.9) |
| Laboratory investigation | ||
| SGOT (IU/L) | 41 (35.5, 51) | 41 (32, 55) |
| SGPT (IU/L) | 31 (19, 42) | 26 (18, 49.5) |
| GGT (IU/L) | 24 (18, 63) | 34 (22, 83.5) |
| Albumin (g/dL) | 4.2 (4, 4.4) | 4.1 (3.85, 4.2) |
| Hb (g/dL) | 12 (10.8, 12.7) | 10.8 (9.9, 12.2) |
| WBC (106/L) | 8290 (6840, 12,150) | 7760 (5605, 9835) |
| Lymphocyte count (×106/L) | 1365 (288, 6180) | 2900 (660, 4480) |
| Platelet count (×109) | 244 (203.5, 313) | 230 (178.5, 320) |
| Table | Time from Enrolment (Days) | GMT (95% CI), mIU/mL | p-Value | |
|---|---|---|---|---|
| Standard Dose (n = 31) | Double Dose (n = 30) | |||
| 1 | 0 | 3.71 (2.20–6.25) | 4.70 (2.76–7.99) | 0.498 |
| 2 | 28 (28, 35) | 151.93 (54.67–422.26) ** | 163.75 (57.74–464.38) ** | 0.160 |
| 3 | 193 (175, 210) | 193.58 (100.79–371.79) | 123.39 (56.52–269.34) | 0.124 |
| 4 | 280 (262, 329) | 871.65 (358.42–2119.78) * | 677.18 (251.80–2401.82) * | 0.207 |
| 5 | 392 (306, 448) | 443.33 (200.75–979.07) * | 446.17 (155.58–1279.50) * | 0.164 |
| Time Point | IFN-γ-Secreting Cells (SFCs/106 PBMCs) | p-Value | IFN-γ-Secreting Cells (SFCs/106 PBMCs) | p-Value | ||
|---|---|---|---|---|---|---|
| Standard Dose (n = 21) | Double Dose (n = 21) | Hypo-/Responders (n = 37) | Nonresponders (n = 5) | |||
| 1 (first dose) | 4 (0, 16) | 0 (0, 12) | 0.559 | 0 (0, 12) | 8 (0, 12) | 0.690 |
| 2 (second dose) | 8 (0, 28) * | 0 (0, 24) | 0.420 | 8 (0, 24) | 0 (0, 12) | 0.624 |
| 3 (third dose) | - | - | - | - | - | - |
| 4 (after third dose) | 8 (0, 28) | 20 (0, 28) * | 0.778 | 20 (8, 28) * | 0 (0, 0) | 0.003 |
| Parameters | Hyporesponders/ Responders (n = 38) | Nonresponders (n = 5) |
|---|---|---|
| Age at transplantation (years) | 1.35 (0.83, 3.01) | 3.45 (0.71, 13.58) |
| Male (n, % male) | 20 (52.6) | 2 (50) |
| Time at hepatitis B revaccination (years) | 1.95 (0.66, 4.95) | 0.58 (0.54, 0.65) |
| Vaccination protocol, standard dose (n, %) | 21 (55.3%) | 2 (40%) |
| Number of immunosuppressants administered (n, %) | ||
| None | 1 (2.4) | - |
| One | 30 (50) | 5 |
| Two | 6 (38.1) | - |
| Type of immunosuppressants administered (n, %) | ||
| Tacrolimus | 30 (78.6) | 5 (93.3) |
| Cyclosporin | 6 (19) | - |
| None | 1 (2.4) | - |
| Level of immunosuppression (ng/mL) prevaccination | ||
| Tacrolimus | 3.6 (2.6, 5.7) | 6.7 (5.8–7.8) |
| Cyclosporin | 209 (94, 941) | - |
| Pretransplant diseases (n, %) | ||
| BA | 28 (73.8) | 4 (80) |
| Others | 9 (26.2) | 1 (20) |
| Complications (PTLD, rejection, and surgical conditions) (n, %) | 20 (52.4) | 1 (40) |
| Anti-HBs level (mIU/mL) prevaccination | 5.5 (0.6, 13.9) | 1 (0.2, 1.7) |
| Laboratory investigation | ||
| SGOT (IU/L) | 41 (33, 54) | 42 (28, 50) |
| SGPT (IU/L) | 30 (19, 46) | 28 (18, 56) |
| GGT (IU/L) | 30 (21, 66) | 113 (28, 181) |
| Albumin (g/dL) | 4.1 (3.9, 4.3) | 3.9 (3.6, 4.4) |
| Hb (g/dL) | 11.8 (10.4, 12.7) | 10.2 (8.8, 14.2) |
| WBC (×106/L) | 7810 (6332, 10,862) | 8675 (3642, 9627) |
| Lymphocyte count (×106/L) | 619 (266, 3808) | 4020 (941, 5527) |
| Platelet count (×109) | 232 (174, 313) | 298 (204, 425) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sintusek, P.; Buranapraditkun, S.; Wanawongsawad, P.; Posuwan, N.; Thantiworasit, P.; Wanlapakorn, N.; Klaewsongkram, J.; Suratannon, N.; Chaijitraruch, N.; Chongsrisawat, V.; et al. Safety and Immunogenicity of Standard and Double Doses of Hepatitis B Vaccine in Children after Liver Transplantation: An Open-Label, Randomised Controlled Trial. Vaccines 2022, 10, 92. https://doi.org/10.3390/vaccines10010092
Sintusek P, Buranapraditkun S, Wanawongsawad P, Posuwan N, Thantiworasit P, Wanlapakorn N, Klaewsongkram J, Suratannon N, Chaijitraruch N, Chongsrisawat V, et al. Safety and Immunogenicity of Standard and Double Doses of Hepatitis B Vaccine in Children after Liver Transplantation: An Open-Label, Randomised Controlled Trial. Vaccines. 2022; 10(1):92. https://doi.org/10.3390/vaccines10010092
Chicago/Turabian StyleSintusek, Palittiya, Supranee Buranapraditkun, Piyaporn Wanawongsawad, Nawarat Posuwan, Pattarawat Thantiworasit, Nasamon Wanlapakorn, Jettanong Klaewsongkram, Narissara Suratannon, Nataruks Chaijitraruch, Voranush Chongsrisawat, and et al. 2022. "Safety and Immunogenicity of Standard and Double Doses of Hepatitis B Vaccine in Children after Liver Transplantation: An Open-Label, Randomised Controlled Trial" Vaccines 10, no. 1: 92. https://doi.org/10.3390/vaccines10010092







