A Comparative Study of the Effect of Commonly Used Pesticides on Cervical Contractions in Pregnant Cows, In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material
2.3. Preliminary Experiment: Effect of Pesticides on Cell Viability
2.4. Experiment: Effect of Pesticides on Cervical Smooth Muscle Contractility
2.5. Determination of Cell Viability
2.6. Measurement of Smooth Muscle Contractions
2.7. Statistical Analysis
3. Results
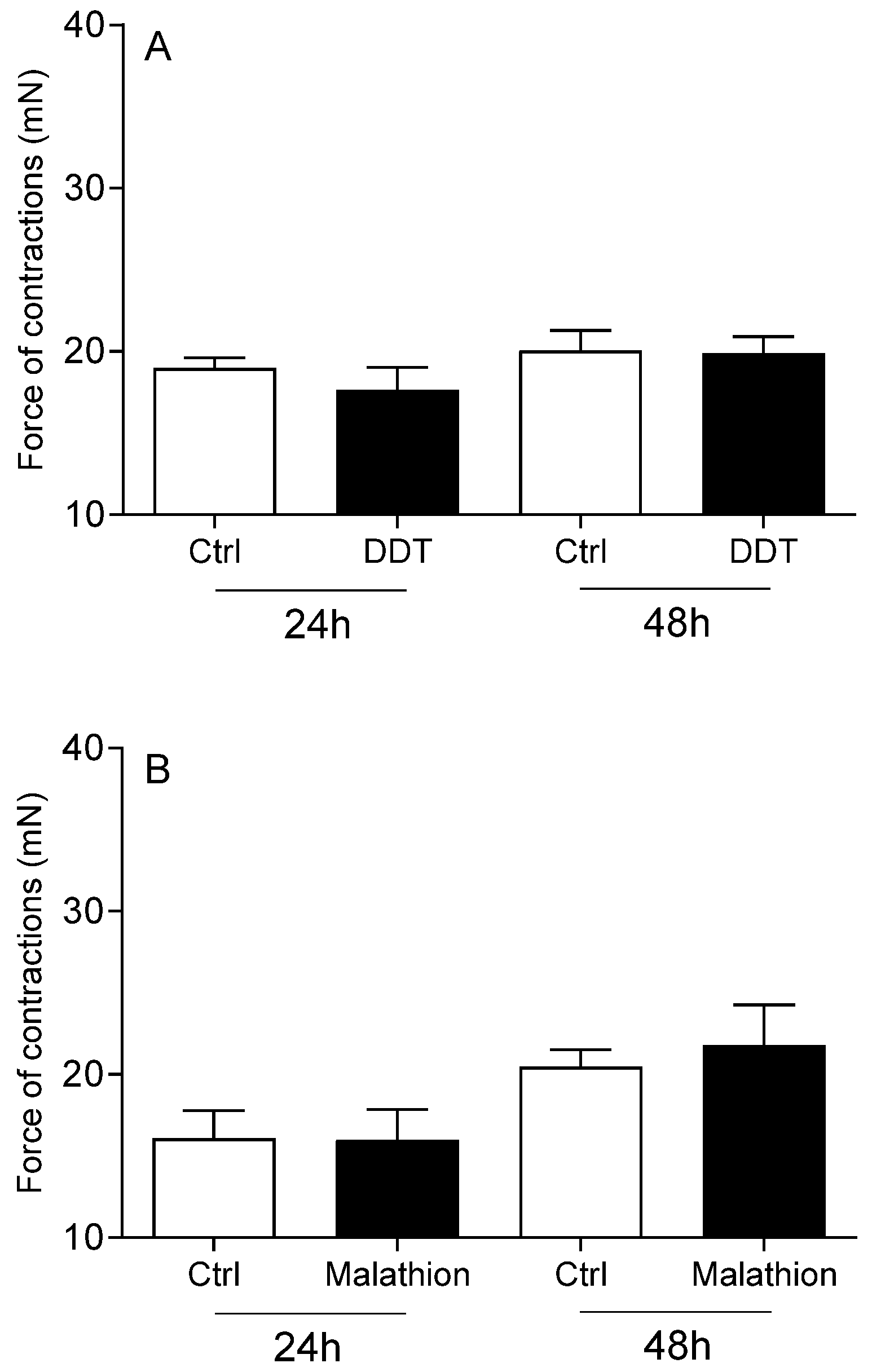
- The changes in cervical contractions were not evoked by the cytotoxic effects of the applied pesticides.
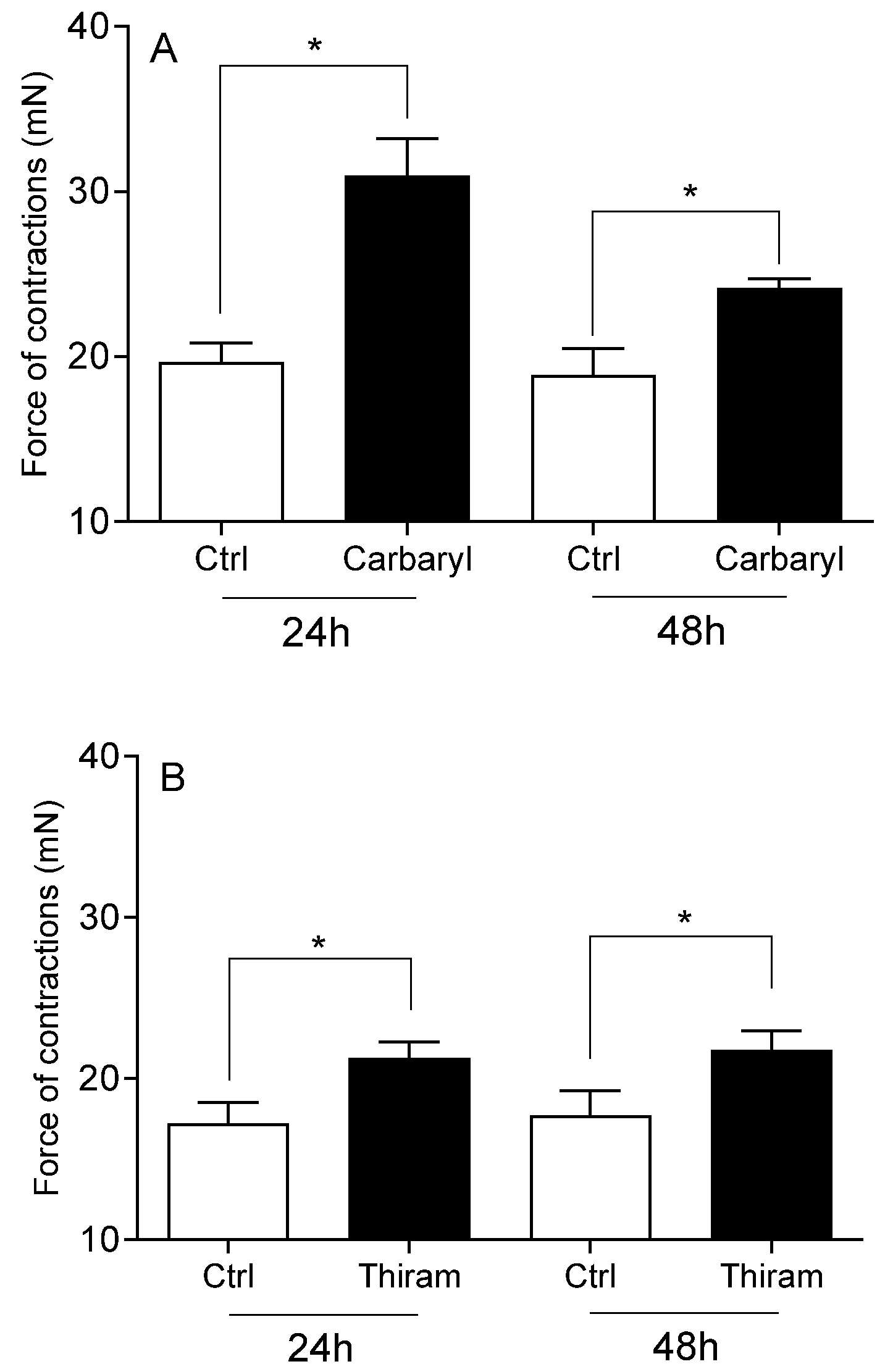
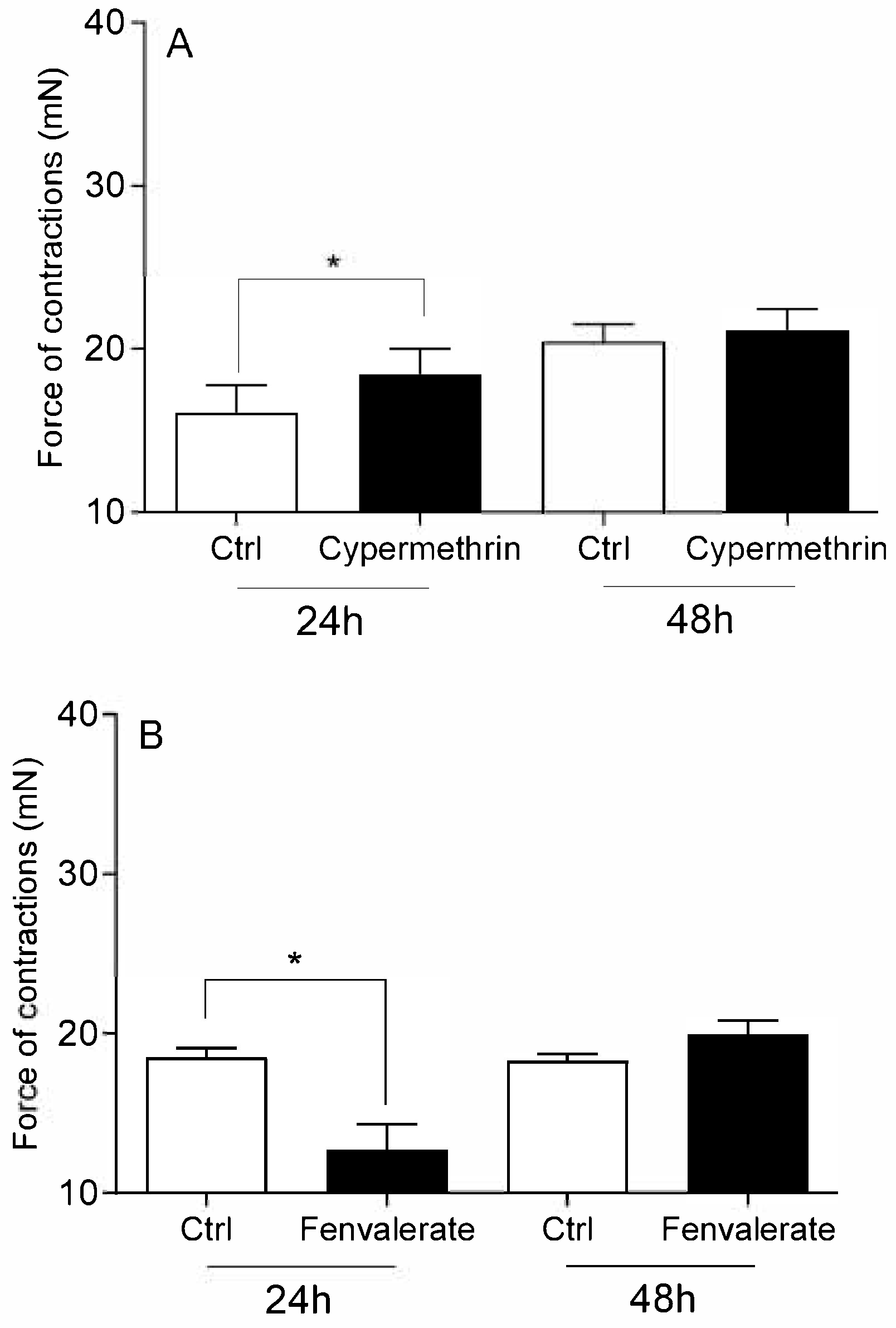
- Carbaryl, cypermethrin, thiram, and glyphosate increased the force of contraction of cervical strips.
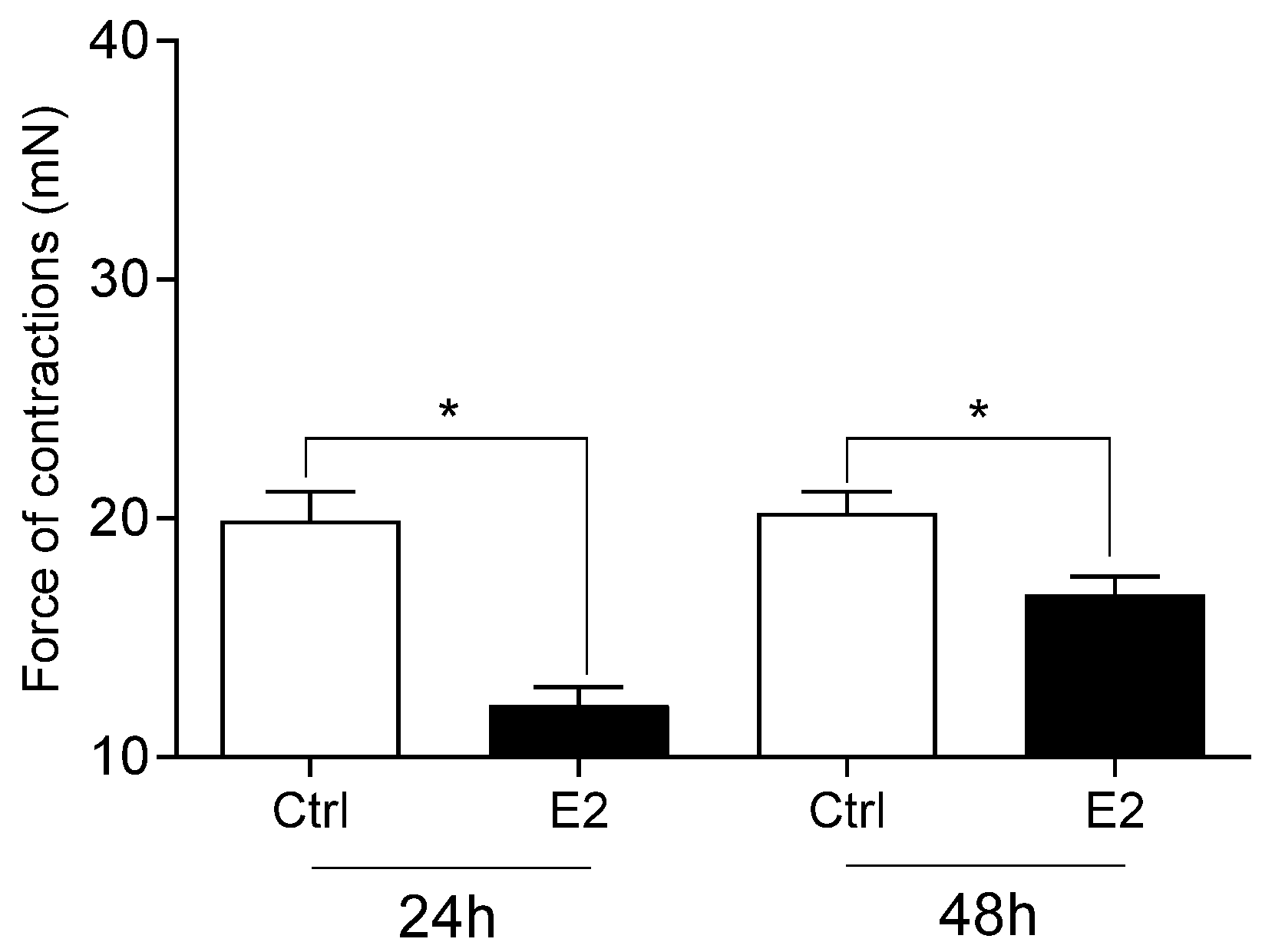
- The administration of fenvalerate and E2 reduced in the intensity of cervical contractions.
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogan, W.J.; Chen, A. Health risks and benefits of bis(4-chlophenyl)-1,1,1-trichloroethane (DDT). Lancet 2005, 366, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Zhulidovb, A.V.; Robarts, R.D.; Korotovad, L.G.; Zhulidovb, D.A.; Gurtovayab, T.Y.; Gee, L.P. Dichlorodiphenyltrichloroethane usage in the former Soviet Union. Sci. Total Environ. 2006, 357, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kaindoa, E.W.; Matowo, N.S.; Ngowo, H.S.; Mkandawile, G.; Mmbando, A.; Finda, M.; Okumu, F.O. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS ONE 2017, 12, e0177807. [Google Scholar] [CrossRef]
- Sardar, A.A.; Pabitra, S.; Moytrey, C.; Bera, D.K.; Biswas, P.; Maji, D.; Guha, S.K.; Basu, N.; Maji, A.K. Insecticide susceptibility status of Phlebotomus argentipes and polymorphisms in voltage-gated sodium channel (vgsc) gene in Kala-azar endemic areas of West Bengal, India. Acta Tropica 2018, 185, 285–293. [Google Scholar] [CrossRef]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J.B. Pesticides with potential thyroid hormone-disrupting effects: A review of recent data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef]
- Asghar, M.; Yaqoob, M.; Haque, N.; Nabi, A. Determination of thiram and aminocarb pesticides in natural water samples using flow injection with tris(2,2′-bipyridyl)ruthenium(II)-diperiodatoargentate(III) chemiluminescence detection. Anal. Sci. 2013, 29, 1061–1066. [Google Scholar] [CrossRef][Green Version]
- Liu, K.; Li, Y.; Iqbal, M.; Tang, Z.; Zhang, H. Thiram exposure in environment: A critical review on cytotoxicity. Chemosphere 2022, 295, 133928. [Google Scholar] [CrossRef]
- Marettova, E.; Maretta, M.; Legáth, J. Effect of pyrethroids on female genital system. Anim. Reprod. Sci. 2017, 184, 132–138. [Google Scholar] [CrossRef]
- Kadian, N.; Gupta, A.; Satya, S.; Mehta, R.K.; Malik, A. Biodegradation of herbicide (atrazine) in contaminated soil using various bioprocessed materials. Bioresource Technol. 2008, 99, 4642–4647. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, F.; Whited, M.; Knight, P. Would banning atrazine benefit farmers? Int. J. Occup. Environ. Health 2014, 20, 61–70. [Google Scholar] [CrossRef][Green Version]
- Lizotte, R.; Locke, M.; Bingner, R.; Steinriede, R.W.; Smith, S. Effectiveness of integrated best management practices on mitigation of atrazine and metolachlor in an agricultural lake watershed. Bull. Environ. Contam. Toxicol. 2017, 98, 447–543. [Google Scholar] [CrossRef]
- Derbalah, A.; Chidya, R.; Kaonga, C.; Iwamoto, Y.; Takeda, K.; Sakugawa, H. Carbaryl residue concentrations, degradation, and major sinks in the Seto Inland Sea, Japan. Environ. Sci. Pollut. Res. Int. 2020, 27, 14668–14678. [Google Scholar] [CrossRef]
- Kanyika-Mbewe, C.; Thole, B.; Makwinja, R.; Kaonga, C.C. Monitoring of carbaryl and cypermethrin concentrations in water and soil in Southern Malawi. Environ. Monit. Assess. 2020, 192, 595. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Finger, B.J.; Scott, A.W.; Harvey, A.J.; Green, M.P. Acute in vitro exposure to environmentally relevant atrazine levels perturbs bovine preimplantation embryo metabolism and cell number. Reprod Toxicol. 2019, 87, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Patra, K.; Jana, S.; Mandal, D.P.; Bhattacharjee, S. Exposure to environmentally relevant concentrations of malathion induces significant cellular, biochemical and histological alterations in Labeo rohita. Pestic. Biochem. Physiol. 2016, 126, 49–57. [Google Scholar] [CrossRef]
- Kongtip, P.; Nankongnab, N.; Phupancharoensuk, R.; Palarach, C.; Sujirarat, D.; Sangprasert, S.; Sermsuk, M.; Sawattrakool, N.; Woskie, S.R. Glyphosate and paraquat in maternal and fetal serums in Thai women. Agromedicine 2017, 22, 282–289. [Google Scholar] [CrossRef]
- Kamarianos, A.; Karamanlis, X.; Goulas, P.; Theodosiadou, E.; Smokovitis, A. The presence of environmental pollutants in follicular fluid of farm animals (cattle, sheep, goats and pigs). Reprod. Toxicol. 2003, 17, 185–195. [Google Scholar] [CrossRef]
- Lederman, S.A. Environmental contaminants in breast milk from the central Asian republics. Reprod. Toxicol. 1996, 10, 93–104. [Google Scholar] [CrossRef]
- Chen, X.; Panuwet, P.; Hunter, R.E.; Riederer, A.M.; Bernoudy, G.C.; Barr, D.B.; Ryan, P.B. Method for the quantification of current use and persistent pesticides in cow milk, human milk and baby formula using gas chromatography tandem mass spectrometry. J. Chromatogr. B 2014, 970, 121–130. [Google Scholar] [CrossRef]
- Shan, G.; Wengatz, I.; Stoutamire, D.W.; Gee, S.J.; Hammock, B.D. An enzyme-linked immunosorbent assay for the detection of esfenvalerate metabolites in human urine. Chemi. Res. Toxicol. 1999, 12, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Simaremare, S.; Hung, C.; Hsieh, C.; Yiin, L. Relationship between Organophosphate and Pyrethroid Insecticides in Blood and Their Metabolites in Urine: A Pilot Study. Int. J. Environ. Res. Public Health 2019, 17, 34. [Google Scholar] [CrossRef]
- Brander, S.M.; Gabler, M.K.; Fowler, N.L.; Connon, R.E.; Schlenk, D. Pyrethroid pesticides as endocrine disruptors: Molecular mechanisms in vertebrates with a focus on fishes. Environ. Sci. Technol. 2016, 50, 8977–8992. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, G.; Figà-Talamanca, I.; Lauria, L.; Mantovani, A. Spontaneous abortion in spouses of greenhouse workers exposed to pesticides. Environ. Health Prev. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.C.; Siddiqui, M.K.; Bhargava, A.K.; Seth, T.D.; Krishnamurti, C.R.; Kutty, D. Role of chlorinated hydrocarbon pesticides in abortions and premature labour. Toxicology 1980, 17, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, I.; Daniel, V.; Link, S.; Monga, B.; Runnebaum, B. Chlorinated hydrocarbons in women with repeated miscarriages. Environ. Health Perspect. 1998, 106, 675–681. [Google Scholar] [CrossRef]
- Korrick, S.A.; Chen, C.; Damokosh, A.I.; Ni, J.; Liu, X.; Cho, S.; Altshul, L.; Ryan, L.; Xu, X. Association of DDT with spontaneous abortion: A case-control study. Ann. Epidemiol. 2001, 11, 491–496. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Klebanoff, M.A.; Zhou, H.; Brock, J.W. Association between maternal serum concentration of the DDT metabolite DDE and preterm and small-for-gestational-age babies at birth. Lancet 2001, 358, 110–114. [Google Scholar] [CrossRef]
- Torres-Arreola, L.; Berkowitz, G.; Torres-Sánchez, L.; López-Cervantes, M.; Cebrián, M.E.; Uribe, M.; López-Carrillo, L. Preterm birth in relation to maternal organochlorine serum levels. Ann. Epidemiol. 2003, 13, 158–162. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Klebanoff, M.A.; Dunson, D.B.; Guo, X.; Chen, Z.; Zhou, H.; Brock, J.W. Maternal serum level of the DDT metabolite DDE in relation to fetal loss in previous pregnancies. Environ. Res. 2005, 97, 127–133. [Google Scholar] [CrossRef]
- Venners, S.A.; Korrick, S.; Xu, X.; Chen, C.; Guang, W.; Huang, A.; Altshul, L.; Perry, M.; Fu, L.; Wang, X. Preconception serum DDT and pregnancy loss: A prospective study using a biomarker of pregnancy. Am. J. Epidemiol. 2005, 162, 709–716. [Google Scholar] [CrossRef]
- Pathak, R.; Suke, S.G.; Ahmed, T.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K.; Sharma, C.S.; Makhijani, S.D.; Banerjee, B.D. Organochlorine pesticide residue levels and oxidative stress in preterm delivery cases. Hum. Exp. Toxicol. 2010, 29, 351–358. [Google Scholar] [CrossRef]
- Mustafa, M.D.; Banerjee, B.D.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K. Gene-environment interaction in preterm delivery with special reference to organochlorine pesticides. Mol. Hum. Reprod. 2013, 19, 35–42. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, A.; Zhang, J.; Hu, S. Maternal exposure to the mixture of organophosphorus pesticides induces reproductive dysfunction in the offspring. Environ. Toxicol. 2011, 28, 507–515. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Baez, G.M.; Garcia-Guerra, A.; Toledo, M.Z.; Monteiro, P.L.; Melo, L.F.; Ochoa, J.C.; Santos, J.E.; Sartori, R. Pivotal periods for pregnancy loss during the first trimester of gestation in lactating dairy cows. Theriogenology 2016, 86, 239–253. [Google Scholar] [CrossRef]
- Shennan, A.; Jones, B. The cervix and prematurity: Aetiology, prediction and prevention. Semin. Fetal Neonatal Med. 2004, 9, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Bekana, M.; Jonsson, P.; Kindahl, H. Bacterial isolates associated with retained fetal membranes and subsequent ovarian activity in cattle. Vet. Rec. 1997, 140, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Martyn, F.; McAuliffe, F.M.; Wingfield, M. The role of the cervix in fertility: Is it time for a reappraisal? Hum. Reprod. 2014, 29, 2092–2098. [Google Scholar] [CrossRef]
- Breeveld-Dwarkasing, V.N.A. The Bovine Cervix Explored: The Cow as a Model for Studies on Functional Changes in the Uterine Cervix. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2002. [Google Scholar]
- Vink, J.; Feltovich, H. Cervical etiology of spontaneous preterm birth. Semin. Fetal. Neonatal Med. 2016, 21, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.Y.; Qin, S.; Brock, C.O.; Zork, N.M.; Feltovich, H.M.; Chen, X.; Urie, P.; Myers, K.M.; Hall, T.J.; Wapner, R.; et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am. J. Obstet. Gynecol. 2016, 215, 478.e1–478.e11. [Google Scholar] [CrossRef]
- Mlynarczuk, J.; Wróbel, M.H.; Kotwica, J. Effect of environmental pollutants on oxytocin synthesis and secretion from corpus luteum and on contractions of uterus from pregnant cows. TAAP 2010, 247, 243–249. [Google Scholar] [CrossRef]
- Breeveld-Dwarkasing, V.N.A.; van der Weijden, G.C.; Taverne, M.A.M.; van Dissel-Emiliani, F.M.F. The bovine cervix during the oestrous cycle: Regional differences in morphology and density of steroid hormone receptors. Reprod. Dom. Anim. 2000, 35, 120–124. [Google Scholar] [CrossRef]
- van Engelen, E.; Taverne, M.A.; Everts, M.E.; van der Weijden, G.C.; Doornenbal, A.; Breeveld-Dwarkasing, V.N. EMB activity of the muscular and stromal layer of the cervix in relation to EMG activity of the myometrium and cervical dilatation in PGF2α induced parturition in the cow. Theriogenology 2007, 67, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.O.; Fitzpatrick, R.J.; Nixon, W.C. Activity of the human cervix and corpus uteri. Their response to drugs in early pregnancy. Lancet 1951, 1, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Huszar, G.; Frederick, N. The myometrium and uterine cervix in normal and preterm labor. N. Engl. J. Med. 1984, 311, 571–581. [Google Scholar] [CrossRef]
- Egarter, C.H.; Husslein, P. Biochemistry of myometrial contractility. Baillieres Clin. Obstet. Gynaecol. 1992, 6, 755–769. [Google Scholar] [CrossRef]
- Calder, A.A.; Greer, I.A. Pharmacological modulation of cervical compliance in the first and second trimesters of pregnancy. Semin. Perinatol. 1991, 15, 162–172. [Google Scholar]
- Candappa, I.B.R.; Bartlewski, P.M. A review of advances in artificial insemination (AI) and embryo transfer (ET) in sheep, with the special reference to hormonal induction of cervical dilation and its implications for controlled animal reproduction and surgical techniques. Open Reprod. Sci. J. 2011, 311, 162–175. [Google Scholar] [CrossRef]
- Juberg, D.R.; Loch-Caruso, R. Investigation prostaglandin of the role of estrogenic action and E2 in DDT-stimulated rat uterine contractions ex vivo. Toxicology 1992, 74, 161–172. [Google Scholar] [CrossRef]
- Bolt, H.M.; Degen, G.H. Comparative assessment of endocrine modulators with oestrogenic activity. II. Persistent organochlorine pollutants. Arch. Toxicol. 2002, 76, 187–193. [Google Scholar] [CrossRef]
- Tange, S.; Fujimoto, N.; Uramaru, N.; Wong, F.F.; Sugihara, K.; Ohta, S.; Kitamura, S. In vitro metabolism of methiocarb and carbaryl in rats, and its effect on their estrogenic and antiandrogenic activities. Environ. Toxicol. Pharmacol. 2016, 41, 289–297. [Google Scholar] [CrossRef]
- Taylor, J.S.; Thomson, B.M.; Lang, C.N.; Sin, F.Y.T.; Podivinsky, E. Estrogenic pyrethroid pesticides regulate expression of estrogen receptor transcripts in mouse Sertoli cells differently from 17beta-estradiol. J. Toxicol. Environ. Health A 2010, 73, 1075–1089. [Google Scholar] [CrossRef]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Phedonos, A.; Biserni, M.; Arno, M.; Balu, S.; Corton, J.C.; Ugarte, R.; Antoniou, M.N. Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem. Toxicol. 2017, 108, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.H.; Jung, D.W.; Lee, H.S. Confirmation of the steroid hormone receptor-mediated endocrine disrupting potential of fenvalerate following the Organization for Economic Cooperation and Development test guidelines, and its estrogen receptor alpha-dependent effects on lipid accumulation. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 283, 109955. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.J.; Lee, J.; Park, H.L. Organophosphate pesticide exposure and breast cancer risk: A rapid review of human, animal, and cell-based studies. Int. J. Environ. Res. Public Health 2020, 17, 5030. [Google Scholar] [CrossRef]
- Albanito, L.; Lappano, R.; Madeo, A.; Chimento, A.; Prossnitz, E.R.; Cappello, A.R.; Dolce, V.; Abonante, S.; Pezzi, V.; Maggiolini, M. Effects of atrazine on estrogen receptor α- and G protein-coupled receptor 30-mediated signaling and proliferation in cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 2015, 123, 493–499. [Google Scholar] [CrossRef]
- Cragin, L.A.; Kesner, J.S.; Bachand, A.M.; Barr, D.B.; Meadows, J.W.; Krieg, E.F.; Reif, J.S. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ. Res. 2011, 111, 1293–1301. [Google Scholar] [CrossRef]
- Wrobel, M.H.; Mlynarczuk, J.; Rekawiecki, R. Effects of commonly used carbamates (carbaryl and thiram) on the regulatory, secretory and motor functions of bovine cervixes in vitro. Theriogenology 2024, 218, 183–192. [Google Scholar] [CrossRef]
- Wrobel, M.H.; Bedziechowski, P.; Mlynarczuk, J.; Kotwica, J. Impairment of uterine smooth muscle contractions and prostaglandin secretion from cattle myometrium and corpus luteum in vitro is influenced by DDT, DDE and HCH. Environ. Res. 2014, 132, 54–61. [Google Scholar] [CrossRef]
- Wrobel, M.H.; Grzeszczyk, M.; Mlynarczuk, J.; Kotwica, J. The adverse effects of aldrin and dieldrin on both myometrial contractions and the secretory functions of bovine ovaries and uterus in vitro. TAAP 2015, 85, 23–31. [Google Scholar] [CrossRef]
- Wrobel, M.H.; Mlynarczuk, J. The inhibition of myometrial contractions by chlorinated herbicides (atrazine and linuron), and their disruptive effect on the secretory functions of uterine and ovarian cells in cow, in vitro. Pestic. Biochem. Physiol. 2017, 142, 44–52. [Google Scholar] [CrossRef]
- Wrobel, M.H. Glyphosate affects the secretion of regulators of uterine contractions in cows, while it does not directly impair the motoric function of myometrium in vitro. TAAP 2018, 349, 55–61. [Google Scholar] [CrossRef]
- Wrobel, M.H.; Mlynarczuk, J. Chloroorganic (DDT) and organophosphate (malathion) insecticides impair the motor function of the bovine cervix. TAAP 2021, 427, 115667. [Google Scholar] [CrossRef]
- Wrobel, M.H.; Mlynarczuk, J.; Rekawiecki, R. Do commonly used herbicides (atrazine and glyphosate) have the potential to impair the contractions, prostaglandin releasing and conducting of oxytocin signal at the bovine cervix in vitro? Theriogenology 2022, 183, 26–35. [Google Scholar] [CrossRef]
- Wrobel, M.H.; Mlynarczuk, J.; Kowalik, M.K. Do cypermethrin and fenvalerate disturb the function of the bovine cervix in vitro? J. Endocrinol. 2022, 253, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.S.E. Reproduction in Farm Animals; Lea & Febiger: Philadelphia, PA, USA, 1968; pp. 173–205. ISBN 9781119265306. [Google Scholar]
- de Carvalho, M.T.; Celotto, A.C.; Albuquerque, A.A.; Ferreira, L.G.; Capellini, V.K.; Silveira, A.P.; de Nadai, T.R.; Evora, P.R. In vitro effects of the organophosphorus pesticide malathion on the reactivity of rat aorta. Pharmacology 2014, 94, 157–162. [Google Scholar] [CrossRef]
- Juberg, D.R.; Webb, R.C.; Loch-Caruso, R. Characterization of o,p′-DDT-stimulated contraction frequency in rat uterus in vitro. Fundam. Appl. Toxicol. 1991, 17, 543–549. [Google Scholar] [CrossRef]
- Wróbel, M.H.; Młynarczuk, J.; Kotwica, J. The effect of DDT and its metabolite (DDE) on prostaglandin secretion from epithelial cells and on contractions of the smooth muscle of the bovine oviduct in vitro. TAAP 2012, 259, 152–159. [Google Scholar] [CrossRef]
- Eskenazi, B.; Harley, K.; Bradman, A.; Weltzien, E.; Jewell, N.P.; Barr, D.B.; Furlong, C.E.; Holland, N.T. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population children’s health. Environ. Health Perspect. 2004, 112, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Hirsbrunner, G.; Reist, M.; Keller, C.; Steiner, A. An in vitro study on spontaneous cervical contractility in the cow during oestrus and diestrus. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Falchi, L.; Scaramuzzi, R.J. An in vitro investigation of the actions of reproductive hormones on the cervix of the ewe in the follicular stage: The effects of 17β-estradiol, oxytocin, FSH, and arachidonic acid on the cervical pathway for the synthesis of prostaglandin E2. Theriogenology 2015, 83, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, M.; Kaminski, K.; Kotwica, J. In vitro effects of polychlorinated biphenyls (PCBs) on the contractility of bovine myometrium from the periovulatory stage of the estrous cycle. Reprod. Biol. 2005, 5, 303–319. [Google Scholar] [PubMed]
- Silva, L.D.; Onclin, K.; Verstegen, J.P. Cervical opening in relation to progesterone and oestradiol during heat in beagle bitches. J. Reprod. Fertil. 1995, 104, 85–90. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrobel, M.H. A Comparative Study of the Effect of Commonly Used Pesticides on Cervical Contractions in Pregnant Cows, In Vitro. Toxics 2025, 13, 793. https://doi.org/10.3390/toxics13090793
Wrobel MH. A Comparative Study of the Effect of Commonly Used Pesticides on Cervical Contractions in Pregnant Cows, In Vitro. Toxics. 2025; 13(9):793. https://doi.org/10.3390/toxics13090793
Chicago/Turabian StyleWrobel, Michal Hubert. 2025. "A Comparative Study of the Effect of Commonly Used Pesticides on Cervical Contractions in Pregnant Cows, In Vitro" Toxics 13, no. 9: 793. https://doi.org/10.3390/toxics13090793
APA StyleWrobel, M. H. (2025). A Comparative Study of the Effect of Commonly Used Pesticides on Cervical Contractions in Pregnant Cows, In Vitro. Toxics, 13(9), 793. https://doi.org/10.3390/toxics13090793







