Effects of Diethylstilbestrol on the Structure and Function of the Spleen in Male Golden Hamsters
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Sample Collection
2.2. Hematoxylin and Eosin Staining
2.3. Immunohistochemical Staining
2.4. Assessment of Lymphocyte Proliferation
2.5. Quantification of Peripheral Blood Cytokines
2.6. Measurement of Antioxidant Enzyme Activity
2.7. Quantitative Real-Time PCR (RT-qPCR)
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. DES Reduced Body Weight and Spleen Index in Male Golden Hamsters
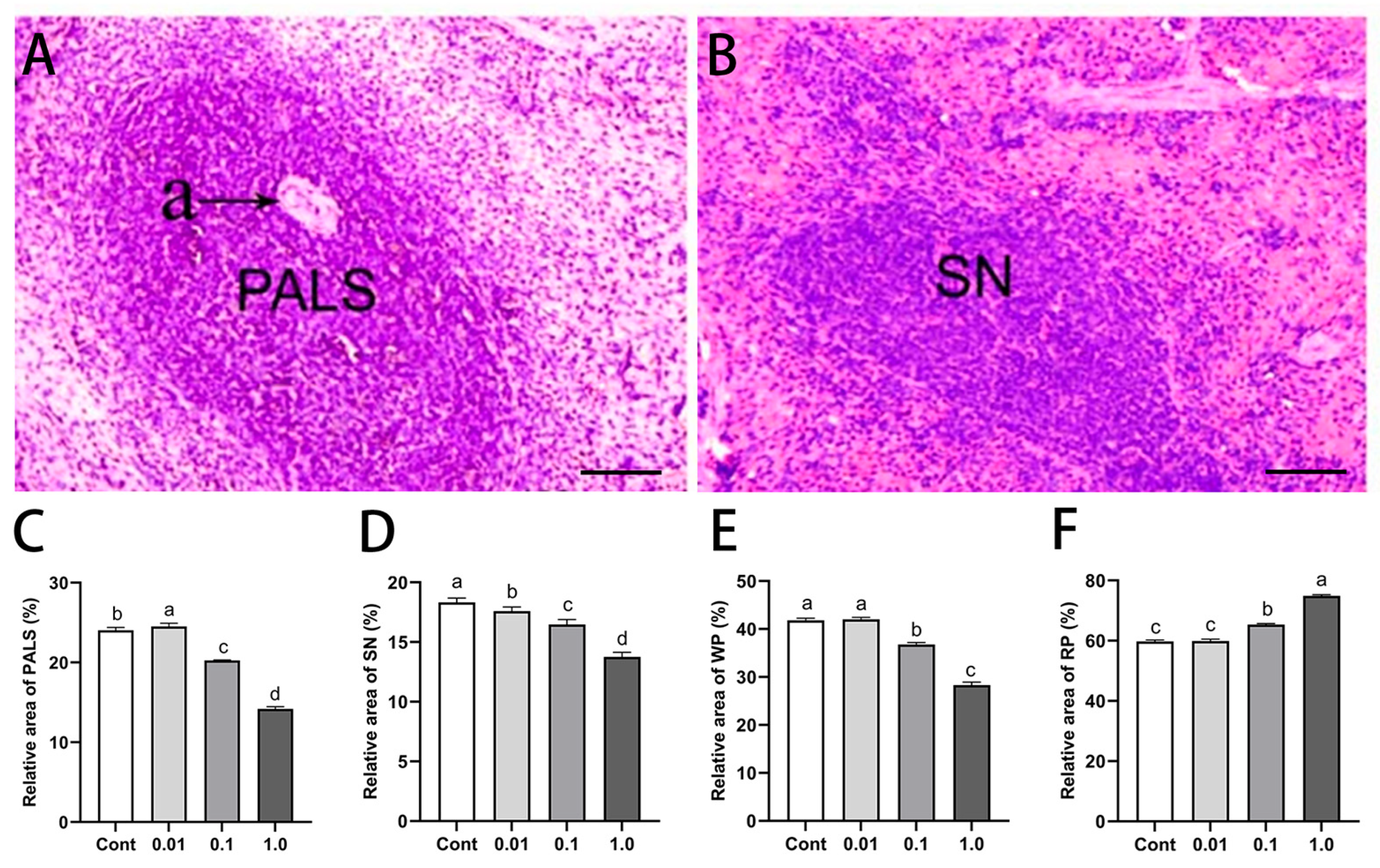
3.2. DES Altered Splenic Histological Architecture in Male Golden Hamsters
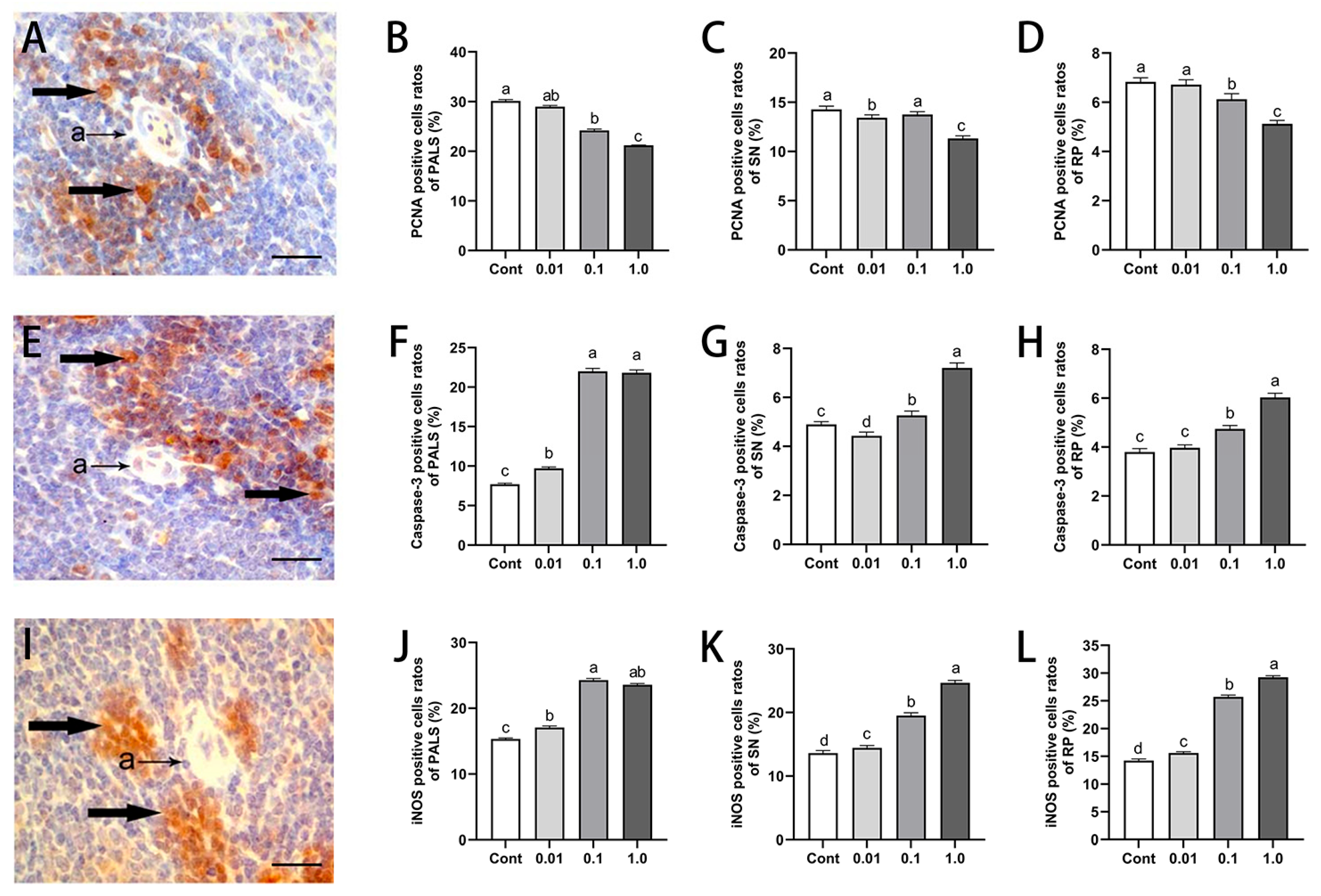
3.3. DES Inhibited Splenocyte Proliferation While Inducing Apoptosis and iNOS Expression in Male Golden Hamsters
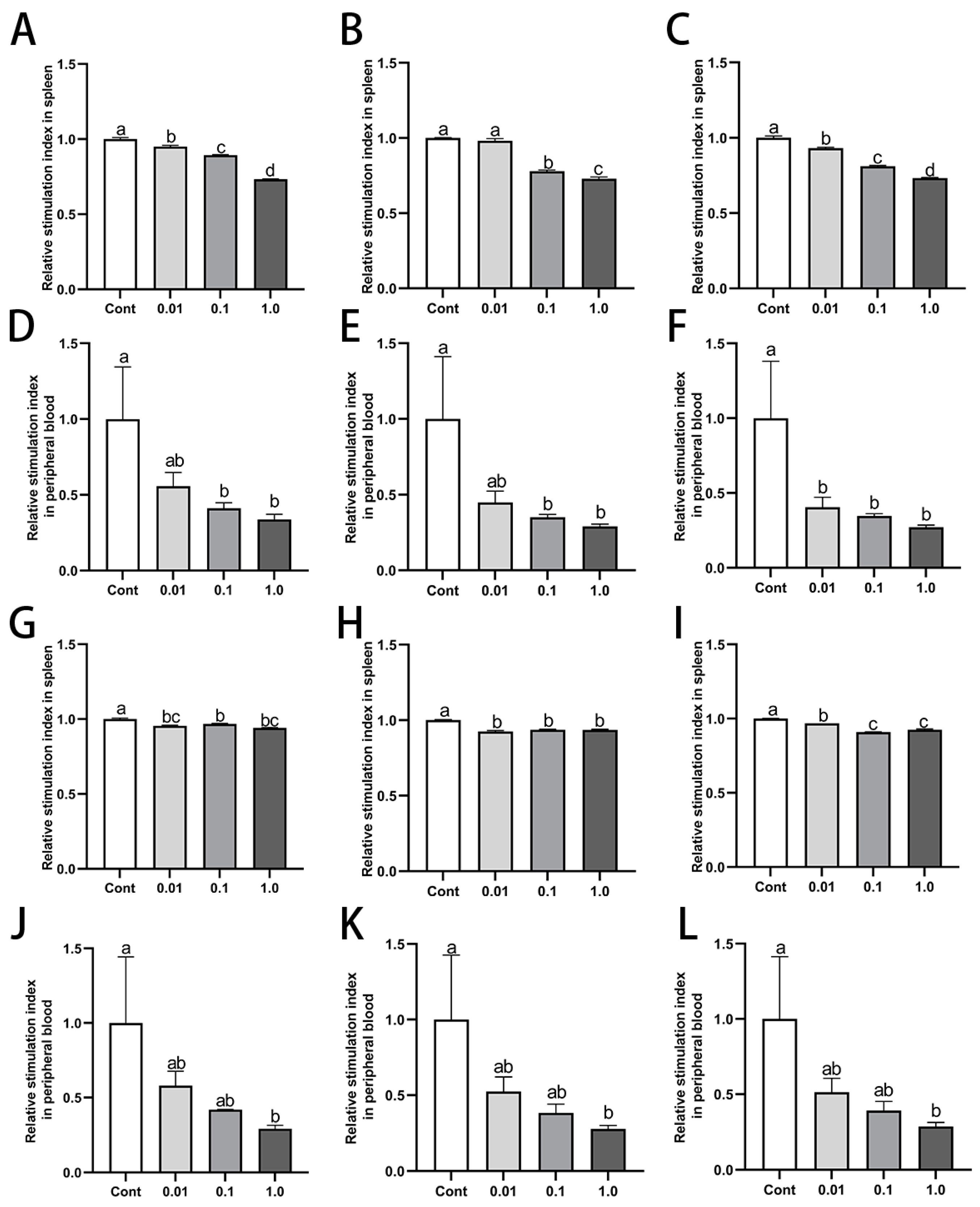
3.4. DES Inhibited Proliferation of Splenic and Peripheral Blood Lymphocytes in Male Golden Hamsters
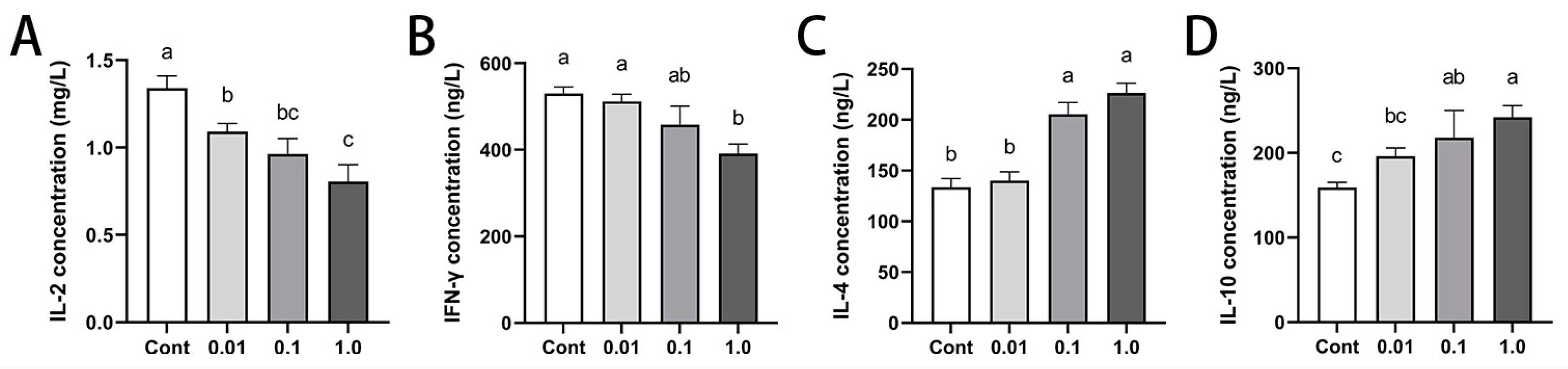
3.5. DES Modulated Peripheral Cytokine Profiles in Male Golden Hamsters
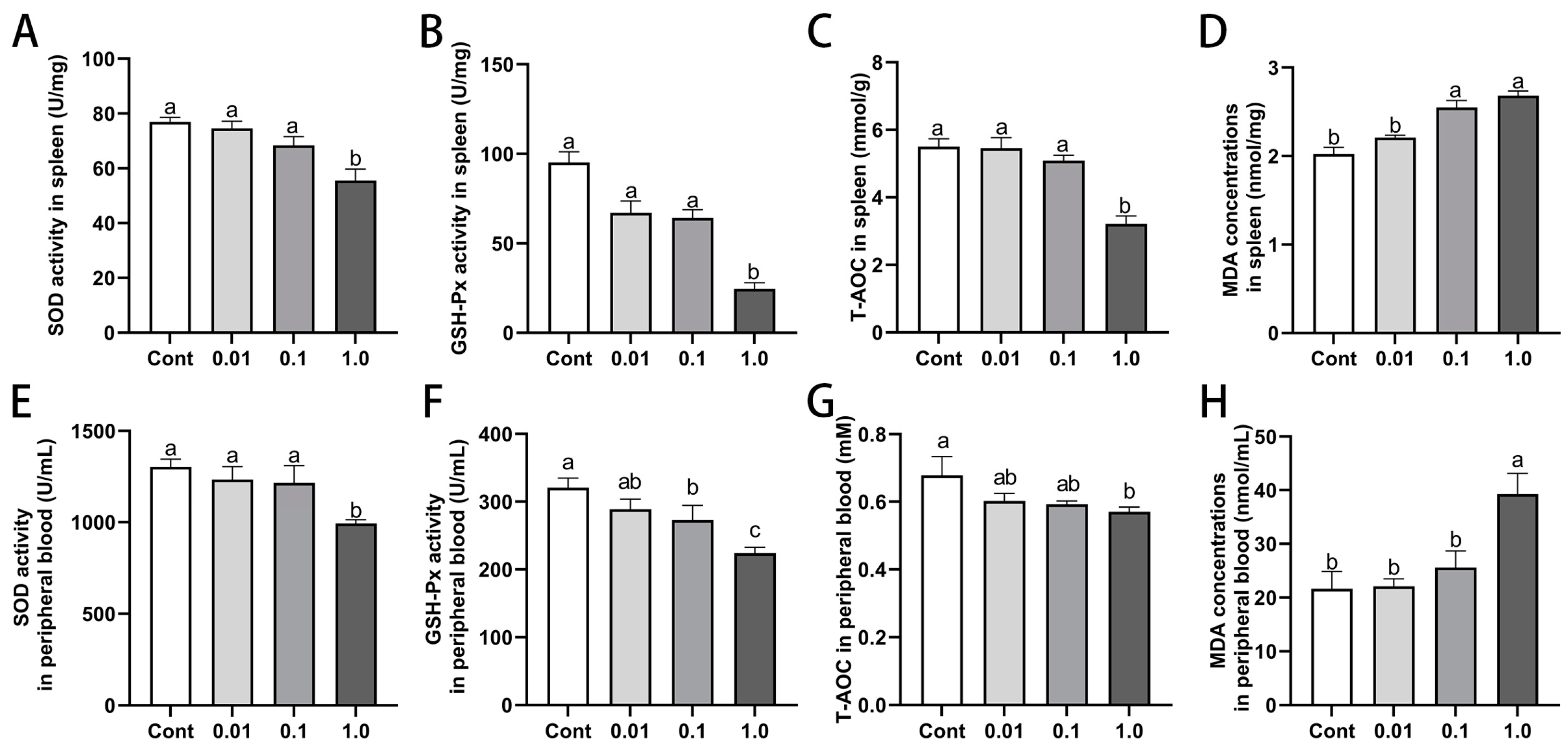
3.6. DES Impaired Antioxidant Capacity in Spleen and Peripheral Blood of Male Golden Hamsters
3.7. DES Modulated Estrogen Receptor Expression in the Spleen of Male Golden Hamsters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Gea, M.; Toso, A.; Schilirò, T. Estrogenic activity of biological samples as a biomarker. Sci. Total Environ. 2020, 740, 140050. [Google Scholar] [CrossRef] [PubMed]
- Kallon, S.; Li, X.; Ji, J.; Chen, C.; Xi, Q.; Chang, S.; Xue, C.; Ma, J.; Xie, Q.; Zhang, Y. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. Anim. Sci. Biotechnol. 2013, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Robles-Matos, N.; Artis, T.; Simmons, R.A.; Bartolomei, M.S. Environmental exposure to endocrine disrupting chemicals influences genomic imprinting, growth, and metabolism. Genes 2021, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Noller, K.L.; Fish, C.R. Diethylstilbestrol usage: Its interesting past, important present, and questionable future. Med. Clin. N. Am. 1974, 58, 793–810. [Google Scholar] [CrossRef]
- Stefanick, M.L. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am. J. Med. 2005, 118, 64–73. [Google Scholar] [CrossRef]
- Glimp, H.A.; Cundiff, L.V. Effects of oral Melengestrol Acetate and a testosterone-diethylstilbestrol implant, breed and age on growth and carcass traits of beef heifers. J. Anim. Sci. 1971, 57, 957–961. [Google Scholar] [CrossRef]
- Roberson, R.H.; Trujillo, V. The effect of methionine, thiouracil, dienestrol diacetate and thyroprotein on the development and prevention of fatty liver in pullets. Poult. Sci. 1975, 54, 715–721. [Google Scholar] [CrossRef]
- Hoover, R.N.; Hyer, M.; Pfeiffer, R.M.; Adam, E.; Bond, B.; Cheville, A.L.; Colton, T.; Hartge, P.; Hatch, E.E.; Herbst, A.L. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N. Engl. J. Med. 2011, 365, 1304–1314. [Google Scholar] [CrossRef]
- Chighizola, C.; Meroni, P.L. The role of environmental estrogens and autoimmunity. Autoimmun. Rev. 2012, 11, A493–A501. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex hormones in acquired immunity and autoimmune disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Mendoza, M.; Morales-Montor, J. Immune tumor microenvironment in breast cancer and the participation of estrogen and its receptors in cancer physiopathology. Front. Immunol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A. The immune system as a potential target for environmental estrogens (endocrine disrupters): A new emerging field. Toxicology 2000, 150, 191–206. [Google Scholar] [CrossRef]
- Sakazaki, H.; Ueno, H.; Nakamuro, K. Estrogen receptor α in mouse splenic lymphocytes: Possible involvement in immunity. Toxicol. Lett. 2002, 133, 221–229. [Google Scholar] [CrossRef]
- Chen, F.; Reheman, A.; Cao, J.; Wang, Z.; Dong, Y.; Zhang, Y.; Chen, Y. Effect of melatonin on monochromatic light-induced T-lymphocyte proliferation in the thymus of chickens. J. Photochem. Photobiol. B 2016, 161, 9–16. [Google Scholar] [CrossRef]
- Strohsnitter, W.C.; Noller, K.L.; Hoover, R.N.; Robboy, S.J.; Palmer, J.R.; Titus-Ernstoff, L.; Kaufman, R.H.; Adam, E.; Herbst, A.L.; Hatch, E.E. Cancer risk in men exposed in utero to diethylstilbestrol. J. Natl. Cancer Inst. 2001, 93, 545–551. [Google Scholar] [CrossRef]
- Mueller, S.N.; Germain, R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 2009, 9, 618–629. [Google Scholar] [CrossRef]
- Wierońska, J.M.; Cieślik, P.; Kalinowski, L. Nitric oxide-dependent pathways as critical factors in the consequences and recovery after brain ischemic hypoxia. Biomolecules 2021, 11, 1097. [Google Scholar] [CrossRef]
- Ranadive, S.M.; Dillon, G.A.; Mascone, S.E.; Alexander, L.M. Vascular Health Triad in Humans With Hypertension—Not the Usual Suspects. Front. Physiol. 2021, 12, 746278. [Google Scholar] [CrossRef]
- Chokshi, N.K.; Guner, Y.S.; Hunter, C.J.; Upperman, J.S.; Grishin, A.; Ford, H.R. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin. Perinatol. 2008, 32, 92–99. [Google Scholar] [CrossRef]
- Dias, A.S.; Porawski, M.; Alonso, M.; Marroni, N.; Collado, P.S.; Gonzalez-Gallego, J. Quercetin decreases oxidative stress, NF-κB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J. Nutr. 2005, 135, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Di Penta, A.; Moreno, B.; Reix, S.; Fernandez-Diez, B.; Villanueva, M.; Errea, O.; Escala, N.; Vandenbroeck, K.; Comella, J.X.; Villoslada, P. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS ONE 2013, 8, e54722. [Google Scholar] [CrossRef]
- Albina, J.E.; Abate, J.A.; Henry, W.J. Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J. Immunol. 1991, 147, 144–148. [Google Scholar] [CrossRef]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Röllinghoff, M.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nance, D.M.; Sanders, V.M. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 2007, 21, 736–745. [Google Scholar] [CrossRef]
- Gruetter, C.A.; Gruetter, D.; Lyon, J.E.; Kadowitz, P.J.; Ignarro, L.J. Relationship between cyclic guanosine 3′:5′-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: Effects of methylene blue and methemoglobin. J. Pharmacol. Exp. Ther. 1981, 219, 181–186. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Brown, N.; Nagarkatti, M.; Nagarkatti, P.S. Diethylstilbestrol alters positive and negative selection of T cells in the thymus and modulates T-cell repertoire in the periphery. Toxicol. Appl. Pharmacol. 2006, 212, 119–126. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Prenatal exposure of mice to diethylstilbestrol disrupts T-cell differentiation by regulating Fas/Fas ligand expression through estrogen receptor element and nuclear factor-κB motifs. J. Pharmacol. Exp. Ther. 2012, 343, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Sullivan, B.M.; Peng, S.L.; Glimcher, L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003, 21, 713–758. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Hirahara, K.; Onodera, A.; Endo, Y.; Hosokawa, H.; Shinoda, K.; Tumes, D.J.; Okamoto, Y. Th2 cells in health and disease. Annu. Rev. Immunol. 2017, 35, 53–84. [Google Scholar] [CrossRef]
- Phadwal, K.; Vrahnas, C.; Ganley, I.G.; MacRae, V.E. Mitochondrial dysfunction: Cause or consequence of vascular calcification? Front. Cell Dev. Biol. 2021, 9, 611922. [Google Scholar] [CrossRef]
- Villanueva-Paz, M.; Morán, L.; López-Alcántara, N.; Freixo, C.; Andrade, R.J.; Lucena, M.I.; Cubero, F.J. Oxidative stress in drug-induced liver injury (DILI): From mechanisms to biomarkers for use in clinical practice. Antioxidants 2021, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Efimova, O.; Szankasi, P.; Kelley, T.W. Ncf1 (p47phox) is essential for direct regulatory T cell mediated suppression of CD4+ effector T cells. PLoS ONE 2011, 6, e16013. [Google Scholar] [CrossRef]
- Zhang, Y.; Mi, K.; Xue, W.; Wei, W.; Yang, H. Acute BPA exposure-induced oxidative stress, depressed immune genes expression and damage of hepatopancreas in red swamp crayfish Procambarus clarkii. Fish Shellfish Immunol. 2020, 103, 95–102. [Google Scholar] [CrossRef]
- Xu, H.; Yang, M.; Qiu, W.; Pan, C.; Wu, M. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environ. Toxicol. Chem. 2013, 32, 1793–1799. [Google Scholar] [CrossRef]
- Fan, D.; Liu, S.Y.; Van Hasselt, C.A.; Vlantis, A.C.; Ng, E.K.; Zhang, H.; Dong, Y.; Ng, S.K.; Chu, R.; Chan, A.B. Estrogen receptor α induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J. Clin. Endocrinol. Metab. 2015, 100, E561–E571. [Google Scholar] [CrossRef]
- Konduri, S.D.; Medisetty, R.; Liu, W.; Kaipparettu, B.A.; Creighton, C.J.; Rao, M.K.; Alsarraj, J.; Manakkalalli, K.; Bhat, N.; Komaki, R.; et al. Mechanisms of estrogen receptor antagonism toward p53 and its implications in breast cancer therapeutic response and stem cell regulation. Proc. Natl. Acad. Sci. USA 2010, 107, 15081–15086. [Google Scholar] [CrossRef]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Sivaprasad, U.; Terai, K.; Amador, V.; Pagano, M.; Dutta, A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 2008, 22, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Le May, C.; Wong, W.P.; Ward, R.D.; Clegg, D.J.; Marcelli, M.; Korach, K.S.; Mauvais-Jarvis, F. Importance of extranuclear estrogen receptor-α and membrane G protein–coupled estrogen receptor in pancreatic islet survival. Diabetes 2009, 58, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Xiang, W.; Ping, Y. Activation of novel estrogen receptor GPER results in inhibition of cardiocyte apoptosis and cardioprotection. Mol. Med. Rep. 2015, 12, 2425–2430. [Google Scholar] [CrossRef]
- Mills, C. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef]

| Genes | Primer Sequence (5′-3′) | Produce Size (bp) | Accession No. |
|---|---|---|---|
| Esr1 | CAGCAGCAGCATCGTCGTCTG AGCATCCAACATCTCCAGCAACAG | 147 | NM_001281325.1 |
| Esr2 | TAGAACACACCTTGCCTGGGAG ACATCCTTCGCACGACCAGA | 170 | XM_013115381.2 |
| gper | TTCCGCACCAAGCACCAT AGCCACTGCACCTCTCTGACA | 149 | XM_013120473.2 |
| β-actin | GGCTCCCAGCACCATGAA GCCACCGATCCACACAGAGT | 70 | NM_001281595.1 |
| DES Dose (mg/kg·BW) | Initial Weight (g) | Final Weight (g) | Average Daily Gain (g) | Spleen Index (%) |
|---|---|---|---|---|
| control | 129.3 ± 1.467 a | 142.8 ± 0.630 a | 1.930 ± 0.195 a | 0.1120 ± 0.0035 a |
| 0.01 | 129.9 ± 1.941 a | 139.3 ± 1.378 ab | 1.347 ± 0.083 ab | 0.1291 ± 0.0103 a |
| 0.1 | 128.3 ± 1.263 a | 136.2 ± 2.845 ab | 1.023 ± 0.164 b | 0.0956 ± 0.0052 a |
| 1.0 | 128.6 ± 0.440 a | 132.3 ± 1.135 b | 0.533 ± 0.129 c | 0.0857 ± 0.0064 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, R.; Wang, Q.; Bai, X.; Su, Y.; Chen, Y.; Cao, J. Effects of Diethylstilbestrol on the Structure and Function of the Spleen in Male Golden Hamsters. Toxics 2025, 13, 397. https://doi.org/10.3390/toxics13050397
Li J, Xu R, Wang Q, Bai X, Su Y, Chen Y, Cao J. Effects of Diethylstilbestrol on the Structure and Function of the Spleen in Male Golden Hamsters. Toxics. 2025; 13(5):397. https://doi.org/10.3390/toxics13050397
Chicago/Turabian StyleLi, Jian, Ruiping Xu, Qingwei Wang, Xue Bai, Yanhua Su, Yaoxing Chen, and Jing Cao. 2025. "Effects of Diethylstilbestrol on the Structure and Function of the Spleen in Male Golden Hamsters" Toxics 13, no. 5: 397. https://doi.org/10.3390/toxics13050397
APA StyleLi, J., Xu, R., Wang, Q., Bai, X., Su, Y., Chen, Y., & Cao, J. (2025). Effects of Diethylstilbestrol on the Structure and Function of the Spleen in Male Golden Hamsters. Toxics, 13(5), 397. https://doi.org/10.3390/toxics13050397






