A Review of Biogenic Volatile Organic Compounds from Plants: Research Progress and Future Prospects
Abstract
:1. Introduction
2. Methodology
3. BVOC Emissions Characterization Methods
3.1. Methods of Emission Measurement
3.1.1. Single-Plant Scale Sampling Methods
- (1)
- On-line measurement
- (2)
- Off-line measurement
- (1)
- Sample collection
- (2)
- Sample analysis
| Collection Containers | Pretreatment Requirements | Storage Conditions | Maximum Preservation Time | Applicable Compound Range | Ref. |
|---|---|---|---|---|---|
| Tedlar Bags | Nitrogen flushed 3 times, light-proof aluminum foil wrapped | 4 °C, <30% humidity | 48 h | Nonpolar terpenes (C5-C10) | [76,77] |
| Tenax Tubes | Aging (320 °C, 2 h), vacuum sealed in aluminum foil pouch | −20 °C with desiccant | 7–30 days | Semi-volatile VOCs (C10-C15) | [78,79] |
| Activated Charcoal Tubes | Aged 1 h at 400 °C, sealed in moisture-proof container | 4 °C, avoid condensation | 14 days | Highly volatile VOCs (C2-C8) | [80,81] |
| SUMMA Canisters | Vacuum baked at 160 °C for 12 h, nitrogen filled to 30 psi | Protected from light at room temperature, pressure monitoring | 30 days | Broad spectrum VOCs (C2-C15) | [77,82] |
3.1.2. Canopy Scale Flux Measurement Methods
3.2. Model-Based Estimation of BVOCs Emission
3.2.1. Guenther
3.2.2. BEIS
3.2.3. MEGAN
4. Emission Mechanisms of BVOCs from Plants
4.1. Emission Sources
4.2. Compositions
| BVOCs Categories | Synthesis Method | Main Emission Plants Category | Contribution Rate | Global c (Tg C yr−1) | Ref. |
|---|---|---|---|---|---|
| 1. Isoprenoids | MEP | Broadleaf trees | 40–60% | 400–600 | [148,149,150] |
| 2. Monoterpenes | MVA | Coniferous trees | 25–40% | 40–180 | [151,152] |
| 3. Sesquiterpenes | MEP and MVA | Tropical rainforest plants Herbaceous plants | 5–15% | 20–40 | [153,154,155] |
| 4. OBVOCs | LOX/MEP/MVA | Herbaceous plants; trees; grasses crops; flowers | 10–25% | –280 | [156,157] |
| Study Area | Seasonal Mean Concentrations (μg/m3) | Annual Mean Concentrations (μg/m3) | Ref. | |||
|---|---|---|---|---|---|---|
| Spring | Summer | Autumn | Winter | |||
| Isoprene | ||||||
| Urban area of Beijing | 1.83 | 2.82 | 1.48 | 0.43 | 1.66 | [133] |
| Suburban areas of Tianjin | - | - | 1.61 | - | - | [158] |
| Urban area of Shenzhen | 2.07 | 4.26 | 1.09- | 1.06 | - | [159] |
| Village of Guangzhou | 6.69 | - | 4.26 | 0.40 | 3.41 | [160] |
| Urban area of Shanghai | - | - | - | - | 0.10 | [161] |
| Urban area of Houston | - | - | - | - | 1.92 | [162] |
| Urban area of Lille | - | 0.88 | - | 0.33 | 0.58 | [162] |
| Zurich | - | 0.49 | - | 0.24 | - | [162] |
| α-Pinene | ||||||
| Typical urban area of Beijing | 0.05 | 0.11 | 0.18 | 0.04 | 0.10 | [133] |
| Mountainous areas of Sichuan | - | - | - | - | 0.30 | [163] |
| Guangzhou Forest Park | - | 1.12 | - | - | [164] | |
| β-Pinene | ||||||
| Typical urban area of Beijing | 0.05 | 0.003 | 0.006 | 0.002 | 0.02 | [133] |
| Mountainous areas of Sichuan | - | - | - | - | 0.30 | [163] |
4.3. Synthetic Pathways
5. Influencing Factors of BVOC Emissions
5.1. Intrinsic Plant Factors Influencing BVOC Emissions
5.1.1. Plant Types
5.1.2. Physiological Differences
5.1.3. Biotic Stresses
5.2. Environmental Factors
5.2.1. Temperature and Light
5.2.2. Humidity
5.2.3. Concentration of CO2
5.2.4. O3 Stress
6. Atmospheric Oxidation Mechanisms of BVOCs
6.1. Initial Oxidation Reactions
6.2. O3 Formation
6.3. SOA Formation
6.4. Synergistic Effects of BVOCs with AVOCs
7. Atmospheric Impacts of BVOCs
7.1. Ozone Formation Potential (OFP) and Climate Feedbacks
7.2. Secondary Organic Aerosol Formation Potential (SOAP) and Environmental Impacts
8. Future Research Challenges
9. Recommendations and Prospects
9.1. Recommendations of BVOC Prevention and Control
9.2. Prospects of BVOC Monitor Strategies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, M.; An, C.; Guy, C. A scientometric analysis and review of biogenic volatile organic compound emissions: Research hotspots, new frontiers, and environmental implications. Renew. Sustain. Energy Rev. 2021, 149, 111317. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; Mckay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. 1995, 100, 8873. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240034228 (accessed on 27 April 2025).
- Eastman, J.R.; Sangermano, F.; Machado, E.A.; Rogan, J.; Anyamba, A. Global Trends in Seasonality of Normalized Difference Vegetation Index (NDVI), 1982–2011. Remote Sens. 2013, 5, 4799–4818. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, X.; Chen, C.; Tan, J.; Li, Y.; Chen, H.; Wang, Y.; Li, L.; Guenther, A.; Huang, H. Uncertainties of biogenic VOC emissions caused by land cover data and implications on ozone mitigation strategies for the Yangtze river Delta region. Atmos. Environ. 2024, 337, 120765. [Google Scholar] [CrossRef]
- Steinbrecher, R.; Smiatek, G.; Köble, R.; Seufert, G.; Theloke, J.; Hauff, K.; Ciccioli, P.; Vautard, R.; Curci, G. Intra- and inter-annual variability of VOC emissions from natural and semi-natural vegetation in Europe and neighbouring countries. Atmos. Environ. 2009, 43, 1380–1391. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y.; Hu, J.; Zhang, H.; Ying, Q. Source apportionment of summertime ozone in China using a source-oriented chemical transport model. Atmos. Environ. 2019, 211, 79–90. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, B.; Meng, H.; Song, S.; Dai, Q.; Shi, L.; Feng, Y.; Hopke, P.K. Source apportionment of consumed volatile organic compounds in the atmosphere. J. Hazard. Mater. 2023, 459, 132138. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Cao, J.; Situ, S.; Hao, Y.; Xie, S.; Li, L. Enhanced summertime ozone and SOA from biogenic volatile organic compound (BVOC) emissions due to vegetation biomass variability during 1981–2018 in China. Atmos. Chem. Phys. 2022, 22, 2351–2364. [Google Scholar] [CrossRef]
- Qin, M.; Hu, Y.; Wang, X.; Vasilakos, P.; Boyd, C.M.; Xu, L.; Song, Y.; Ng, N.L.; Nenes, A.; Russell, A.G. Modeling biogenic secondary organic aerosol (BSOA) formation from monoterpene reactions with NO3: A case study of the SOAS campaign using CMAQ. Atmos. Environ. 2018, 184, 146–155. [Google Scholar] [CrossRef]
- Rap, A.; Scott, C.E.; Reddington, C.L.; Mercado, L.; Ellis, R.J.; Garraway, S.; Evans, M.J.; Beerling, D.J.; MacKenzie, A.R.; Hewitt, C.N.; et al. Enhanced global primary production by biogenic aerosol via diffuse radiation fertilization. Nature Geosci. 2018, 11, 640–644. [Google Scholar] [CrossRef]
- Yang, K.; Llusià, J.; Preece, C.; Ogaya, R.; Márquez Tur, L.; Mu, Z.; You, C.; Xu, Z.; Tan, Y.; Peñuelas, J. Impacts of seasonality, drought, nitrogen fertilization, and litter on soil fluxes of biogenic volatile organic compounds in a Mediterranean forest. Sci. Total Environ. 2024, 906, 167354. [Google Scholar] [CrossRef] [PubMed]
- Sanadze, G.A. Emission of Gaseous Organic Sub-stances from Plants. Rep. Akad. Nauk. GruzSSR 1956, 17, 429–433. [Google Scholar]
- Sanadze, G.A. Biogenic Isoprene (A Review). Russ. J. Plant Physiol. 2004, 51, 729–741. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, Y.; Ou, W.; Wei, L.; Li, Z.; Deng, X.; Gao, Q. The characteristics and environmental significance of BVOCs released by aquatic macrophytes. Chemosphere 2024, 361, 142574. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. BVOCs: Plant defense against climate warming? Trends Plant Sci. 2003, 8, 105–109. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Helmig, D.; Klinger, L.F.; Guenther, A.; Vierling, L.; Geron, C.; Zimmerman, P. Biogenic volatile organic compound emissions (BVOCs) II. Landscape flux potentials from three continental sites in the U.S. Chemosphere 1999, 38, 2189–2204. [Google Scholar] [CrossRef]
- Otter, L.B.; Guenther, A.; Greenberg, J. Seasonal and spatial variations in biogenic hydrocarbon emissions from southern African savannas and woodlands. Atmos. Environ. 2002, 36, 4265–4275. [Google Scholar] [CrossRef]
- Papiez, M.R.; Potosnak, M.J.; Goliff, W.S.; Guenther, A.B.; Matsunaga, S.N.; Stockwell, W.R. The impacts of reactive terpene emissions from plants on air quality in Las Vegas, Nevada. Atmos. Environ. 2009, 43, 4109–4123. [Google Scholar] [CrossRef]
- Kramshøj, M.; Vedel-Petersen, I.; Schollert, M.; Rinnan, Å.; Nymand, J.; Ro-Poulsen, H.; Rinnan, R. Large increases in Arctic biogenic volatile emissions are a direct effect of warming. Nature Geosci. 2016, 9, 349–352. [Google Scholar] [CrossRef]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. J. Geophys. Res. Atmos. 1993, 98, 12609–12617. [Google Scholar] [CrossRef]
- Guenther, A.B.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Observations with eucalyptus and emission rate algorithm development. J. Geophys. Res. Atmos. 1991, 96, 10799–10808. [Google Scholar] [CrossRef]
- Pierce, T.E. PC-BEIS: A personal computer version of the biogenic emissions inventory system. J. Air Waste Manag. Assoc. 1991, 41, 937–941. [Google Scholar] [CrossRef]
- Ng, N.L.; Brown, S.S.; Archibald, A.T.; Atlas, E.; Cohen, R.C.; Crowley, J.N.; Day, D.A.; Donahue, N.M.; Fry, J.L.; Fuchs, H.; et al. Nitrate radicals and biogenic volatile organic compounds: Oxidation, mechanisms, and organic aerosol. Atmos. Chem. Phys. 2017, 17, 2103–2162. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, P.; Miller, P.A.; Schurgers, G.; Gustafson, A.; Makkonen, R.; Fu, Y.H.; Rinnan, R. High-latitude vegetation changes will determine future plant volatile impacts on atmospheric organic aerosols. npj Clim. Atmos. Sci. 2023, 6, 1–13. [Google Scholar] [CrossRef]
- Lun, X.; Lin, Y.; Chai, F.; Fan, C.; Li, H.; Liu, J. Reviews of emission of biogenic volatile organic compounds (BVOCs) in Asia. J. Environ. Sci. 2020, 95, 266–277. [Google Scholar] [CrossRef]
- Yang, W.; Cao, J.; Wu, Y.; Kong, F.; Li, L. Review on plant terpenoid emissions worldwide and in China. Sci. Total Environ. 2021, 787, 147454. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P. Direct, rapid quantitative analyses of BVOCs using SIFT-MS and PTR-MS obviating sample collection. TrAC Trends Anal. Chem. 2011, 30, 945–959. [Google Scholar] [CrossRef]
- Bacsik, Z.; Mink, J.; Keresztury, G. FTIR Spectroscopy of the Atmosphere. I. Principles and Methods. Appl. Spectrosc. Rev. 2004, 39, 295–363. [Google Scholar]
- Gómez, M.C.; Durana, N.; García, J.A.; de Blas, M.; Sáez de Cámara, E.; García-Ruiz, E.; Gangoiti, G.; Torre-Pascual, E.; Iza, J. Long-term measurement of biogenic volatile organic compounds in a rural background area: Contribution to ozone formation. Atmos. Environ. 2020, 224, 117315. [Google Scholar]
- Kalalian, C.; Roth, E.; El Dib, G.; Singh, H.J.; Rao, P.K.; Chakir, A. Product investigation of the gas phase ozonolysis of 1-penten-3-ol, cis-2-penten-1-ol and trans-3-hexen-1-ol. Atmos. Environ. 2020, 238, 117732. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, B.; Yin, C.; Li, Z.; Yu, Y. Performance improvement: A lightweight gas information classification method combined with an electronic nose system. Sens. Actuators B Chem. 2023, 396, 134551. [Google Scholar] [CrossRef]
- Das, P.P. Glass microfiber based method for extraction and detection of BVOCs of Camellia sinensis (L.) O. Kuntze. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100319. [Google Scholar]
- Fattobene, M.; Papa, F.; Russo, R.E.; Zamponi, S.; Conti, P.; Taffetani, F.; Sorci, A.; Liu, F.; Berrettoni, M. ON-SITE monitoring OF BVOCS emission in Tremiti island, Italy. Heliyon 2024, 10, e23822. [Google Scholar] [CrossRef]
- Baroja, O.; Unceta, N.; Sampedro, M.C.; Goicolea, M.A.; Barrio, R.J. Optimization and validation of a method of analysis for fenitrothion and its main metabolites in forestry air samples using sorbent tubes with thermal desorption cold trap injection and gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1059, 165–170. [Google Scholar] [CrossRef]
- Wu, K.; Yang, X.; Chen, D.; Gu, S.; Lu, Y.; Jiang, Q.; Wang, K.; Ou, Y.; Qian, Y.; Shao, P.; et al. Estimation of biogenic VOC emissions and their corresponding impact on ozone and secondary organic aerosol formation in China. Atmos. Res. 2020, 231, 104656. [Google Scholar] [CrossRef]
- Gonçalves, O.C.; Cerqueira, J.S.R.F.; Mestre, A.S.; Neng, N.R.; Nogueira, J.M.F. HS-BAμE: A New Alternative Approach for VOCs Analysis—Application for Monitoring Biogenic Emissions from Tree Species. Molecules 2023, 28, 1179. [Google Scholar] [CrossRef]
- Kim, S.; Karl, T.; Guenther, A.; Tyndall, G.; Orlando, J.; Harley, P.; Rasmussen, R.; Apel, E. Emissions and ambient distributions of Biogenic Volatile Organic Compounds (BVOC) in a ponderosa pine ecosystem: Interpretation of PTR-MS mass spectra. Atmos. Chem. Phys. 2010, 10, 1759–1771. [Google Scholar] [CrossRef]
- Lindinger, W.; Jordan, A. Proton-transfer-reaction mass spectrometry (PTR–MS): On-line monitoring of volatile organic compounds at pptv levels. Chem. Soc. Rev. 1998, 27, 347–375. [Google Scholar] [CrossRef]
- D’Arco, A.; Mancini, T.; Paolozzi, M.C.; Macis, S.; Mosesso, L.; Marcelli, A.; Petrarca, M.; Radica, F.; Tranfo, G.; Lupi, S.; et al. High Sensitivity Monitoring of VOCs in Air through FTIR Spectroscopy Using a Multipass Gas Cell Setup. Sensors 2022, 22, 5624. [Google Scholar] [CrossRef] [PubMed]
- Fathy, A.; Sabry, Y.M.; Amr, M.; Gnambodoe-Capo-chichi, M.; Anwar, M.; Ghoname, A.O.; Amr, A.; Saeed, A.; Gad, M.; Haron, M.A.; et al. MEMS FTIR optical spectrometer enables detection of volatile organic compounds (VOCs) in part-per-billion (ppb) range for air quality monitoring. In MOEMS and Miniaturized Systems XVIII, Proceedings of the SPIE OPTO, San Francisco, CA, USA, 2–7 February 2019; SPIE: Bellingham, WA, USA; Volume 10931, pp. 69–75.
- Mermet, K.; Sauvage, S.; Dusanter, S.; Salameh, T.; Léonardis, T.; Flaud, P.-M.; Perraudin, É.; Villenave, E.; Locoge, N. Optimization of a gas chromatographic unit for measuring biogenic volatile organic compounds in ambient air. Atmos. Meas. Tech. 2019, 12, 6153–6171. [Google Scholar] [CrossRef]
- Chang, Y.; Tang, N.; Qu, H.; Liu, J.; Zhang, D.; Zhang, H.; Pang, W.; Duan, X. Detection of Volatile Organic Compounds by Self-assembled Monolayer Coated Sensor Array with Concentration-independent Fingerprints. Sci. Rep. 2016, 6, 23970. [Google Scholar] [CrossRef]
- Fair, J.D.; Bailey, W.F.; Felty, R.A.; Gifford, A.E.; Shultes, B.; Volles, L.H. Method for rapid on-site identification of VOCs. J. Environ. Sci. 2009, 21, 1005–1008. [Google Scholar] [CrossRef]
- Stierlin, É.; Michel, T.; Fernandez, X. Field analyses of lavender volatile organic compounds: Performance evaluation of a portable gas chromatography–mass spectrometry device. Phytochem. Anal. 2020, 31, 778–785. [Google Scholar] [CrossRef]
- Santos, F.J.; Galceran, M.T. The application of gas chromatography to environmental analysis. TrAC Trends Anal. Chem. 2002, 21, 672–685. [Google Scholar] [CrossRef]
- Santhanakrishnan, V.P.; Shoba, N.; Senthamizh Selvi, B.; Varun, E.; Mohankumar, S.; Raveendran, M. Aromatic profiling of Murraya koenigii leaves by Thermal Desorption Gas chromatography-Mass Spectroscopy (TD-GC-MS). Heliyon 2023, 9, e17832. [Google Scholar]
- Ren, Q.; Jin, Y.; Hu, Y.; Chen, H.; Li, Z. Rapid changes of induced volatile organic compounds in Pinus massoniana. Front. Forest. China 2007, 2, 459–465. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, K.-H. Ultimate Detectability of Volatile Organic Compounds: How Much Further Can We Reduce Their Ambient Air Sample Volumes for Analysis? Anal. Chem. 2012, 84, 8284–8293. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Hwang, S.-M.; Lee, D.-W.; Heo, G.-S. Determination of Volatile Organic Compounds (VOCs) Using Tedlar Bag/Solid-phase Microextraction/Gas Chromatography/Mass Spectrometry (SPME/GC/MS) in Ambient and Workplace Air. Bull. Korean Chem. Soc. 2002, 23, 488–496. [Google Scholar]
- Feng, J.; Zhang, B.; Zhang, H.; Wu, Z.; Li, M.; Wang, D.; Wang, C. Combining with E-nose, GC-MS, GC-IMS and chemometrics to explore volatile characteristics during the different stages of Zanthoxylum bungeanum maxim fruits. Food Res. Int. 2024, 195, 114964. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.; Clarisse, L.; Van Damme, M.; Coheur, P.-F.; Xie, Y.; Shan, C.; Hu, Q.; Sun, Y.; Jones, N. Ground-based measurements of atmospheric NH3 by Fourier transform infrared spectrometry at Hefei and comparisons with IASI data. Atmos. Environ. 2022, 287, 119256. [Google Scholar] [CrossRef]
- Yuan, B.; Koss, A.R.; Warneke, C.; Coggon, M.; Sekimoto, K.; de Gouw, J.A. Proton-Transfer-Reaction Mass Spectrometry: Applications in Atmospheric Sciences. Chem. Rev. 2017, 117, 13187–13229. [Google Scholar] [CrossRef]
- Hansel, A.; Jordan, A.; Holzinger, R.; Prazeller, P.; Vogel, W.; Lindinger, W. Proton transfer reaction mass spectrometry: On-line trace gas analysis at the ppb level. Int. J. Mass Spectrom. Ion Process. 1995, 149–150, 609–619. [Google Scholar] [CrossRef]
- Zhu, B.; Han, Y.; Wang, C.; Huang, X.; Xia, S.; Niu, Y.; Yin, Z.; He, L. Understanding primary and secondary sources of ambient oxygenated volatile organic compounds in Shenzhen utilizing photochemical age-based parameterization method. J. Environ. Sci. 2019, 75, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bessala, L.F.B.; Gao, J.; He, Z.; Wang, Z.; Yi, S. Effects of different heat treatment media on odorous constituents, chemical decomposition and mechanical properties of two hardwoods. RSC Adv. 2024, 14, 7414–7429. [Google Scholar] [CrossRef]
- Fathy, A.; Pivert, M.L.; Kim, Y.J.; Ba, M.O.; Erfan, M.; Sabry, Y.M.; Khalil, D.; Leprince-Wang, Y.; Bourouina, T.; Gnambodoe-Capochichi, M. Continuous Monitoring of Air Purification: A Study on Volatile Organic Compounds in a Gas Cell. Sensors 2020, 20, 934. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, G.; Guo, H.; Liao, L.; Huang, X.; Yi, Z. Transient interaction effects of temperature and light intensity on isoprene and monoterpene emissions from Schima superba and Phoebe bournei. Sci. Total Environ. 2023, 894, 165082. [Google Scholar] [CrossRef]
- Rhew, R.C.; Deventer, M.J.; Turnipseed, A.A.; Warneke, C.; Ortega, J.; Shen, S.; Martinez, L.; Koss, A.; Lerner, B.M.; Gilman, J.B.; et al. Ethene, propene, butene and isoprene emissions from a ponderosa pine forest measured by relaxed eddy accumulation. Atmos. Chem. Phys. 2017, 17, 13417–13438. [Google Scholar] [CrossRef]
- Copolovici, L.; Niinemets, Ü. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ. 2010, 33, 1582–1594. [Google Scholar] [CrossRef]
- You, D.-W.; Seon, Y.-S.; Jang, Y.; Bang, J.; Oh, J.-S.; Jung, K.-W. A portable gas chromatograph for real-time monitoring of aromatic volatile organic compounds in air samples. J. Chromatogr. A 2020, 1625, 461267. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Zhou, M.; Hunter, M.D.; Fan, X. Rapid In Situ Analysis of Plant Emission for Disease Diagnosis Using a Portable Gas Chromatography Device. J. Agric. Food Chem. 2019, 67, 7530–7537. [Google Scholar] [CrossRef] [PubMed]
- Ciccioli, P.; Silibello, C.; Finardi, S.; Pepe, N.; Ciccioli, P.; Rapparini, F.; Neri, L.; Fares, S.; Brilli, F.; Mircea, M.; et al. The potential impact of biogenic volatile organic compounds (BVOCs) from terrestrial vegetation on a Mediterranean area using two different emission models. Agric. For. Meteorol. 2023, 328, 109255. [Google Scholar] [CrossRef]
- Anisimov, D.S.; Chekusova, V.P.; Trul, A.A.; Abramov, A.A.; Borshchev, O.V.; Agina, E.V.; Ponomarenko, S.A. Fully integrated ultra-sensitive electronic nose based on organic field-effect transistors. Sci. Rep. 2021, 11, 10683. [Google Scholar] [CrossRef]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC–MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chang, T.-C.; Chen, Y.-H.; Chen, Y.-J.; Cheng, S.-S.; Chang, S.-T. Monitoring the dynamic emission of biogenic volatile organic compounds from Cryptomeria japonica by enclosure measurement. Atmos. Environ. 2015, 122, 163–170. [Google Scholar] [CrossRef]
- Li, L.; Guenther, A.B.; Xie, S.; Gu, D.; Seco, R.; Nagalingam, S.; Yan, D. Evaluation of semi-static enclosure technique for rapid surveys of biogenic volatile organic compounds (BVOCs) emission measurements. Atmos. Environ. 2019, 212, 1–5. [Google Scholar] [CrossRef]
- Aydin, Y.M.; Yaman, B.; Koca, H.; Dasdemir, O.; Kara, M.; Altiok, H.; Dumanoglu, Y.; Bayram, A.; Tolunay, D.; Odabasi, M.; et al. Biogenic volatile organic compound (BVOC) emissions from forested areas in Turkey: Determination of specific emission rates for thirty-one tree species. Sci. Total Environ. 2014, 490, 239–253. [Google Scholar] [CrossRef]
- Vedel-Petersen, I.; Schollert, M.; Nymand, J.; Rinnan, R. Volatile organic compound emission profiles of four common arctic plants. Atmos. Environ. 2015, 120, 117–126. [Google Scholar] [CrossRef]
- Jing, X.; Lun, X.; Fan, C.; Ma, W. Emission patterns of biogenic volatile organic compounds from dominant forest species in Beijing, China. J. Environ. Sci. 2020, 95, 73–81. [Google Scholar] [CrossRef]
- Yuan, X.; Calatayud, V.; Gao, F.; Fares, S.; Paoletti, E.; Tian, Y.; Feng, Z. Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ. 2016, 39, 2276–2287. [Google Scholar] [CrossRef]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the use of Tedlar® bags for breath-gas sampling and analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-C.; Horng, S.-H.; Liao, P.-N. Stability of Trace-level Volatile Organic Compounds Stored in Canisters and Tedlar Bags. Aerosol Air Qual. Res. 2003, 3, 17–28. [Google Scholar] [CrossRef]
- Arnts, R.R. Evaluation of adsorbent sampling tube materials and Tenax-TA for analysis of volatile biogenic organic compounds. Atmos. Environ. 2010, 44, 1579–1584. [Google Scholar] [CrossRef]
- Hellén, H.; Tykkä, T.; Schallhart, S.; Stratigou, E.; Salameh, T.; Iturrate-Garcia, M. Measurements of atmospheric C10–C15 biogenic volatile organic compounds (BVOCs) with sorbent tubes. Atmos. Meas. Tech. 2024, 17, 315–333. [Google Scholar] [CrossRef]
- Nassiri, P.; Golbabaie, F.; Mehrain, K.; Hematian, A. Evaluation of activated charcoal sampler tubes. Iran. J. Public Health 1994, 23, 11–20. [Google Scholar]
- Prado, C.; Alcaraz, M.J.; Fuentes, A.; Garrido, J.; Periago, J.F. Storage stability of ketones on carbon adsorbents. J. Chromatogr. A 2006, 1129, 82–87. [Google Scholar] [CrossRef]
- Wang, D.K.W.; Austin, C.C. Determination of complex mixtures of volatile organic compounds in ambient air: Canister methodology. Anal. Bioanal. Chem. 2006, 386, 1099–1120. [Google Scholar] [CrossRef]
- Acton, W.J.F.; Schallhart, S.; Langford, B.; Valach, A.; Rantala, P.; Fares, S.; Carriero, G.; Tillmann, R.; Tomlinson, S.J.; Dragosits, U.; et al. Canopy-scale flux measurements and bottom-up emission estimates of volatile organic compounds from a mixed oak and hornbeam forest in northern Italy. Atmos. Chem. Phys. 2016, 16, 7149–7170. [Google Scholar] [CrossRef]
- Karl, T.; Misztal, P.K.; Jonsson, H.H.; Shertz, S.; Goldstein, A.H.; Guenther, A.B. Airborne Flux Measurements of BVOCs above Californian Oak Forests: Experimental Investigation of Surface and Entrainment Fluxes, OH Densities, and Damkohler Numbers. J. Atmos. Sci. 2013, 70, 3277–3287. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Hincks, B.B.; Meyers, T.P. Measuring Biosphere-Atmosphere Exchanges of Biologically Related Gases with Micrometeorological Methods. Ecology 1988, 69, 1331–1340. [Google Scholar] [CrossRef]
- Spirig, C.; Neftel, A.; Ammann, C.; Dommen, J.; Grabmer, W.; Thielmann, A.; Schaub, A.; Beauchamp, J.; Wisthaler, A.; Hansel, A. Eddy covariance flux measurements of biogenic VOCs during ECHO 2003 using proton transfer reaction mass spectrometry. Atmos. Chem. Phys. 2005, 5, 465–481. [Google Scholar] [CrossRef]
- Misztal, P.K.; Nemitz, E.; Langford, B.; Di Marco, C.F.; Phillips, G.J.; Hewitt, C.N.; MacKenzie, A.R.; Owen, S.M.; Fowler, D.; Heal, M.R.; et al. Direct ecosystem fluxes of volatile organic compounds from oil palms in South-East Asia. Atmos. Chem. Phys. 2011, 11, 8995–9017. [Google Scholar] [CrossRef]
- Bai, J.; Guenther, A.; Turnipseed, A.; Duhl, T.; Greenberg, J. Seasonal and interannual variations in whole-ecosystem BVOC emissions from a subtropical plantation in China. Atmos. Environ. 2017, 161, 176–190. [Google Scholar] [CrossRef]
- Park, J.-H.; Fares, S.; Weber, R.; Goldstein, A.H. Biogenic volatile organic compound emissions during BEARPEX 2009 measured by eddy covariance and flux-gradient similarity methods. Atmos. Chem. Phys. 2014, 14, 231–244. [Google Scholar] [CrossRef]
- Holst, T.; Arneth, A.; Hayward, S.; Ekberg, A.; Mastepanov, M.; Jackowicz-Korczynski, M.; Friborg, T.; Crill, P.M.; Backstrand, K. BVOC ecosystem flux measurements at a high latitude wetland site. Atmos. Chem. Phys. 2010, 10, 1617–1634. [Google Scholar] [CrossRef]
- Bachy, A.; Aubinet, M.; Schoon, N.; Amelynck, C.; Bodson, B.; Moureaux, C.; Heinesch, B. Are BVOC exchanges in agricultural ecosystems overestimated? Insights from fluxes measured in a maize field over a whole growing season. Atmos. Chem. Phys. 2016, 16, 5343–5356. [Google Scholar] [CrossRef]
- Edwards, G.D.; Martins, D.K.; Starn, T.; Pratt, K.; Shepson, P.B. A disjunct eddy accumulation system for the measurement of BVOC fluxes: Instrument characterizations and field deployment. Atmos. Meas. Tech. 2012, 5, 2115–2132. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wu, X.; Li, J.; Qiu, Y.; Wang, H.; Cheng, Z.; Zheng, C.; Yang, F. Volatile Organic Compound Sampling through Rotor Unmanned Aerial Vehicle Technique for Environmental Monitoring. Atmosphere 2022, 13, 1442. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Ye, J.; Jia, T.; Khuzestani, R.B.; Sun, J.Y.; Cheng, X.; Zheng, Y.; Li, X.; Wu, C.; et al. Unmanned Aerial Vehicle Measurements of Volatile Organic Compounds over a Subtropical Forest in China and Implications for Emission Heterogeneity. ACS Earth Space Chem. 2021, 5, 247–256. [Google Scholar] [CrossRef]
- McKinney, K.A.; Wang, D.; Ye, J.; de Fouchier, J.-B.; Guimarães, P.C.; Batista, C.E.; Souza, R.A.F.; Alves, E.G.; Gu, D.; Guenther, A.B.; et al. A sampler for atmospheric volatile organic compounds by copter unmanned aerial vehicles. Atmos. Meas. Tech. 2019, 12, 3123–3135. [Google Scholar] [CrossRef]
- Ashworth, K.; Boissard, C.; Folberth, G.; Lathière, J.; Schurgers, G. Global Modelling of Volatile Organic Compound Emissions. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Tree Physiology; Springer: Dordrecht, The Netherlands, 2013; pp. 451–487. ISBN 978-94-007-6606-8. [Google Scholar]
- Müller, J.-F.; Stavrakou, T.; Wallens, S.; De Smedt, I.; Van Roozendael, M.; Potosnak, J.; Rinne, J.; Munger, B.; Goldstein, A.; Guenther, B. Global isoprene emissions estimated using MEGAN, ECMWF analyses and a detailed canopy environment model. Atmos. Chem. Phys. 2008, 8, 1329–1341. [Google Scholar] [CrossRef]
- Chi, Y.Q.; Xie, S.D. Spatiotemporal Inventory of Biogenic Volatile Organic Compound Emissions in China Based on Vegetation Volume and Production. Adv. Mater. Res. 2012, 356–360, 2579–2582. [Google Scholar] [CrossRef]
- Wang, L.; Lun, X.; Wu, J.; Wang, Q.; Tao, J.; Dou, X.; Zhang, Z. Investigation of biogenic volatile organic compounds emissions in the Qinghai-Tibetan Plateau. Sci. Total Environ. 2023, 902, 165877. [Google Scholar] [CrossRef]
- Pierce, T.; Geron, C.; Bender, L.; Dennis, R.; Tonnesen, G.; Guenther, A. Influence of increased isoprene emissions on regional ozone modeling. J. Geophys. Res. Atmos. 1998, 103, 25611–25629. [Google Scholar]
- Feldman, M.S.; Howard, T.; McDonald-Buller, E.; Mullins, G.; Allen, D.T.; Hansel, A.; Wisthaler, A. Applications of satellite remote sensing data for estimating biogenic emissions in southeastern Texas. Atmos. Environ. 2010, 44, 917–929. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, Z.; Yu, Y.; Zhong, L. Temporal, spatial characteristics and uncertainty of biogenic VOC emissions in the Pearl River Delta region, China. Atmos. Environ. 2010, 44, 1960–1969. [Google Scholar] [CrossRef]
- Sakulyanontvittaya, T.; Duhl, T.; Wiedinmyer, C.; Helmig, D.; Matsunaga, S.; Potosnak, M.; Milford, J.; Guenther, A. Monoterpene and Sesquiterpene Emission Estimates for the United States. Environ. Sci. Technol. 2008, 42, 1623–1629. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Guenther, A.; Jiang, X.; Shah, T.; Huang, L.; Kemball-Cook, S.; Yarwood, G. Model of Emissions of Gases and Aerosol from Nature Version 3 (MEGAN3) for Estimating Biogenic Emissions. In Air Pollution Modeling and its Application XXVI, Proceedings of the ITM 2018, Ottawa, ON, Canada, 14–18 May 2018; Mensink, C., Gong, W., Hakami, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 187–192. [Google Scholar]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.-F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Wang, P.; Schade, G.; Estes, M.; Ying, Q. Improved MEGAN predictions of biogenic isoprene in the contiguous United States. Atmos. Environ. 2017, 148, 337–351. [Google Scholar] [CrossRef]
- Jiang, X.; Guenther, A.; Potosnak, M.; Geron, C.; Seco, R.; Karl, T.; Kim, S.; Gu, L.; Pallardy, S. Isoprene emission response to drought and the impact on global atmospheric chemistry. Atmos. Environ. 2018, 183, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Duhl, T. A primary generalized empirical model of BVOC emissions for some typical forests in China. Atmos. Pollut. Res. 2021, 12, 101126. [Google Scholar] [CrossRef]
- Benjamin, M.T.; Winer, A.M. Estimating the ozone-forming potential of urban trees and shrubs. Atmos. Environ. 1998, 32, 53–68. [Google Scholar] [CrossRef]
- Churkina, G.; Kuik, F.; Bonn, B.; Lauer, A.; Grote, R.; Tomiak, K.; Butler, T.M. Effect of VOC Emissions from Vegetation on Air Quality in Berlin during a Heatwave. Environ. Sci. Technol. 2017, 51, 6120–6130. [Google Scholar] [CrossRef]
- Bao, X.; Zhou, W.; Wang, W.; Yao, Y.; Xu, L. Tree species classification improves the estimation of BVOCs from urban greenspace. Sci. Total Environ. 2024, 914, 169762. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public. Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and Spatial Variability of Volatile Organic Compounds in the Forest Atmosphere. Int. J. Environ. Res. Public. Health 2019, 16, 4915. [Google Scholar] [CrossRef]
- Smiatek, G.; Steinbrecher, R. Temporal and spatial variation of forest VOC emissions in Germany in the decade 1994–2003. Atmos. Environ. 2006, 40, 166–177. [Google Scholar] [CrossRef]
- Liu, Y.; Schallhart, S.; Tykkä, T.; Räsänen, M.; Merbold, L.; Hellén, H.; Pellikka, P. Biogenic volatile organic compounds in different ecosystems in Southern Kenya. Atmos. Environ. 2021, 246, 118064. [Google Scholar] [CrossRef]
- Chang, J.; Ren, Y.; Shi, Y.; Zhu, Y.; Ge, Y.; Hong, S.; Jiao, L.; Lin, F.; Peng, C.; Mochizuki, T.; et al. An inventory of biogenic volatile organic compounds for a subtropical urban-rural complex. Atmos. Environ. 2012, 56, 115–123. [Google Scholar] [CrossRef]
- Okumura, M.; Kosugi, Y.; Tani, A. Biogenic volatile organic compound emissions from bamboo species in Japan. J. Agric. Meteorol. 2018, 74, 40–44. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Wu, J.; Lun, X.; Wang, X.; Li, X. Emission Pattern of Biogenic Volatile Organic Compounds from Wetland Vegetation. Atmosphere 2024, 15, 651. [Google Scholar] [CrossRef]
- Mannisto, E.; Ylanne, H.; Losoi, M.; Keinanen, M.; Yli-Pirila, P.; Korrensalo, A.; Back, J.; Hellen, H.; Virtanen, A.; Tuittila, E.-S. Emissions of biogenic volatile organic compounds from adjacent boreal fen and bog as impacted by vegetation composition. Sci. Total Environ. 2023, 858, 159809. [Google Scholar] [CrossRef]
- Gomez, L.G.; Loubet, B.; Lafouge, F.; Ciuraru, R.; Buysse, P.; Durand, B.; Gueudet, J.-C.; Fanucci, O.; Fortineau, A.; Zurfluh, O.; et al. Comparative study of biogenic volatile organic compounds fluxes by wheat, maize and rapeseed with dynamic chambers over a short period in northern France. Atmos. Environ. 2019, 214, 116855. [Google Scholar] [CrossRef]
- Mozaffar, A.; Schoon, N.; Digrado, A.; Bachy, A.; Delaplace, P.; du Jardin, P.; Fauconnier, M.-L.; Aubinet, M.; Heinesch, B.; Amelynck, C. Methanol emissions from maize: Ontogenetic dependence to varying light conditions and guttation as an additional factor constraining the flux. Atmos. Environ. 2017, 152, 405–417. [Google Scholar] [CrossRef]
- Bäck, J.; Aaltonen, H.; Hellén, H.; Kajos, M.K.; Patokoski, J.; Taipale, R.; Pumpanen, J.; Heinonsalo, J. Variable emissions of microbial volatile organic compounds (MVOCs) from root-associated fungi isolated from Scots pine. Atmos. Environ. 2010, 44, 3651–3659. [Google Scholar] [CrossRef]
- Bourtsoukidis, E.; Behrendt, T.; Yañez-Serrano, A.M.; Hellén, H.; Diamantopoulos, E.; Catão, E.; Ashworth, K.; Pozzer, A.; Quesada, C.A.; Martins, D.L.; et al. Strong sesquiterpene emissions from Amazonian soils. Nat. Commun. 2018, 9, 2226. [Google Scholar] [CrossRef]
- Mäki, M. Volatile Organic Compound Fluxes from Northern Forest Soils. Ph.D. Thesis, Institute for Atmospheric and Earth System Research, Forest Sciences Faculty of Agriculture and Forestry, University of Helsinki, Helsinki, Finland, 2019. [Google Scholar]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil. Plant Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef] [PubMed]
- Wester-Larsen, L.; Kramshøj, M.; Albers, C.N.; Rinnan, R. Biogenic Volatile Organic Compounds in Arctic Soil: A Field Study of Concentrations and Variability with Vegetation Cover. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005551. [Google Scholar] [CrossRef]
- Sindelarova, K.; Markova, J.; Simpson, D.; Huszar, P.; Karlicky, J.; Darras, S.; Granier, C. High-resolution biogenic global emission inventory for the time period 2000–2019 for air quality modelling. Earth Syst. Sci. Data 2022, 14, 251–270. [Google Scholar] [CrossRef]
- Yáñez-Serrano, A.M.; Bourtsoukidis, E.; Alves, E.G.; Bauwens, M.; Stavrakou, T.; Llusià, J.; Filella, I.; Guenther, A.; Williams, J.; Artaxo, P.; et al. Amazonian biogenic volatile organic compounds under global change. Global Change Biol. 2020, 26, 4722–4751. [Google Scholar] [CrossRef] [PubMed]
- Yee, L.D.; Isaacman-VanWertz, G.; Wernis, R.A.; Meng, M.; Rivera, V.; Kreisberg, N.M.; Hering, S.V.; Bering, M.S.; Glasius, M.; Upshur, M.A.; et al. Observations of sesquiterpenes and their oxidation products in central Amazonia during the wet and dry seasons. Atmos. Chem. Phys. 2018, 18, 10433–10457. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Tan, Y.; Tan, Y.; Bai, J.; Gu, D.; Ma, Z.; Du, J.; Han, Z. Effects of light on the emissions of biogenic isoprene and monoterpenes: A review. Atmos. Pollut. Res. 2022, 13, 101397. [Google Scholar] [CrossRef]
- Liu, L.; Seyler, B.C.; Liu, H.; Zhou, L.; Chen, D.; Liu, S.; Yan, C.; Yang, F.; Song, D.; Tan, Q.; et al. Biogenic volatile organic compound emission patterns and secondary pollutant formation potentials of dominant greening trees in Chengdu, southwest China. J. Environ. Sci. 2022, 114, 179–193. [Google Scholar] [CrossRef]
- Cheng, X.; Li, H.; Zhang, Y.; Li, Y.; Zhang, W.; Wang, X.; Bi, F.; Zhang, H.; Gao, J.; Chai, F.; et al. Atmospheric isoprene and monoterpenes in a typical urban area of Beijing: Pollution characterization, chemical reactivity and source identification. J. Environ. Sci. 2018, 71, 150–167. [Google Scholar] [CrossRef]
- Liu, J.; Chu, B.; Chen, T.; Liu, C.; Wang, L.; Bao, X.; He, H. Secondary Organic Aerosol Formation from Ambient Air at an Urban Site in Beijing: Effects of OH Exposure and Precursor Concentrations. Environ. Sci. Technol. 2018, 52, 6834–6841. [Google Scholar] [CrossRef]
- Ndah, F.; Valolahti, H.; Schollert, M.; Michelsen, A.; Rinnan, R.; Kivimäenpää, M. Influence of increased nutrient availability on biogenic volatile organic compound (BVOC) emissions and leaf anatomy of subarctic dwarf shrubs under climate warming and increased cloudiness. Ann. Bot. 2022, 129, 443–455. [Google Scholar] [CrossRef]
- Lugo, P.L.; Straccia, V.G.; Rivela, C.B.; Patroescu-Klotz, I.; Illmann, N.; Teruel, M.A.; Wiesen, P.; Blanco, M.B. Diurnal photodegradation of fluorinated diketones (FDKs) by OH radicals using different atmospheric simulation chambers: Role of keto-enol tautomerization on reactivity. Chemosphere 2022, 286, 131562. [Google Scholar] [CrossRef] [PubMed]
- Frazier, G.; McGlynn, D.F.; Barry, L.E.; Lerdau, M.; Pusede, S.E.; Isaacman-VanWertz, G. Composition, concentration, and oxidant reactivity of sesquiterpenes in the southeastern U.S. Environ. Sci. Atmos. 2022, 2, 1208–1220. [Google Scholar] [CrossRef]
- McGlynn, D.F.; Barry, L.E.R.; Lerdau, M.T.; Pusede, S.E.; Isaacman-VanWertz, G. Measurement report: Variability in the composition of biogenic volatile organic compounds in a Southeastern US forest and their role in atmospheric reactivity. Atmos. Chem. Phys. 2021, 21, 15755–15770. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Gas-phase tropospheric chemistry of biogenic volatile organic compounds: A review. Atmos. Environ. 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Bouvier-Brown, N.C.; Goldstein, A.H.; Gilman, J.B.; Kuster, W.C.; de Gouw, J.A. In-situ ambient quantification of monoterpenes, sesquiterpenes, and related oxygenated compounds during BEARPEX 2007: Implications for gas- and particle-phase chemistry. Atmos. Chem. Phys. 2009, 9, 5505–5518. [Google Scholar] [CrossRef]
- Calvert, J.; Mellouki, A.; Orlando, J.; Pilling, M.; Wallington, T. Mechanisms of Atmospheric Oxidation of the Oxygenates; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Isidorov, V.A.; Masłowiecka, J. Chemical Composition of Volatile and Extractive Organic Compounds in the Inflorescence Litter of Five Species of Woody Plants. Plants 2024, 13, 1829. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Wiberley, A.E.; Donohue, A.R. Isoprene Emission from Plants: Why and How. Ann. Bot. 2008, 101, 5–18. [Google Scholar] [CrossRef]
- Ma, S.X.; Rindelaub, J.D.; McAvey, K.M.; Gagare, P.D.; Nault, B.A.; Ramachandran, P.V.; Shepson, P.B. α-Pinene nitrates: Synthesis, yields and atmospheric chemistry. Atmos. Chem. Phys. 2011, 11, 6337–6347. [Google Scholar] [CrossRef]
- Dash, M.R.; Rajakumar, B. Experimental and theoretical rate coefficients for the gas phase reaction of β-Pinene with OH radical. Atmos. Environ. 2013, 79, 161–171. [Google Scholar] [CrossRef]
- Chen, J.; Griffin, R.J. Modeling secondary organic aerosol formation from oxidation of α-pinene, β-pinene, and d-limonene. Atmos. Environ. 2005, 39, 7731–7744. [Google Scholar] [CrossRef]
- Chen, X.; Hopke, P.K. A chamber study of secondary organic aerosol formation by linalool ozonolysis. Atmos. Environ. 2009, 43, 3935–3940. [Google Scholar] [CrossRef]
- Arneth, A.; Schurgers, G.; Lathiere, J.; Duhl, T.; Beerling, D.J.; Hewitt, C.N.; Martin, M.; Guenther, A. Global terrestrial isoprene emission models: Sensitivity to variability in climate and vegetation. Atmos. Chem. Phys. 2011, 11, 8037–8052. [Google Scholar] [CrossRef]
- Cao, Y.; Yue, X.; Liao, H.; Yang, Y.; Zhu, J.; Chen, L.; Tian, C.; Lei, Y.; Zhou, H.; Ma, Y. Ensemble projection of global isoprene emissions by the end of 21st century using CMIP6 models. Atmos. Environ. 2021, 267, 118766. [Google Scholar] [CrossRef]
- Weng, H.; Lin, J.; Martin, R.; Millet, D.B.; Jaeglé, L.; Ridley, D.; Keller, C.; Li, C.; Du, M.; Meng, J. Global high-resolution emissions of soil NOx, sea salt aerosols, and biogenic volatile organic compounds. Sci. Data 2020, 7, 148. [Google Scholar] [CrossRef]
- Rabin, S.S.; Alexander, P.; Henry, R.; Anthoni, P.; Pugh, T.A.M.; Rounsevell, M.; Arneth, A. Impacts of future agricultural change on ecosystem service indicators. Earth Syst. Dyn. 2020, 11, 357–376. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Gil-Pelegrin, E.; Pedro Ferrio, J.; Alonso-Forn, D.; Martin-Sanchez, R.; dos Santos Silva, J.V.; Imanishi, J.; Javier Peguero-Pina, J.; Angeles Sanz, M. Changes in the Abundance of Monoterpenes from Breathable Air of a Mediterranean Conifer Forest: When Is the Best Time for a Human Healthy Leisure Activity? Forests 2022, 13, 965. [Google Scholar] [CrossRef]
- Acosta Navarro, J.C.; Smolander, S.; Struthers, H.; Zorita, E.; Ekman, A.M.L.; Kaplan, J.O.; Guenther, A.; Arneth, A.; Riipinen, I. Global emissions of terpenoid VOCs from terrestrial vegetation in the last millennium. J. Geophys. Res. Atmos. 2014, 119, 6867–6885. [Google Scholar] [CrossRef]
- Llusia, J.; Penuelas, J.; Guenther, A.; Rapparini, F. Seasonal variations in terpene emission factors of dominant species in four ecosystems in NE Spain. Atmos. Environ. 2013, 70, 149–158. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Wu, C.; Lin, G. Regional to global distributions, trends, and drivers of biogenic volatile organic compound emission from 2001 to 2020. Atmos. Chem. Phys. 2024, 24, 3309–3328. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Lathière, J.; Hauglustaine, D.A.; Friend, A.D.; De Noblet-Ducoudré, N.; Viovy, N.; Folberth, G.A. Impact of climate variability and land use changes on global biogenic volatile organic compound emissions. Atmos. Chem. Phys. 2006, 6, 2129–2146. [Google Scholar] [CrossRef]
- Xin-min, Z.; Fa-he, C.; Ting-ting, Y.; Kai, Z.; Yi-zhen, C.; Jing-chun, D.; Zhi-gang, X. Photochemical Characteristics and Sources of Volatile Organic Compounds in Wuqing, Tianjin. Res. Environ. Sci. 2012, 25, 1085–1091. [Google Scholar]
- Zhu, S.; Huang, X.; He, L.; Lu, S.; Feng, N. Variation characteristics and chemical reactivity of ambient VOCs in Shenzhen. China Environ. Sci. 2012, 32, 2140–2148. [Google Scholar]
- Zou, Y.; Deng, X.; Li, F.; Wang, B.; Tan, H.; Deng, T.; Mai, B.; Liu, X. Variation characteristics, chemical reactivity and sources of isoprene in the atmosphere of Guangzhou. Huanjing Kexue Xuebao 2015, 35, 647–655. [Google Scholar]
- Ran, L.; Zhao, C.; Geng, F.; Tie, X.; Tang, X.; Peng, L.; Zhou, G.; Yu, Q.; Xu, J.; Guenther, A. Ozone photochemical production in urban Shanghai, China: Analysis based on ground level observations. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Park, C.; Schade, G.W.; Boedeker, I. Characteristics of the flux of isoprene and its oxidation products in an urban area. J. Geophys. Res. Atmos. 2011, 116. [Google Scholar] [CrossRef]
- Zhang, J.-K.; Wang, Y.-S.; Wu, F.-K.; Sun, J. Study on atmospheric VOCs in Gongga Mountain base station. Huanjing Kexue 2012, 33, 4159–4166. [Google Scholar]
- Ying-xin, Y.U.; Sheng, W.E.N.; Hui-xiong, L.V.; Xin-ming, W.; Guo-ying, S.; Jia-mo, F.U. Diurnal Variations of VOCs and Relative Contribution to Ozone in Forest of Guangzhou. Huanjing Kexue Yu Jishu 2009, 32, 94–98. [Google Scholar]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Li, Z.; Sharkey, T.D. Molecular and Pathway Controls on Biogenic Volatile Organic Compound Emissions. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 119–151. ISBN 978-94-007-6606-8. [Google Scholar]
- Ma, M.; Li, M.; Wu, Z.; Liang, X.; Zheng, Q.; Li, D.; Wang, G.; An, T. The microbial biosynthesis of noncanonical terpenoids. Appl. Microbiol. Biotechnol. 2024, 108, 226. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. In Biotechnology of Isoprenoids; Schrader, J., Bohlmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 63–106. ISBN 978-3-319-20107-8. [Google Scholar]
- Arigoni, D.; Sagner, S.; Latzel, C.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Terpenoid biosynthesis from 1-deoxy-D-xylulose in higher plants by intramolecular skeletal rearrangement. Proc. Natl. Acad. Sci. USA 1997, 94, 10600–10605. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.; Perez-Gil, J.; Hodson, M.P.; Schrübbers, L.; Wulff, T.; Sommer, M.O.; Nielsen, L.K.; Vickers, C.E. Adaptation of hydroxymethylbutenyl diphosphate reductase enables volatile isoprenoid production. eLife 2020, 9, e48685. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Sun, Z.; Talts, E. Controls of the quantum yield and saturation light of isoprene emission in different-aged aspen leaves. Plant Cell Environ. 2015, 38, 2707–2720. [Google Scholar] [CrossRef]
- Sun, Z.; Shen, Y.; Niinemets, Ü. Responses of isoprene emission and photochemical efficiency to severe drought combined with prolonged hot weather in hybrid Populus. J. Exp. Bot. 2020, 71, 7364–7381. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, D.; Hemmerlin, A.; Bach, T.J.; Rohmer, M. Plant isoprenoid biosynthesis via the MEP pathway: In vivo IPP/DMAPP ratio produced by (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase in tobacco BY-2 cell cultures. FEBS Lett. 2010, 584, 129–134. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Boronat, A. Breaking new ground in the regulation of the early steps of plant isoprenoid biosynthesis. Curr. Opin. Plant Biol. 2015, 25, 17–22. [Google Scholar] [CrossRef]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.-L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Bergman, M.E.; Kortbeek, R.W.J.; Gutensohn, M.; Dudareva, N. Plant terpenoid biosynthetic network and its multiple layers of regulation. Prog. Lipid Res. 2024, 95, 101287. [Google Scholar] [CrossRef]
- Lange, B.M.; Ahkami, A. Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes—Current status and future opportunities. Plant Biotechnol. J. 2013, 11, 169–196. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, L.; Rusalepp, L.; Kaurilind, E.; Sulaiman, H.Y.; Püssa, T.; Niinemets, Ü. Heat priming improved heat tolerance of photosynthesis, enhanced terpenoid and benzenoid emission and phenolics accumulation in. Plant Cell Environ. 2021, 44, 2365–2385. [Google Scholar] [CrossRef]
- Vellosillo, T.; Martínez, M.; López, M.A.; Vicente, J.; Cascón, T.; Dolan, L.; Hamberg, M.; Castresana, C. Oxylipins Produced by the 9-Lipoxygenase Pathway in Arabidopsis Regulate Lateral Root Development and Defense Responses through a Specific Signaling Cascade. The Plant Cell 2007, 19, 831–846. [Google Scholar] [PubMed]
- Wan, S.; Xin, X.-F. Regulation and integration of plant jasmonate signaling: A comparative view of monocot and dicot. J. Genet. Genom. 2022, 49, 704–714. [Google Scholar] [CrossRef]
- Wang, M.; Fan, X.; Ding, F. Jasmonate: A Hormone of Primary Importance for Temperature Stress Response in Plants. Plants 2023, 12, 4080. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Geron, C.; Pierce, T.; Lamb, B.; Harley, P.; Fall, R. Natural emissions of non-methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America. Atmos. Environ. 2000, 34, 2205–2230. [Google Scholar] [CrossRef]
- Potosnak, M.J.; LeStourgeon, L.; Pallardy, S.G.; Hosman, K.P.; Gu, L.; Karl, T.; Geron, C.; Guenther, A.B. Observed and modeled ecosystem isoprene fluxes from an oak-dominated temperate forest and the influence of drought stress. Atmos. Environ. 2014, 84, 314–322. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, B.; Wu, Y.; Liu, S.; Kong, F.; Li, L. Effects of soil drought and nitrogen deposition on BVOC emissions and their O3 and SOA formation for Pinus thunbergii. Environ. Pollut. 2023, 316, 120693. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mouat, A.P.; Hu, Y.; Li, M.; McDonald, B.C.; Kaiser, J. Source appointment of volatile organic compounds and evaluation of anthropogenic monoterpene emission estimates in Atlanta, Georgia. Atmos. Environ. 2022, 288, 119324. [Google Scholar]
- Xu, L.; Pye, H.O.T.; He, J.; Chen, Y.; Murphy, B.N.; Ng, N.L. Experimental and model estimates of the contributions from biogenic monoterpenes and sesquiterpenes to secondary organic aerosol in the southeastern United States. Atmos. Chem. Phys. 2018, 18, 12613–12637. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Zenone, T.; Hendriks, C.; Brilli, F.; Fransen, E.; Gioli, B.; Portillo-Estrada, M.; Schaap, M.; Ceulemans, R. Interaction between isoprene and ozone fluxes in a poplar plantation and its impact on air quality at the European level. Sci. Rep. 2016, 6, 32676. [Google Scholar] [CrossRef]
- Zou, Y.; Deng, X.J.; Deng, T.; Yin, C.Q.; Li, F. One-Year Characterization and Reactivity of Isoprene and Its Impact on Surface Ozone Formation at A Suburban Site in Guangzhou, China. Atmosphere 2019, 10, 201. [Google Scholar] [CrossRef]
- Schade, G.W.; Goldstein, A.H.; Lamanna, M.S. Are monoterpene emissions influenced by humidity? Geophys. Res. Lett. 1999, 26, 2187–2190. [Google Scholar] [CrossRef]
- Street, R.A.; Owen, S.; Duckham, S.C.; Boissard, C.; Hewitt, C.N. Effect of habitat and age on variations in volatile organic compound (VOC) emissions from Quercus ilex and Pinus pinea. Atmos. Environ. 1997, 31, 89–100. [Google Scholar] [CrossRef]
- Kuzma, J.; Fall, R. Leaf Isoprene Emission Rate Is Dependent on Leaf Development and the Level of Isoprene Synthase. Plant Physiol. 1993, 101, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Bichele, I.; Laisk, A.; Niinemets, Ü. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant Cell Environ. 2014, 37, 724–741. [Google Scholar] [CrossRef]
- Lin, P.-A.; Chen, Y.; Ponce, G.; Acevedo, F.E.; Lynch, J.P.; Anderson, C.T.; Ali, J.G.; Felton, G.W. Stomata-mediated interactions between plants, herbivores, and the environment. Trends Plant Sci. 2022, 27, 287–300. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Reichstein, M. Controls on the emission of plant volatiles through stomata: A sensitivity analysis. J. Geophys. Res. Atmos. 2003, 108, 4211. [Google Scholar] [CrossRef]
- Yin, Q.; Tian, T.; Kou, M.; Liu, P.; Wang, L.; Hao, Z.; Yue, M. The relationships between photosynthesis and stomatal traits on the Loess Plateau. Glob. Ecol. Conserv. 2020, 23, e01146. [Google Scholar] [CrossRef]
- Chuong, B.; Zhang, J.; Donahue, N.M. Cycloalkene Ozonolysis: Collisionally Mediated Mechanistic Branching. J. Am. Chem. Soc. 2004, 126, 12363–12373. [Google Scholar] [CrossRef]
- Nemecek-Marshall, M.; MacDonald, R.C.; Franzen, J.J.; Wojciechowski, C.L.; Fall, R. Methanol Emission from Leaves (Enzymatic Detection of Gas-Phase Methanol and Relation of Methanol Fluxes to Stomatal Conductance and Leaf Development). Plant Physiol. 1995, 108, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Mild versus severe stress and BVOCs: Thresholds, priming and consequences. Trends Plant Sci. 2010, 15, 145–153. [Google Scholar] [CrossRef]
- Riedlmeier, M.; Ghirardo, A.; Wenig, M.; Knappe, C.; Koch, K.; Georgii, E.; Dey, S.; Parker, J.E.; Schnitzler, J.-P.; Vlot, A.C. Monoterpenes Support Systemic Acquired Resistance within and between Plants. Plant Cell 2017, 29, 1440–1459. [Google Scholar] [CrossRef] [PubMed]
- Imbiscuso, G.; Trotta, A.; Maffei, M.; Bossi, S. Herbivory induces a ROS burst and the release of volatile organic compounds in the fern Pteris vittata L. J. Plant Interact. 2009, 4, 15–22. [Google Scholar] [CrossRef]
- Joutsensaari, J.; Yli-Pirilä, P.; Korhonen, H.; Arola, A.; Blande, J.D.; Heijari, J.; Kivimäenpää, M.; Mikkonen, S.; Hao, L.; Miettinen, P.; et al. Biotic stress accelerates formation of climate-relevant aerosols in boreal forests. Atmos. Chem. Phys. 2015, 15, 12139–12157. [Google Scholar] [CrossRef]
- Penuelas, J.; Llusia, J. The Complexity of Factors Driving Volatile Organic Compound Emissions by Plants. Biol. Plant. 2001, 44, 481–487. [Google Scholar] [CrossRef]
- Litvak, M.E.; Monson, R.K. Patterns of induced and constitutive monoterpene production in conifer needles in relation to insect herbivory. Oecologia 1998, 114, 531–540. [Google Scholar] [CrossRef]
- Abis, L.; Kalalian, C.; Lunardelli, B.; Wang, T.; Zhang, L.; Chen, J.; Perrier, S.; Loubet, B.; Ciuraru, R.; George, C. Measurement report: Biogenic volatile organic compound emission profiles of rapeseed leaf litter and its secondary organic aerosol formation potential. Atmos. Chem. Phys. 2021, 21, 12613–12629. [Google Scholar] [CrossRef]
- Monson, R.K.; Jaeger, C.H.; Adams, W.W.; Driggers, E.M.; Silver, G.M.; Fall, R. Relationships among Isoprene Emission Rate, Photosynthesis, and Isoprene Synthase Activity as Influenced by Temperature. Plant Physiol. 1992, 98, 1175–1180. [Google Scholar] [CrossRef]
- Li, T.; Holst, T.; Michelsen, A.; Rinnan, R. Amplification of plant volatile defence against insect herbivory in a warming Arctic tundra. Nat. Plants 2019, 5, 568–574. [Google Scholar] [CrossRef]
- Loreto, F.; Barta, C.; Brilli, F.; Nogues, I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006, 29, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Lerdau, M.; Gray, D. Ecology and evolution of light-dependent and light-independent phytogenic volatile organic carbon. New Phytol. 2003, 157, 199–211. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Monson, R.K. Isoprene research—60 years later, the biology is still enigmatic. Plant Cell Environ. 2017, 40, 1671–1678. [Google Scholar] [CrossRef]
- Yu, S.-J.; Seo, Y.-S.; Son, Y.-S.; Kim, J.-C. Characteristics of BVOCs emitted from representative tree species in South Korea according to environmental influences. Atmos. Environ. 2024, 318, 120238. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; van Meeteren, U. Can prolonged exposure to low VPD disturb the ABA signalling in stomatal guard cells? J. Exp. Bot. 2013, 64, 3551–3566. [Google Scholar] [CrossRef]
- Arve, L.E.; Terfa, M.T.; Gislerød, H.R.; Olsen, J.E.; Torre, S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant Cell Environ. 2013, 36, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Asensio, D.; Llusià, J.; Filella, I.; Ogaya, R.; Yi, Z.; Peñuelas, J. Annual and seasonal variations in soil volatile organic compound concentrations in a Mediterranean shrubland and holm oak forest. Geoderma 2022, 405, 115401. [Google Scholar] [CrossRef]
- Yáñez-Serrano, A.M.; Nölscher, A.C.; Williams, J.; Wolff, S.; Alves, E.; Martins, G.A.; Bourtsoukidis, E.; Brito, J.; Jardine, K.; Artaxo, P.; et al. Diel and seasonal changes of biogenic volatile organic compounds within and above an Amazonian rainforest. Atmos. Chem. Phys. 2015, 15, 3359–3378. [Google Scholar] [CrossRef]
- Fanourakis, D.; Giday, H.; Hyldgaard, B.; Bouranis, D.; Körner, O.; Ottosen, C.-O. Low air humidity during cultivation promotes stomatal closure ability in rose. Eur. J. Hortic. Sci. 2019, 84, 245–252. [Google Scholar] [CrossRef]
- Ning, P.; Guo, X.; Tian, S.; Shi, J.; Sun, C. Emission of main BVOCS for typical landscape trees in Kunming. Zhongnan Daxue Xuebao (Ziran Kexue Ban)/J. Cent. South Univ. (Sci. Technol.) 2013, 44, 1290–1296. [Google Scholar]
- Constable, J.V.; Litvak, M.E.; Greenberg, J.P.; Monson, R.K. Monoterpene emission from coniferous trees in response to elevated CO2 concentration and climate warming. Glob. Change Biol. 1999, 5, 252–267. [Google Scholar] [CrossRef]
- Jasoni, R.; Kane, C.; Green, C.; Peffley, E.; Tissue, D.; Thompson, L.; Payton, P.; Paré, P.W. Altered leaf and root emissions from onion (Allium cepa L.) grown under elevated CO2 conditions. Environ. Exp. Bot. 2004, 51, 273–280. [Google Scholar] [CrossRef]
- Rapparini, F.; Baraldi, R.; Miglietta, F.; Loreto, F. Isoprenoid emission in trees of Quercus pubescens and Quercus ilex with lifetime exposure to naturally high CO2 environment. Plant Cell Environ. 2004, 27, 381–391. [Google Scholar] [CrossRef]
- Heiden, A.C.; Kobel, K.; Langebartels, C.; Schuh-Thomas, G.; Wildt, J. Emissions of Oxygenated Volatile Organic Compounds from Plants Part I: Emissions from Lipoxygenase Activity. J. Atmos. Chem. 2003, 45, 143–172. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Jiang, X.; Lee, M.; Turnipseed, A.; Guenther, A.; Kim, J.-C.; Lee, S.-J.; Kim, S. Impact of biogenic volatile organic compounds on ozone production at the Taehwa Research Forest near Seoul, South Korea. Atmos. Environ. 2013, 70, 447–453. [Google Scholar] [CrossRef]
- Sha, T.; Ma, X.; Zhang, H.; Janechek, N.; Wang, Y.; Wang, Y.; Castro García, L.; Jenerette, G.D.; Wang, J. Impacts of Soil NOx Emission on O3 Air Quality in Rural California. Environ. Sci. Technol. 2021, 55, 7113–7122. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, P.; Baldauf, R.; Zhang, K.M.; Pilla, F.; Di Sabatino, S.; Brattich, E.; Pulvirenti, B. Considerations for evaluating green infrastructure impacts in microscale and macroscale air pollution dispersion models. Sci. Total Environ. 2019, 672, 410–426. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J.; Gimeno, B.S. Seasonal and species-specific response of VOC emissions by Mediterranean woody plant to elevated ozone concentrations. Atmos. Environ. 2002, 36, 3931–3938. [Google Scholar] [CrossRef]
- Li, L.; Cao, J.; Hao, Y. Spatial and species-specific responses of biogenic volatile organic compound (BVOC) emissions to elevated ozone from 2014–2020 in China. Sci. Total Environ. 2023, 868, 161636. [Google Scholar] [CrossRef]
- Vo, T.; Faiola, C.L. Acute ozone exposure decreases terpene emissions from Canary Island pines. Agric. For. Meteorol. 2023, 333, 109416. [Google Scholar] [CrossRef]
- Barreira, L.M.F.; Ylisirniö, A.; Pullinen, I.; Buchholz, A.; Li, Z.; Lipp, H.; Junninen, H.; Hõrrak, U.; Noe, S.M.; Krasnova, A.; et al. The importance of sesquiterpene oxidation products for secondary organic aerosol formation in a springtime hemiboreal forest. Atmos. Chem. Phys. 2021, 21, 11781–11800. [Google Scholar] [CrossRef]
- Salvador, C.M.; Chou, C.C.-K.; Ho, T.-T.; Tsai, C.-Y.; Tsao, T.-M.; Tsai, M.-J.; Su, T.-C. Contribution of Terpenes to Ozone Formation and Secondary Organic Aerosols in a Subtropical Forest Impacted by Urban Pollution. Atmosphere 2020, 11, 1232. [Google Scholar] [CrossRef]
- Charan, S.M.; Huang, Y.; Seinfeld, J.H. Computational Simulation of Secondary Organic Aerosol Formation in Laboratory Chambers. Chem. Rev. 2019, 119, 11912–11944. [Google Scholar] [CrossRef]
- Hellén, H.; Praplan, A.P.; Tykkä, T.; Ylivinkka, I.; Vakkari, V.; Bäck, J.; Petäjä, T.; Kulmala, M.; Hakola, H. Long-term measurements of volatile organic compounds highlight the importance of sesquiterpenes for the atmospheric chemistry of a boreal forest. Atmos. Chem. Phys. 2018, 18, 13839–13863. [Google Scholar] [CrossRef]
- Kawamura, K.; Bikkina, S. A review of dicarboxylic acids and related compounds in atmospheric aerosols: Molecular distributions, sources and transformation. Atmos. Res. 2016, 170, 140–160. [Google Scholar] [CrossRef]
- Yue, H.; Zhang, C.; Lin, X.; Wen, Z.; Zhang, W.; Mostafa, S.; Luo, P.-L.; Zhang, Z.; Hemberger, P.; Fittschen, C.; et al. Dimeric Product of Peroxy Radical Self-Reaction Probed with VUV Photoionization Mass Spectrometry and Theoretical Calculations: The Case of C2H5OOC2H5. Int. J. Mol. Sci. 2023, 24, 3731. [Google Scholar] [CrossRef] [PubMed]
- Dunlea, E.J.; Ravishankara, A.R. Measurement of the rate coefficient for the reaction of O(1D) with H2O and re-evaluation of the atmospheric OH production rate. Phys. Chem. Chem. Phys. 2004, 6, 3333–3340. [Google Scholar] [CrossRef]
- Monks, P.S. Gas-phase radical chemistry in the troposphere. Chem. Soc. Rev. 2005, 34, 376–395. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Fang, W.; Chen, X. Theoretical insight towards the photo-dissociation dynamics of O3–H2O complex: Deep understanding the source of atmospheric hydroxyl radical. Chem. Phys. Lett. 2014, 608, 95–101. [Google Scholar] [CrossRef]
- Atkinson, R. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem. Rev. 1986, 86, 69–201. [Google Scholar] [CrossRef]
- Zhao, Y.; Wingen, L.M.; Perraud, V.; Greaves, J.; Finlayson-Pitts, B.J. Role of the reaction of stabilized Criegee intermediates with peroxy radicals in particle formation and growth in air. Phys. Chem. Chem. Phys. 2015, 17, 12500–12514. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.S.; Stutz, J. Nighttime radical observations and chemistry. Chem. Soc. Rev. 2012, 41, 6405–6447. [Google Scholar] [CrossRef] [PubMed]
- Geyer, A.; Alicke, B.; Konrad, S.; Schmitz, T.; Stutz, J.; Platt, U. Chemistry and oxidation capacity of the nitrate radical in the continental boundary layer near Berlin. J. Geophys. Res.-Atmos. 2001, 106, 8013–8025. [Google Scholar] [CrossRef]
- Schindler, M. A QSAR for the prediction of rate constants for the reaction of VOCs with nitrate radicals. Chemosphere 2016, 154, 23–33. [Google Scholar] [CrossRef]
- Mogensen, D.; Gierens, R.; Crowley, J.N.; Keronen, P.; Smolander, S.; Sogachev, A.; Nölscher, A.C.; Zhou, L.; Kulmala, M.; Tang, M.J.; et al. Simulations of atmospheric OH, O3 and NO3 reactivities within and above the boreal forest. Atmos. Chem. Phys. 2015, 15, 3909–3932. [Google Scholar] [CrossRef]
- Xu, L.; Tsona, N.T.; You, B.; Zhang, Y.; Wang, S.; Yang, Z.; Xue, L.; Du, L. NOx enhances secondary organic aerosol formation from nighttime γ-terpinene ozonolysis. Atmos. Environ. 2020, 225, 117375. [Google Scholar] [CrossRef]
- Waring, M.S.; Wells, J.R. Volatile organic compound conversion by ozone, hydroxyl radicals, and nitrate radicals in residential indoor air: Magnitudes and impacts of oxidant sources. Atmos. Environ. 2015, 106, 382–391. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, P.; Li, C.; Li, X.; Ma, W.; Yin, S.; Yu, Q.; Li, J.; Liu, X. Evolution and variations of atmospheric VOCs and O3 photochemistry during a summer O3 event in a county-level city, Southern China. Atmos. Environ. 2022, 272, 118942. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Ling, Z.; Fang, G.; Ming, L.; Zhao, J.; Zou, S.; Guan, H.; Wang, H.; Wang, X.; et al. Roles of photochemical consumption of VOCs on regional background O3 concentration and atmospheric reactivity over the pearl river estuary, Southern China. Sci. Total Environ. 2024, 928, 172321. [Google Scholar] [CrossRef]
- Ng, N.L.; Canagaratna, M.R.; Zhang, Q.; Jimenez, J.L.; Tian, J.; Ulbrich, I.M.; Kroll, J.H.; Docherty, K.S.; Chhabra, P.S.; Bahreini, R.; et al. Organic aerosol components observed in Northern Hemispheric datasets from Aerosol Mass Spectrometry. Atmos. Chem. Phys. 2010, 10, 4625–4641. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.S.; Wu, W.; Lim, Y.B.; Ziemann, P.J. Contributions of Organic Peroxides to Secondary Aerosol Formed from Reactions of Monoterpenes with O3. Environ. Sci. Technol. 2005, 39, 4049–4059. [Google Scholar] [CrossRef]
- Kroll, J.H.; Seinfeld, J.H. Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 2008, 42, 3593–3624. [Google Scholar] [CrossRef]
- Orlando, J.J.; Tyndall, G.S. Laboratory studies of organic peroxy radical chemistry: An overview with emphasis on recent issues of atmospheric significance. Chem. Soc. Rev. 2012, 41, 6294–6317. [Google Scholar] [CrossRef]
- Berndt, T.; Richters, S.; Kaethner, R.; Voigtländer, J.; Stratmann, F.; Sipilä, M.; Kulmala, M.; Herrmann, H. Gas-Phase Ozonolysis of Cycloalkenes: Formation of Highly Oxidized RO2 Radicals and Their Reactions with NO, NO2, SO2, and Other RO2 Radicals. J. Phys. Chem. A 2015, 119, 10336–10348. [Google Scholar] [CrossRef] [PubMed]
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of Biogenic Volatile Organic Compounds (BVOC) emitted by urban trees on ozone concentration in cities: A review. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef]
- Chen, W.; Guenther, A.B.; Jia, S.; Mao, J.; Yan, F.; Wang, X.; Shao, M. Synergistic effects of biogenic volatile organic compounds and soil nitric oxide emissions on summertime ozone formation in China. Sci. Total Environ. 2022, 828, 154218. [Google Scholar] [CrossRef]
- Xie, X.; Shao, M.; Liu, Y.; Lu, S.; Chang, C.-C.; Chen, Z.-M. Estimate of initial isoprene contribution to ozone formation potential in Beijing, China. Atmos. Environ. 2008, 42, 6000–6010. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.; Kim, S.; Choi, S.; Seok, S.; Kim, S. Photochemical characteristics of high and low ozone episodes observed in the Taehwa Forest observatory (TFO) in June 2011 near Seoul South Korea. Asia-Pac. J. Atmos. Sci. 2013, 49, 325–331. [Google Scholar] [CrossRef]
- Lin, C.; Feng, X.; Heal, M.R. Temporal persistence of intra-urban spatial contrasts in ambient NO2, O3 and Ox in Edinburgh, UK. Atmos. Pollut. Res. 2016, 7, 734–741. [Google Scholar] [CrossRef]
- Pancholi, P.; Kumar, A.; Bikundia, D.S.; Chourasiya, S. An observation of seasonal and diurnal behavior of O3–NOx relationships and local/regional oxidant (OX = O3 + NO2) levels at a semi-arid urban site of western India. Sustain. Environ. Res. 2018, 28, 79–89. [Google Scholar] [CrossRef]
- Simon, H.; Fallmann, J.; Kropp, T.; Tost, H.; Bruse, M. Urban Trees and Their Impact on Local Ozone Concentration—A Microclimate Modeling Study. Atmosphere 2019, 10, 154. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, J.; Li, Q.; Chen, T.; Mu, J.; Brasseur, G.; Wang, T.; Xue, L. Biogenic volatile organic compounds enhance ozone production and complicate control efforts: Insights from long-term observations in Hong Kong. Atmos. Environ. 2023, 309, 119917. [Google Scholar] [CrossRef]
- Liu, C.; Shi, K. A review on methodology in O3-NOx-VOC sensitivity study. Environ. Pollut. 2021, 291, 118249. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Guo, F.; Xie, S. Diagnosing ozone–NOx–VOC sensitivity and revealing causes of ozone increases in China based on 2013–2021 satellite retrievals. Atmos. Chem. Phys. 2022, 22, 15035–15047. [Google Scholar] [CrossRef]
- Iyer, S.; Reiman, H.; Møller, K.H.; Rissanen, M.P.; Kjaergaard, H.G.; Kurtén, T. Computational Investigation of RO2 + HO2 and RO2 + RO2 Reactions of Monoterpene Derived First-Generation Peroxy Radicals Leading to Radical Recycling. J. Phys. Chem. A 2018, 122, 9542–9552. [Google Scholar] [CrossRef]
- Bianchi, F.; Junninen, H.; Bigi, A.; Sinclair, V.A.; Dada, L.; Hoyle, C.R.; Zha, Q.; Yao, L.; Ahonen, L.R.; Bonasoni, P.; et al. Biogenic particles formed in the Himalaya as an important source of free tropospheric aerosols. Nat. Geosci. 2021, 14, 4–9. [Google Scholar] [CrossRef]
- Friedman, B.; Farmer, D.K. SOA and gas phase organic acid yields from the sequential photooxidation of seven monoterpenes. Atmos. Environ. 2018, 187, 335–345. [Google Scholar] [CrossRef]
- Kulmala, M.; Nieminen, T.; Chellapermal, R.; Makkonen, R.; Bäck, J.; Kerminen, V.-M. Climate Feedbacks Linking the Increasing Atmospheric CO2 Concentration, BVOC Emissions, Aerosols and Clouds in Forest Ecosystems. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 489–508. ISBN 978-94-007-6606-8. [Google Scholar]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef]
- Robinson, A.L.; Donahue, N.M.; Shrivastava, M.K.; Weitkamp, E.A.; Sage, A.M.; Grieshop, A.P.; Lane, T.E.; Pierce, J.R.; Pandis, S.N. Rethinking Organic Aerosols: Semivolatile Emissions and Photochemical Aging. Science 2007, 315, 1259–1262. [Google Scholar] [CrossRef]
- Carlton, A.G.; Wiedinmyer, C.; Kroll, J.H. A review of Secondary Organic Aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 2009, 9, 4987–5005. [Google Scholar] [CrossRef]
- Dada, L.; Stolzenburg, D.; Simon, M.; Fischer, L.; Heinritzi, M.; Wang, M.; Xiao, M.; Vogel, A.L.; Ahonen, L.; Amorim, A.; et al. Role of sesquiterpenes in biogenic new particle formation. Sci. Adv. 2023, 9, eadi5297. [Google Scholar] [CrossRef] [PubMed]
- Bates, K.H.; Burke, G.J.P.; Cope, J.D.; Nguyen, T.B. Secondary organic aerosol and organic nitrogen yields from the nitrate radical (NO3) oxidation of alpha-pinene from various RO2 fates. Atmos. Chem. Phys. 2022, 22, 1467–1482. [Google Scholar] [CrossRef]
- Ziemann, P.J.; Atkinson, R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef] [PubMed]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipilä, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A large source of low-volatility secondary organic aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef]
- Perring, A.E.; Pusede, S.E.; Cohen, R.C. An Observational Perspective on the Atmospheric Impacts of Alkyl and Multifunctional Nitrates on Ozone and Secondary Organic Aerosol. Chem. Rev. 2013, 113, 5848–5870. [Google Scholar] [CrossRef]
- Kirkby, J.; Duplissy, J.; Sengupta, K.; Frege, C.; Gordon, H.; Williamson, C.; Heinritzi, M.; Simon, M.; Yan, C.; Almeida, J.; et al. Ion-induced nucleation of pure biogenic particles. Nature 2016, 533, 521–526. [Google Scholar] [CrossRef]
- Tröstl, J.; Chuang, W.K.; Gordon, H.; Heinritzi, M.; Yan, C.; Molteni, U.; Ahlm, L.; Frege, C.; Bianchi, F.; Wagner, R.; et al. The role of low-volatility organic compounds in initial particle growth in the atmosphere. Nature 2016, 533, 527–531. [Google Scholar] [CrossRef]
- Cash, J.M.; Heal, M.R.; Langford, B.; Drewer, J. A review of stereochemical implications in the generation of secondary organic aerosol from isoprene oxidation. Environ. Sci. Process. Impacts 2016, 18, 1369–1380. [Google Scholar] [CrossRef]
- Yang, Z.; Du, L.; Li, Y.; Ge, X. Secondary organic aerosol formation from monocyclic aromatic hydrocarbons: Insights from laboratory studies. Environ. Sci. Process. Impacts 2022, 24, 351–379. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, F.; Ma, M.; Ding, A.; Liao, H.; Wang, S.; Wang, X.; Zhao, B.; Cai, W.; Su, H.; et al. Unveiling the dipole synergic effect of biogenic and anthropogenic emissions on ozone concentrations. Sci. Total Environ. 2022, 818, 151722. [Google Scholar] [CrossRef]
- Shrivastava, M.; Cappa, C.D.; Fan, J.; Goldstein, A.H.; Guenther, A.B.; Jimenez, J.L.; Kuang, C.; Laskin, A.; Martin, S.T.; Ng, N.L.; et al. Recent advances in understanding secondary organic aerosol: Implications for global climate forcing. Rev. Geophys. 2017, 55, 509–559. [Google Scholar] [CrossRef]
- Fierravanti, A.; Fierravanti, E.; Cocozza, C.; Tognetti, R.; Rossi, S. Eligible reference cities in relation to BVOC-derived O3 pollution. Urban For. Urban Green. 2017, 28, 73–80. [Google Scholar] [CrossRef]
- Yenisoy-Karakaş, S.; Dörter, M.; Odabasi, M. Intraday and interday variations of 69 volatile organic compounds (BVOCs and AVOCs) and their source profiles at a semi-urban site. Sci. Total Environ. 2020, 723, 138028. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, Q.; Greenberg, J.; Guenther, A.; Li, J.; Cao, J.; Wang, J.; Liao, H.; Wang, Q.; Zhang, Q. Impacts of biogenic and anthropogenic emissions on summertime ozone formation in the Guanzhong Basin, China. Atmos. Chem. Phys. 2018, 18, 7489–7507. [Google Scholar] [CrossRef]
- Choi, J.-S.; Choi, Y.; Ahn, J.; Park, J.; Oh, J.; Lee, G.; Park, T.; Park, G.; Owen, J.S.; Lee, T. Observation of Secondary Organic Aerosol and New Particle Formation at a Remote Site in Baengnyeong Island, Korea. Asian J. Atmos. Environ. 2017, 11, 300–312. [Google Scholar] [CrossRef]
- Ghirardo, A.; Xie, J.; Zheng, X.; Wang, Y.; Grote, R.; Block, K.; Wildt, J.; Mentel, T.; Kiendler-Scharr, A.; Hallquist, M.; et al. Urban stress-induced biogenic VOC emissions and SOA-forming potentials in Beijing. Atmos. Chem. Phys. 2016, 16, 2901–2920. [Google Scholar] [CrossRef]
- Šimpraga, M.; Ghimire, R.P.; Van Der Straeten, D.; Blande, J.D.; Kasurinen, A.; Sorvari, J.; Holopainen, T.; Adriaenssens, S.; Holopainen, J.K.; Kivimäenpää, M. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. Eur. J. Forest Res. 2019, 138, 763–787. [Google Scholar] [CrossRef]
- Yong, J.; Xie, Y.; Guo, H.; Li, Y.; Sun, S. Unraveling the influence of biogenic volatile organic compounds and their constituents on ozone and SOA formation within the Yellow River Basin, China. Chemosphere 2024, 353, 141549. [Google Scholar] [CrossRef]
- Jia, Y.; Qiao, L.; Xie, W.; Li, L. Spatial-temporal characteristics of ambient isoprene and monoterpene and their ozone and secondary organic aerosol formation potential in China. Environ. Rev. 2024, 32, 203–213. [Google Scholar] [CrossRef]
- Zheng, J.; Shao, M.; Che, W.; Zhang, L.; Zhong, L.; Zhang, Y.; Streets, D. Speciated VOC Emission Inventory and Spatial Patterns of Ozone Formation Potential in the Pearl River Delta, China. Environ. Sci. Technol. 2009, 43, 8580–8586. [Google Scholar] [CrossRef]
- Ryu, Y.-H.; Baik, J.-J.; Kwak, K.-H.; Kim, S.; Moon, N. Impacts of urban land-surface forcing on ozone air quality in the Seoul metropolitan area. Atmos. Chem. Phys. 2013, 13, 2177–2194. [Google Scholar] [CrossRef]
- Li, Y.; Yin, S.; Yu, S.; Bai, L.; Wang, X.; Lu, X.; Ma, S. Characteristics of ozone pollution and the sensitivity to precursors during early summer in central plain, China. J. Environ. Sci. 2021, 99, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Mahilang, M.; Deb, M.K.; Pervez, S. Biogenic secondary organic aerosols: A review on formation mechanism, analytical challenges and environmental impacts. Chemosphere 2021, 262, 127771. [Google Scholar] [CrossRef]
- Yan, F.; Chen, W.; Jia, S.; Zhong, B.; Yang, L.; Mao, J.; Chang, M.; Shao, M.; Yuan, B.; Situ, S.; et al. Stabilization for the secondary species contribution to PM2.5 in the Pearl River Delta (PRD) over the past decade, China: A meta-analysis. Atmos. Environ. 2020, 242, 117817. [Google Scholar] [CrossRef]
- Déméautis, T.; Delles, M.; Tomaz, S.; Monneret, G.; Glehen, O.; Devouassoux, G.; George, C.; Bentaher, A. Pathogenic Mechanisms of Secondary Organic Aerosols. Chem. Res. Toxicol. 2022, 35, 1146–1161. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, Y.; Zhang, H.; Song, W.; Wu, Z.; Wang, X. Design and characterization of a semi-open dynamic chamber for measuring biogenic volatile organic compound (BVOC) emissions from plants. Atmos. Meas. Tech. 2022, 15, 79–93. [Google Scholar] [CrossRef]
- Xu, Y.; Ling, L.; Xie, P.; Hu, R.; Li, Z.; Chen, H. Low cost and high resolution electrochemical sensing for atmospheric NO2 measurement. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2018, 38, 3488–3495. [Google Scholar] [CrossRef]
- Kim, S.; Guenther, A.; Apel, E. Quantitative and qualitative sensing techniques for biogenic volatile organic compounds and their oxidation products. Environ. Sci. Process. Impacts 2013, 15, 1301–1314. [Google Scholar] [CrossRef]


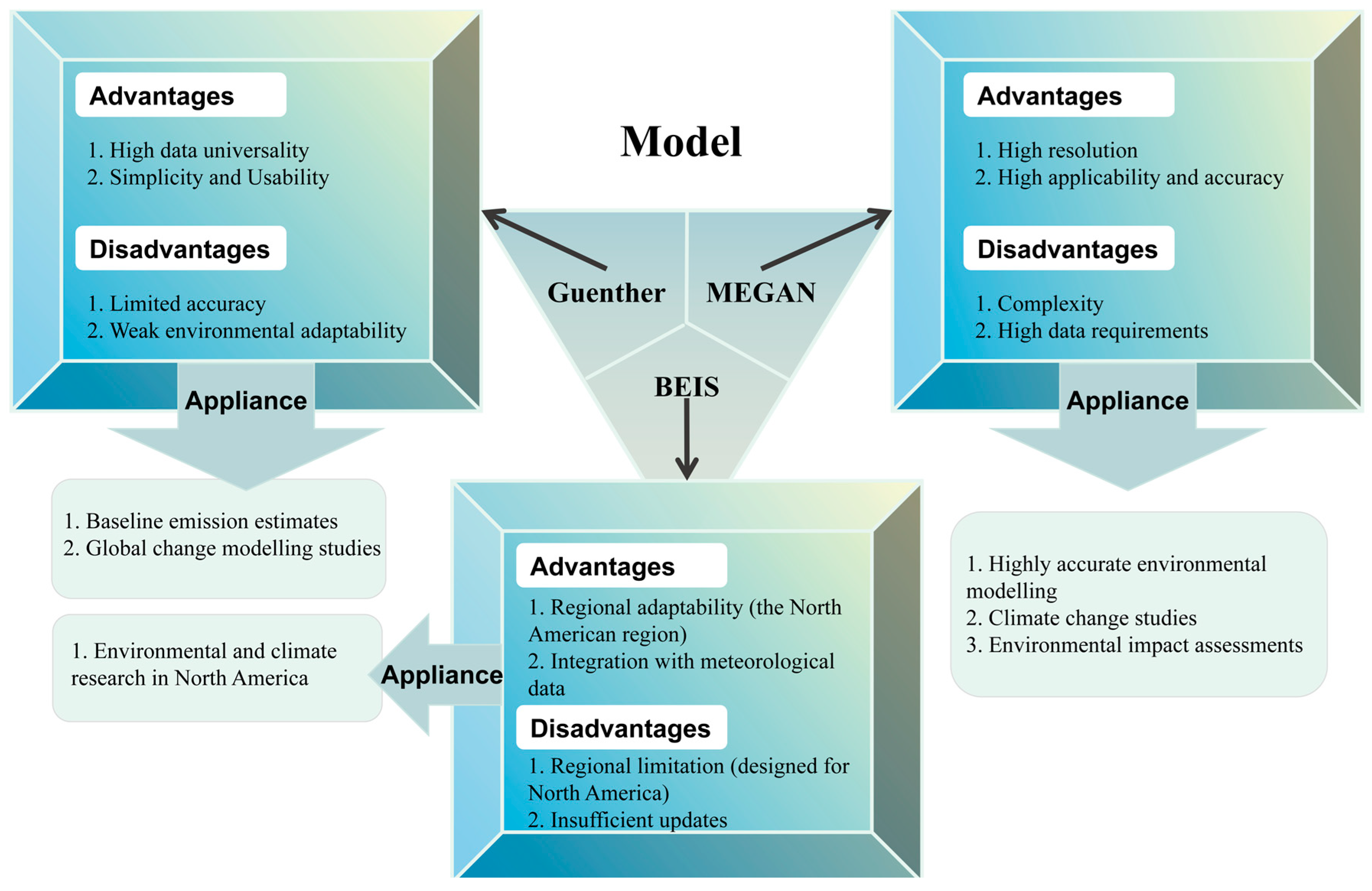

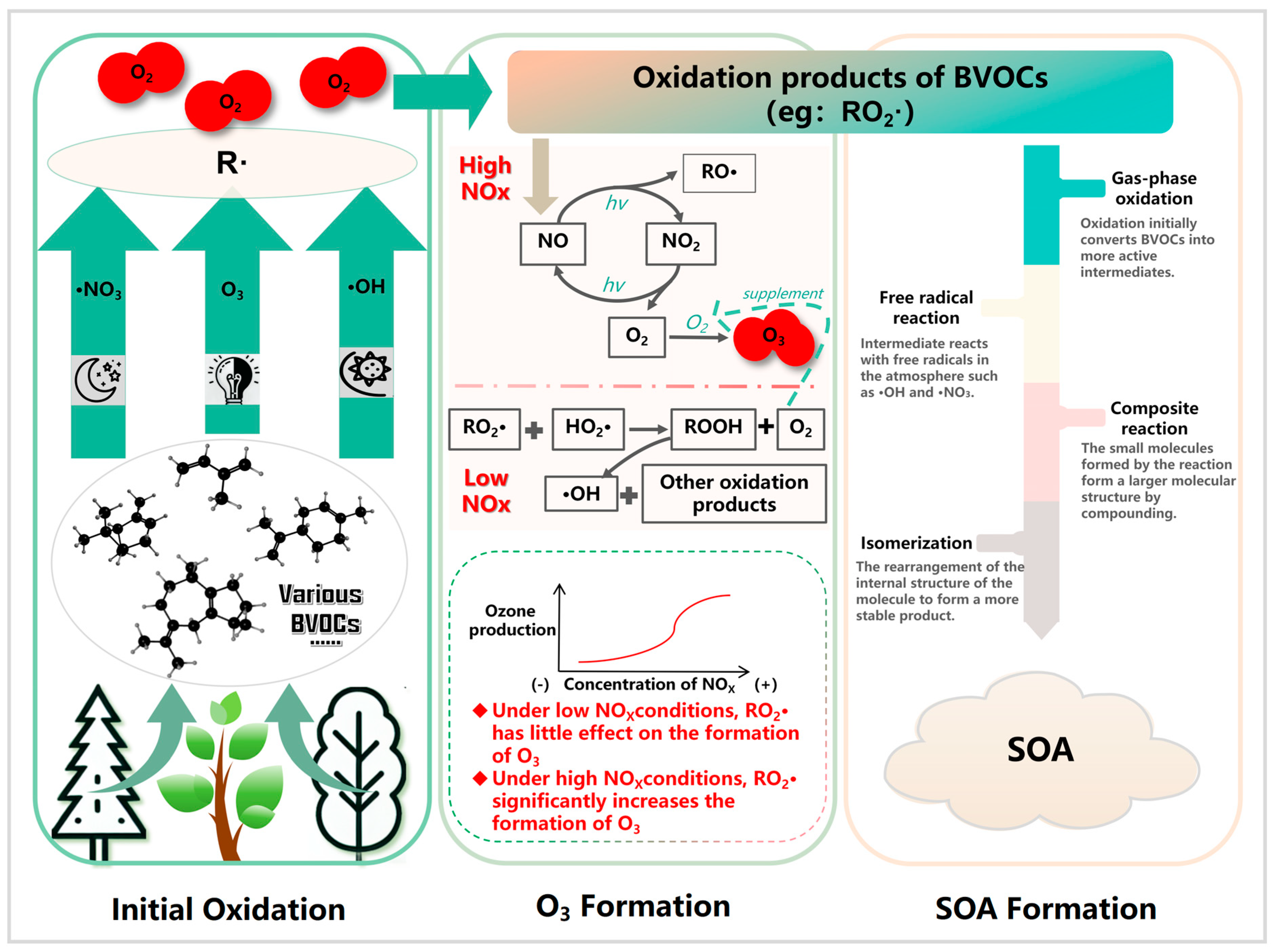
| Measurement Methods | On-Line | Off-Line | |
|---|---|---|---|
| Main technologies | Direct analysis: PTR-MS [32] FTIR [33] GC-FID [34] Portable GC/MS [35] E-noses [36] | Sampling: sampling bags, adsorption tubes, sampling tanks | Analysis: GC-MS [37] TDS-GC/MS [38] TCT-GC/MS [39] TD-GC/TOF-MS [40] SPME-GC/MS [41] |
| Advantages | Real-time monitoring; high temporal resolution; high sensitivity; high degree of automation | Lower equipment costs; high flexibility; diverse and detailed analysis | |
| Disadvantages | Expensive equipment; complexity; high on-site requirements | Time delay; lower temporal resolution; human error | |
| Comprehensive comparison |
| ||
| Analysis Technique | Detector Type | Sensitivity | Time Resolution | Advantages and Disadvantages | Applicable BVOC Types | Ref. |
|---|---|---|---|---|---|---|
| PTR-MS | Mass spectrometry | ppt- ppm | Real-time analysis | Sensitivity and cost | Most BVOCs | [42,43] |
| FTIR | Pyroelectric detector or photoconductive detector | ppb- ppm | Minutes to hours | Versatility and sensitivity | Polar BVOCs | [44,45] |
| GC-FID | Flame ionization detection | ppt | Minutes | High speed and limitations | Nonpolar BVOCs | [34,46] |
| E-noses | Gas sensors | ppb- ppm | Minutes | Speed and resolution | Trace BVOCs | [36,47] |
| Portable GC/MS | Mass spectrometer | ppb | Minutes | Portability and limitations | Most BVOCs | [48,49] |
| GC-MS | Mass spectrometer | ppt | Minutes to hours | Precision and complexity | Most BVOCs | [37,50] |
| TD-GC/MS | Mass spectrometer | ppb | Minutes | Sensitivity and time-consuming | Trace BVOCs | [38,51] |
| TCT-GC/MS | Mass spectrometer | ppb | Minutes | Precision and requirements | Complex BVOCs | [39,52] |
| TD-GC/TOF-MS | Time-of-flight mass spectrometer | ppb | Minutes | Resolution and cost | Trace BVOCs | [40,53] |
| SPME- GC/MS | Mass spectrometer | ppb | Minutes | Simplicity and sensitivity | Most BVOCs | [41,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, R.; Lun, X.; Gao, R.; Wang, L.; Yang, Y.; Su, X.; Habibullah-Al-Mamun, M.; Xu, X.; Li, H.; Li, J. A Review of Biogenic Volatile Organic Compounds from Plants: Research Progress and Future Prospects. Toxics 2025, 13, 364. https://doi.org/10.3390/toxics13050364
Luo R, Lun X, Gao R, Wang L, Yang Y, Su X, Habibullah-Al-Mamun M, Xu X, Li H, Li J. A Review of Biogenic Volatile Organic Compounds from Plants: Research Progress and Future Prospects. Toxics. 2025; 13(5):364. https://doi.org/10.3390/toxics13050364
Chicago/Turabian StyleLuo, Rongrong, Xiaoxiu Lun, Rui Gao, Le Wang, Yuan Yang, Xingqian Su, Md Habibullah-Al-Mamun, Xiaohang Xu, Hong Li, and Jinjuan Li. 2025. "A Review of Biogenic Volatile Organic Compounds from Plants: Research Progress and Future Prospects" Toxics 13, no. 5: 364. https://doi.org/10.3390/toxics13050364
APA StyleLuo, R., Lun, X., Gao, R., Wang, L., Yang, Y., Su, X., Habibullah-Al-Mamun, M., Xu, X., Li, H., & Li, J. (2025). A Review of Biogenic Volatile Organic Compounds from Plants: Research Progress and Future Prospects. Toxics, 13(5), 364. https://doi.org/10.3390/toxics13050364









