A Comprehensive Review of Multifunctional Nanozymes for Degradation and Detection of Organophosphorus Pesticides in the Environment
Abstract
:1. Introduction
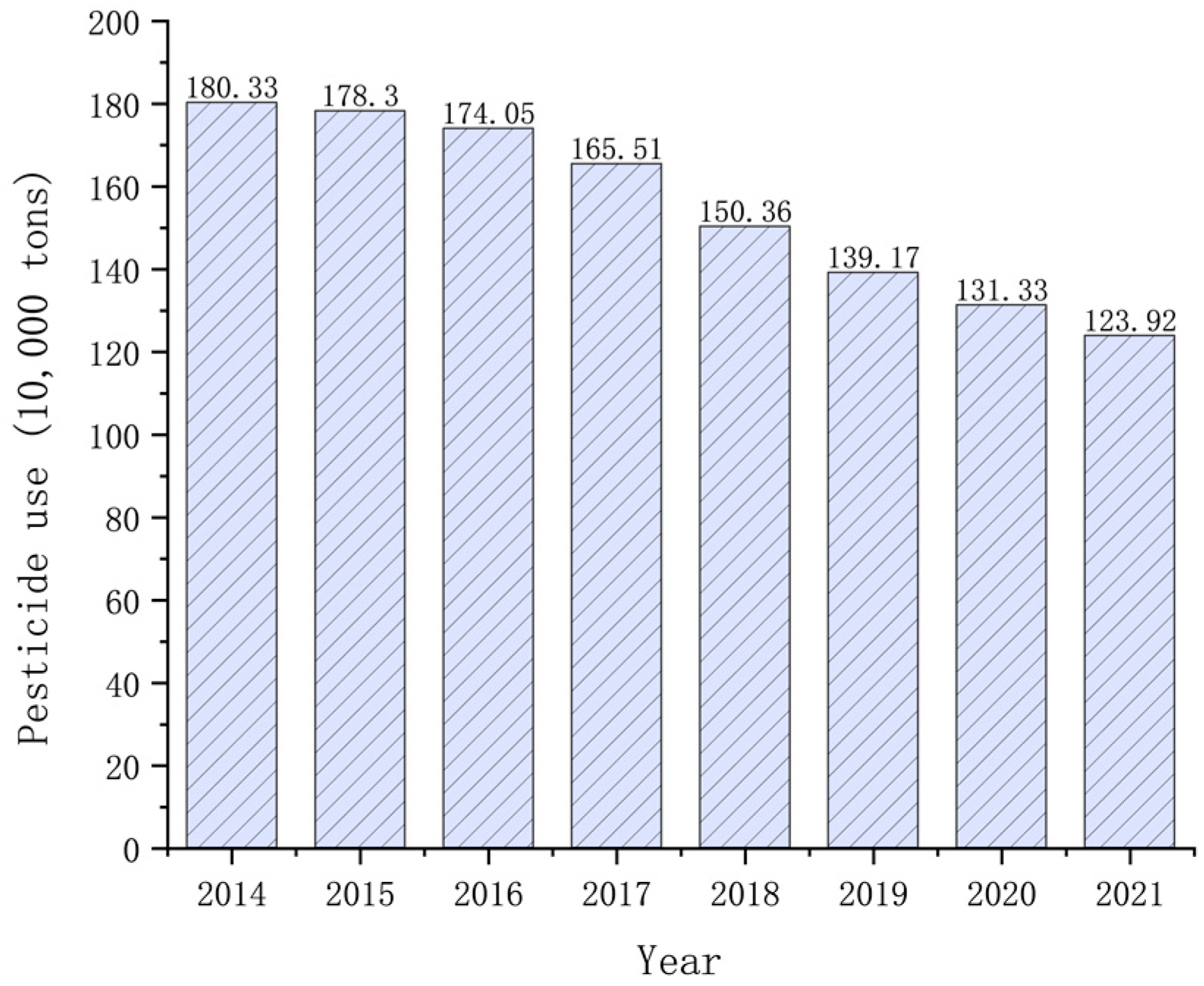
2. Migration and Transformation of Organophosphorus Pesticides in the Environment
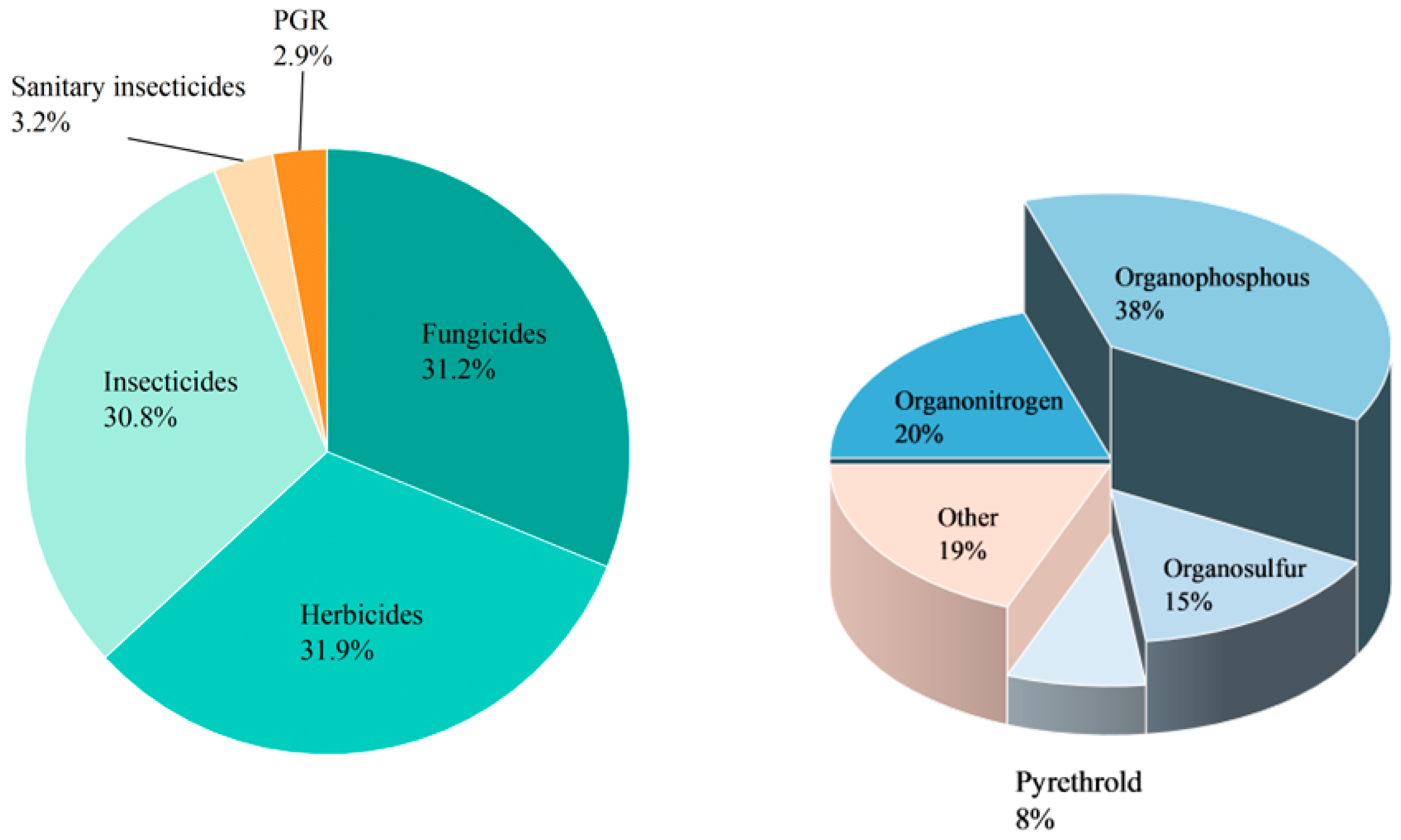
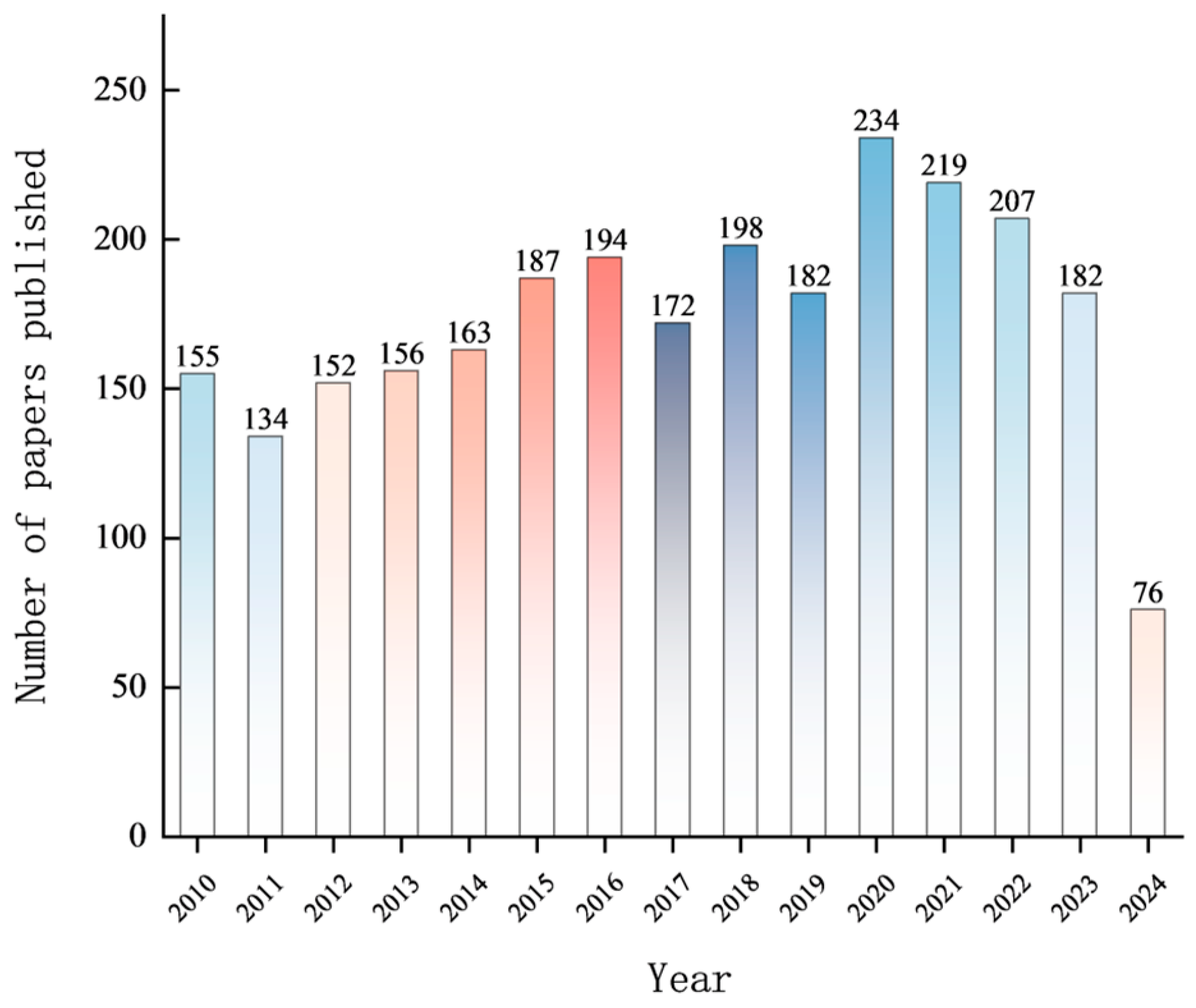
3. Bibliometric Analysis of Research on Degradation and Detection of Organophosphorus Pesticides
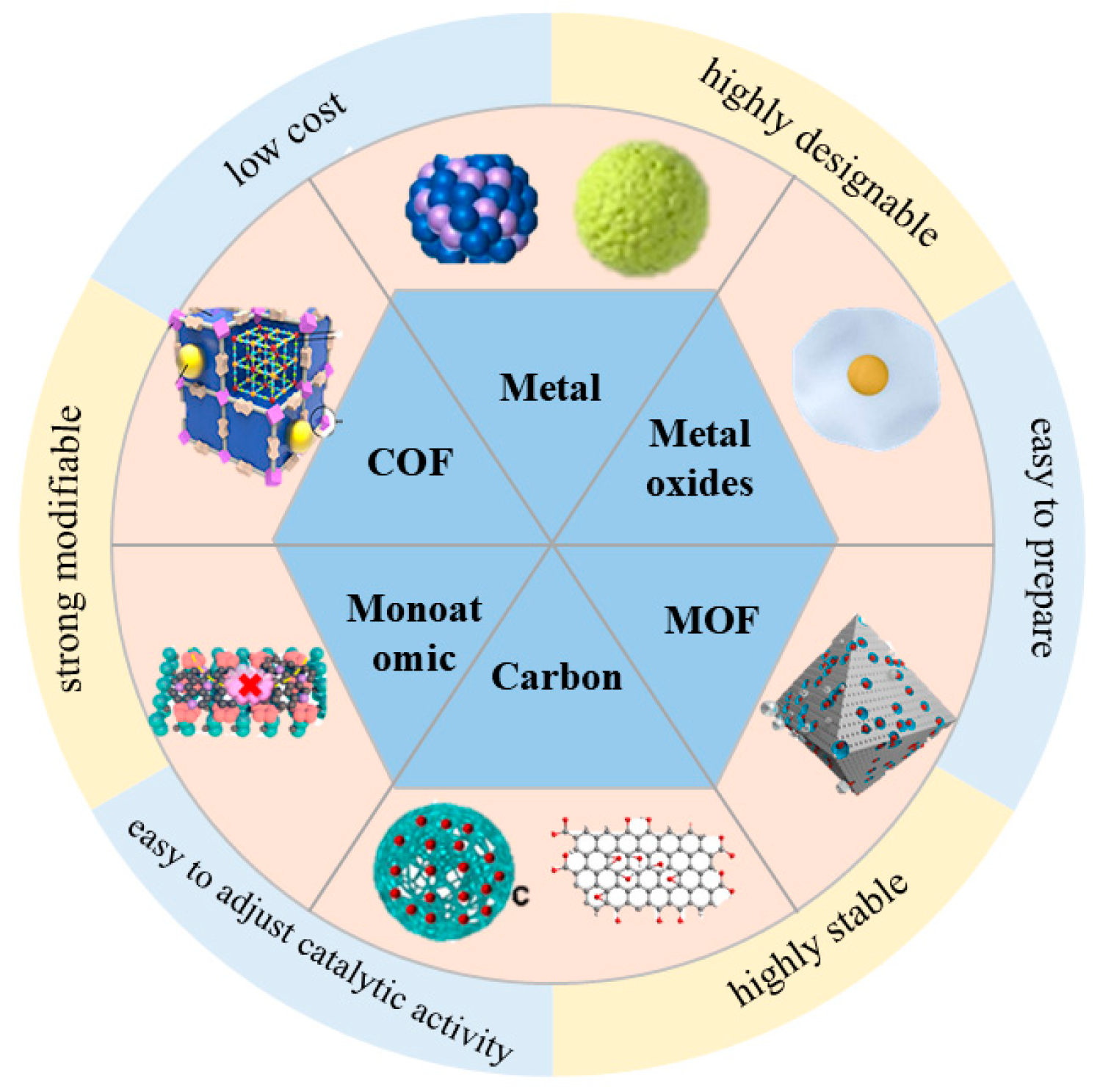
4. Phosphoester Nanozymes and Their Research Progress
4.1. Metal-Based Nanozymes
4.2. Metal Oxide Nanozymes
4.3. MOF-Based Nanozymes
4.3.1. Pure MOFs Materials
4.3.2. Composite MOFs Materials
4.4. Single-Atom Nanozymes (SAzymes)
4.5. Carbon-Based Nanozymes
4.5.1. Carbon Nanosheets
4.5.2. Graphene and Graphene Oxide
4.5.3. Carbon Dots (CDs)
4.6. COF-Based Nanozymes
5. Conclusions and Prospects
- Development of dual-function nanozymes: Efforts should be focused on creating nanozymes that integrate both degradation and detection functionalities, aiming to achieve integrated removal and real-time monitoring. Such approaches would advance the development of innovative functional environmental materials.
- Evaluation of long-term environmental and health impact: assessing and monitoring the long-term effects by using nanozymes on the environment and within the human body is essential to ensure their environmental friendliness and health safety.
- Manufacturing processes for industrial-scale production: investigating manufacturing processes suitably for large-scale industrial production and aligning them with practical applications in areas such as agricultural production, food processing, and environmental safety is crucial for widespread adoption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Washuck, N.; Hanson, M.; Prosser, R. Yield to the Data: Some Perspective on Crop Productivity and Pesticides. Pest Manag. Sci. 2022, 78, 1765–1771. [Google Scholar] [CrossRef]
- Jiao, C.; Chen, L.; Sun, C.; Jiang, Y.; Zhai, L.; Liu, H.; Shen, Z. Evaluating National Ecological Risk of Agricultural Pesticides from 2004 to 2017 in China. Environ. Pollut. 2020, 259, 113778. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.; Dumitriu (Gabur), G.-D.; Teodosiu, C. Pesticides Identification and Sustainable Viticulture Practices to Reduce Their Use: An Overview. Molecules 2022, 27, 8205. [Google Scholar] [CrossRef] [PubMed]
- Malarkodi, C.; Rajeshkumar, S.; Annadurai, G. Detection of Environmentally Hazardous Pesticide in Fruit and Vegetable Samples Using Gold Nanoparticles. Food Control 2017, 80, 11–18. [Google Scholar] [CrossRef]
- Fenik, J.; Tankiewicz, M.; Biziuk, M. Properties and Determination of Pesticides in Fruits and Vegetables. TrAC Trends Anal. Chem. 2011, 30, 814–826. [Google Scholar] [CrossRef]
- Brown, Z.S. Voluntary Programs to Encourage Refuges for Pesticide Resistance Management: Lessons from a Quasi-Experiment. Am. J. Agric. Econ. 2018, 100, 844–867. [Google Scholar] [CrossRef]
- Chau, N.D.G.; Sebesvari, Z.; Amelung, W.; Renaud, F.G. Pesticide Pollution of Multiple Drinking Water Sources in the Mekong Delta, Vietnam: Evidence from Two Provinces. Environ. Sci. Pollut. Res. 2015, 22, 9042–9058. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fantke, P. Toward Harmonizing Global Pesticide Regulations for Surface Freshwaters in Support of Protecting Human Health. J. Environ. Manag. 2022, 301, 113909. [Google Scholar] [CrossRef]
- Memon, Q.U.A.; Wagan, S.A.; Chunyu, D.; Shuangxi, X.; Jingdong, L.; Damalas, C.A. Health Problems from Pesticide Exposure and Personal Protective Measures among Women Cotton Workers in Southern Pakistan. Sci. Total Environ. 2019, 685, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Sharma, P.; Kumar, A.; Walia, Y.; Kumar, R.; Umar, A.; Ibrahim, A.A.; Akhtar, M.S.; Alkhanjaf, A.A.M.; Baskoutas, S. A Review on Ecology Implications and Pesticide Degradation Using Nitrogen Fixing Bacteria under Biotic and Abiotic Stress Conditions. Chem. Ecol. 2023, 39, 753–774. [Google Scholar] [CrossRef]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Dung, T.P. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Zambonin, C.G.; Quinto, M.; De Vietro, N.; Palmisano, F. Solid-Phase Microextraction—Gas Chromatography Mass Spectrometry: A Fast and Simple Screening Method for the Assessment of Organophosphorus Pesticides Residues in Wine and Fruit Juices. Food Chem. 2004, 86, 269–274. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A. Microbial Degradation of Organophosphorus Compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, S.; Tassinari, R.; Maranghi, F.; Eusepi, A.; Di Virgilio, A.; Chiarotti, F.; Ricceri, L.; Pesciolini, A.V.; Gilardi, E.; Moracci, G.; et al. Developmental Exposure to Chlorpyrifos Induces Alterations in Thyroid and Thyroid Hormone Levels Without Other Toxicity Signs in Cd1 Mice. Toxicol. Sci. 2009, 108, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, Monitoring and Biodegradation of Organophosphate Pesticides: A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Wang, J.; Teng, Y.; Zhai, Y.; Yue, W.; Pan, Z. Spatiotemporal Distribution and Risk Assessment of Organophosphorus Pesticides in Surface Water and Groundwater on the North China Plain, China. Environ. Res. 2022, 204, 112310. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, L.; Liu, X.; Zhou, H.; Lu, J.; Huang, S.; Wang, Z. The Occurrence and Spatial Distribution of Organophosphorous Pesticides in Chinese Surface Water. Bull. Environ. Contam. Toxicol. 2009, 82, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, K.; Hassan, M.; Xu, C.; Zhang, B.; Gin, K.Y.-H.; He, Y. Occurrence, Distribution and Risk Assessment of Pesticides in a River-Reservoir System. Ecotoxicol. Environ. Saf. 2018, 166, 320–327. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Liao, X.; Teng, Y.; Zhai, Y.; Yue, W. Influence of Surface-Water Irrigation on the Distribution of Organophosphorus Pesticides in Soil-Water Systems, Jianghan Plain, Central China. J. Environ. Manag. 2021, 281, 111874. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Omar, T.F.T.; Aris, A.Z.; Lee, Y. Surface Water Organophosphorus Pesticides Concentration and Distribution in the Langat River, Selangor, Malaysia. Expo Health 2016, 8, 497–511. [Google Scholar] [CrossRef]
- Osman, R.; Saim, N.; Juahir, H.; Abdullah, M.P. Chemometric Application in Identifying Sources of Organic Contaminants in Langat River Basin. Environ. Monit. Assess. 2012, 184, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Ensminger, M.; Bergin, R.; Spurlock, F.; Goh, K.S. Pesticide Concentrations in Water and Sediment and Associated Invertebrate Toxicity in Del Puerto and Orestimba Creeks, California, 2007–2008. Environ. Monit. Assess. 2011, 175, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Dahshan, H.; Megahed, A.M.; Abd-Elall, A.M.M.; Abd-El-Kader, M.A.-G.; Nabawy, E.; Elbana, M.H. Monitoring of Pesticides Water Pollution-The Egyptian River Nile. J. Environ. Health Sci. Eng. 2016, 14, 15. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Zhao, Y. Nanozymes: Versatile Platforms for Cancer Diagnosis and Therapy. Nano-Micro Lett. 2022, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, D.; Lai, C.; Qin, L.; Zeng, G.; Xu, P.; Li, B.; Yi, H.; Zhang, M. Peroxidase-Like Activity of Smart Nanomaterials and Their Advanced Application in Colorimetric Glucose Biosensors. Small 2019, 15, 1900133. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, D.; Chen, L.; He, H.; Wang, Q.; Hong, C.; He, J.; Gao, X.; Yang, Y.; Jiang, B.; et al. High-Performance Self-Cascade Pyrite Nanozymes for Apoptosis–Ferroptosis Synergistic Tumor Therapy. ACS Nano 2021, 15, 5735–5751. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme Mimicry for Combating Bacteria and Biofilms. Acc. Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef]
- Niu, J.; Sun, Y.; Wang, F.; Zhao, C.; Ren, J.; Qu, X. Photomodulated Nanozyme Used for a Gram-Selective Antimicrobial. Chem. Mater. 2018, 30, 7027–7033. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A. Multienzymes Activity of Metals and Metal Oxide Nanomaterials: Applications from Biotechnology to Medicine and Environmental Engineering. J. Nanobiotechnol. 2021, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Tian, T.; Yang, X.; Wang, L.; Sun, Y.; Li, Y.; Huang, H. Smartphone-Assisted Sensor Array Constructed by Copper-Based Laccase-like Nanozymes for Specific Identification and Discrimination of Organophosphorus Pesticides. Food Chem. 2023, 424, 136477. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Chen, Y.; Wang, H.; Jiao, L.; Chen, H.; Zhu, C. Bismuth Atom-Doped Gold Aerogels for the Detection of Acetylcholinesterase Activity and Organophosphorus Inhibitor. Chem. Eng. J. 2023, 474, 145483. [Google Scholar] [CrossRef]
- Niu, K.; Chen, J.; Lu, X. Versatile Biomimetic Catalyst Functionalized Nanozymes for Electrochemical Sensing. Chem. Eng. J. 2023, 475, 146491. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, B.; Wang, M.; Pan, J.; Xu, L.; Hu, P.; Niu, X. Amorphous Fe-Containing Phosphotungstates Featuring Efficient Peroxidase-like Activity at Neutral pH: Toward Portable Swabs for Pesticide Detection with Tandem Catalytic Amplification. Anal. Chem. 2023, 95, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- Zou, X. Metal-Organic Framework-Derived Fe/C/Bi2O3 as Peroxidase-like Nanozymes for the Detection of Organophosphorus Pesticides. Sens. Actuators 2023, 393, 134121. [Google Scholar] [CrossRef]
- Ji, C.; Tang, X.; Wen, R.; Xu, C.; Wei, J.; Han, B.; Wu, L. A Multienzyme Reaction-Mediated Electrochemical Biosensor for Sensitive Detection of Organophosphorus Pesticides. Biosensors 2024, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, X.; Zhang, Y.; Huang, B.; Han, L. Selective Inhibition toward Dual Enzyme-like Activities of Iridium Nanozymes for a Specific Colorimetric Assay of Malathion without Enzymes. J. Agric. Food Chem. 2022, 70, 3898–3906. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Ding, X.; Liu, J.; Tang, Y.; Chen, H.; Zhou, Y.; Zhu, C.; Yan, H. Trace Amount of Bi-Doped Core–Shell Pd@Pt Mesoporous Nanospheres with Specifically Enhanced Peroxidase-Like Activity Enable Sensitive and Accurate Detection of Acetylcholinesterase and Organophosphorus Nerve Agents. Anal. Chem. 2024, 96, 6072–6078. [Google Scholar] [CrossRef] [PubMed]
- Weerathunge, P.; Behera, B.K.; Zihara, S.; Singh, M.; Prasad, S.N.; Hashmi, S.; Mariathomas, P.R.D.; Bansal, V.; Ramanathan, R. Dynamic Interactions between Peroxidase-Mimic Silver NanoZymes and Chlorpyrifos-Specific Aptamers Enable Highly-Specific Pesticide Sensing in River Water. Anal. Chim. Acta 2019, 1083, 157–165. [Google Scholar] [CrossRef]
- Jiang, W.; Feng, Y.; Jiang, C.; Li, H.; Wang, Z.; Xiao, Y.; Lan, W.; Liu, Y. Platinum-Nickel Nanoparticle-Based Oxidase-like Nanozyme for Colorimetric/Photothermal Dual-Mode Detection of Organophosphorus Pesticides. Sens. Actuators B Chem. 2024, 412, 135861. [Google Scholar] [CrossRef]
- Shen, Y.; Gao, X.; Chen, H.; Wei, Y.; Yang, H.; Gu, Y. Ultrathin C3N4 Nanosheets-Based Oxidase-like 2D Fluorescence Nanozyme for Dual-Mode Detection of Organophosphorus Pesticides. J. Hazard. Mater. 2023, 451, 131171. [Google Scholar] [CrossRef]
- Huang, H.; Song, D.; Zhang, W.; Fang, S.; Zhou, Q.; Zhang, H.; Liang, Z.; Li, Y. Choline Oxidase-Integrated Copper Metal–Organic Frameworks as Cascade Nanozymes for One-Step Colorimetric Choline Detection. J. Agric. Food Chem. 2022, 70, 5228–5236. [Google Scholar] [CrossRef] [PubMed]
- Kute, A.D.; Gaikwad, R.P.; Warkad, I.R.; Gawande, M.B. A Review on the Synthesis and Applications of Sustainable Copper-Based Nanomaterials. Green Chem. 2022, 24, 3502–3573. [Google Scholar] [CrossRef]
- Mortland, M.M.; Raman, K.V. Catalytic Hydrolysis of Some Organic Phosphate Pesticides by Copper(II). J. Agric. Food Chem. 1967, 15, 163–167. [Google Scholar] [CrossRef]
- Liang, X.; Han, L. White Peroxidase-Mimicking Nanozymes: Colorimetric Pesticide Assay without Interferences of O2 and Color. Adv. Funct. Mater. 2020, 30, 2001933. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, K.; Li, H.; Chen, W.; Fu, M.; Yue, K.; Zhu, X.; Liu, Q. Glutathione Detection Based on Peroxidase-like Activity of CoO–Montmorillonite Nanocomposites34. Sens. Actuators B Chem. 2018, 273, 1635–1639. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhao, H.; Zhang, L.; Su, Y.; Lv, Y. BSA-Templated MnO2 Nanoparticles as Both Peroxidase and Oxidase Mimics. Analyst 2012, 137, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- Pedone, D.; Moglianetti, M.; Luca, E.D.; Bardi, G.; Pompa, P.P. Platinum Nanoparticles in Nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef]
- Sharifi, M.; Faryabi, K.; Talaei, A.J.; Shekha, M.S.; Ale-Ebrahim, M.; Salihi, A.; Nanakali, N.M.Q.; Aziz, F.M.; Rasti, B.; Hasan, A.; et al. Antioxidant Properties of Gold Nanozyme: A Review. J. Mol. Liq. 2020, 297, 112004. [Google Scholar] [CrossRef]
- Arsawiset, S.; Sansenya, S.; Teepoo, S. Nanozymes Paper−based Analytical Device for the Detection of Organophosphate Pesticides in Fruits and Vegetables. Anal. Chim. Acta 2023, 1267, 341377. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Qiang, S.; Jia, Z.; Shi, Q.H.; Meng, X.; Yu, M.; Ma, H.; Zhao, K.; Dai, Y. Smartphone-Integrated Nanozymes Sensor Array for High Throughput Recognition of Organophosphorus Pesticides. Sens. Actuators B Chem. 2023, 389, 133857. [Google Scholar] [CrossRef]
- Huang, L.; Sun, D.-W.; Pu, H.; Wei, Q.; Luo, L.; Wang, J. A Colorimetric Paper Sensor Based on the Domino Reaction of Acetylcholinesterase and Degradable γ-MnOOH Nanozyme for Sensitive Detection of Organophosphorus Pesticides. Sens. Actuators B Chem. 2019, 290, 573–580. [Google Scholar] [CrossRef]
- Zuo, M.; Yang, Y.; Jiang, S.; Zhu, C.; Han, Y.; Hu, J.; Ren, K.; Cui, L.; Zhang, C.-Y. Ultrathin-FeOOH-Coated MnO2 Nanozyme with Enhanced Catalase-like and Oxidase-like Activities for Photoelectrochemical and Colorimetric Detection of Organophosphorus Pesticides. Food Chem. 2024, 445, 138716. [Google Scholar] [CrossRef]
- Gai, P.; Pu, L.; Wang, C.; Zhu, D.; Li, F. CeO@NC Nanozyme with Robust Dephosphorylation Ability of Phosphotriester: A Simple Colorimetric Assay for Rapid and Selective Detection of Paraoxon2. Biosens. Bioelectron. 2023, 220, 114841. [Google Scholar] [CrossRef]
- Zhao, F.; Li, M.; Wang, L.; Wang, M. A Colorimetric Sensor Enabled with Heterogeneous Nanozymes with Phosphatase-like Activity for the Residue Analysis of Methyl Parathion. Foods 2023, 12, 2980. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, N.; Liu, S.; Wei, K.; Ma, C.; Pan, J.; Feng, S. Direct and Specific Detection of Methyl-Paraoxon Using a Highly Sensitive Fluorescence Strategy Combined with Phosphatase-like Nanozyme and Molecularly Imprinted Polymer. Talanta 2024, 277, 126434. [Google Scholar] [CrossRef] [PubMed]
- Miura-Stempel, E.; Oregon, A.G.; Harvey, S.M.; De Yoreo, J.J.; Chen, C.-L.; Cossairt, B.M. CeO2 Nanoparticle Doping as a Probe of Active Site Speciation in the Catalytic Hydrolysis of Organophosphates. ACS Appl. Nano Mater. 2024, 7, 15498–15507. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, H.; Yang, B.; Zeng, J.; Meng, L.; Shi, D.; Chen, L.; Huang, Y. Highly-Oxidizing Au@MnO2−X Nanozymes Mediated Homogeneous Electrochemical Detection of Organophosphorus Independent of Dissolved Oxygen. J. Hazard. Mater. 2023, 459, 132116. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, J.; Wu, X.; Ta, N.; Liu, S.; Li, Z.; Lou, W.-Y. Ultrasmall Ce-Based Metal–Organic Frameworks Nanozyme with Hydrolytic Activity for Boosting Antibiofilm Therapy. Chem. Eng. J. 2024, 480, 148246. [Google Scholar] [CrossRef]
- Xiao, J.; Shi, F.; Zhang, Y.; Peng, M.; Xu, J.; Li, J.; Chen, Z.; Yang, Z. A MOF Nanozyme-Mediated Acetylcholinesterase-Free Colorimetric Strategy for Direct Detection of Organophosphorus Pesticides. Chem. Commun. 2024, 60, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Weng, W.; Xu, Y.; Zhou, Y.; Yi, Y.; Zhao, Y.; Zhu, G. Vanadium-Based Metal–Organic Frameworks with Peroxidase-like Activity as a Colorimetric Sensing Platform for Direct Detection of Organophosphorus Pesticides. Inorg. Chem. 2024, 63, 16442–16450. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ou, Y.; Yang, Y.; Liu, G.; Liang, Q.; Ai, X.; Yang, S.; Nian, Y.; Su, L.; Wang, J. Rational Construction of a Robust Metal-Organic Framework Nanozyme with Dual-Metal Active Sites for Colorimetric Detection of Organophosphorus Pesticides. J. Hazard. Mater. 2022, 423, 127253. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, P.; Wang, M.; Sun, X.; Pan, W.; Wang, J. Introducing Mn into ZIF-8 Nanozyme for Enhancing Its Catalytic Activities and Adding Specific Recognizer for Detection of Organophosphorus Pesticides. Microchim. Acta 2023, 190, 437. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kang, Y.; Jiao, L.; Wu, Y.; Yan, H.; Li, J.; Gu, W.; Song, W.; Zhu, C. Tuning Atomically Dispersed Fe Sites in Metal–Organic Frameworks Boosts Peroxidase-Like Activity for Sensitive Biosensing. Nano-Micro Lett. 2020, 12, 184. [Google Scholar] [CrossRef]
- Shen, B.; Wu, Q.; Guo, Y.; Qin, J.; Chen, H.; Yang, Y.; Liu, Z.; Li, L.; Li, W.; Zhu, C. Modulating the Hydrophilic-Hydrophobic Microenvironment of MOF-Stabilized Pt Nanozymes: The Role of H2 O in the Peroxidase-Like Catalyzed Reaction. Adv. Funct. Mater. 2024, 2415854. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, X.; Liao, D.; Hou, J.; Liu, H.; Zhu, G. High Peroxidase-Mimicking Metal–Organic Frameworks Decorated with Platinum Nanozymes for the Colorimetric Detection of Acetylcholine Chloride and Organophosphorus Pesticides via Enzyme Cascade Reaction. Inorg. Chem. 2023, 62, 13929–13936. [Google Scholar] [CrossRef]
- Jin, C.; Yang, S.; Zheng, J.; Chai, F.; Tian, M. A Smartphone-Assisted Portable on-Site Detection System for Organophosphorus Pesticides in Vegetables and Fruits Based on All-in-One Paper-Based Sensors: 2,2-Dichlorovinyl Dimethyl Phosphate as a Model. Food Chem. 2024, 459, 140369. [Google Scholar] [CrossRef]
- Ma, K.; Cheung, Y.H.; Kirlikovali, K.O.; Xie, H.; Idrees, K.B.; Wang, X.; Islamoglu, T.; Xin, J.H.; Farha, O.K. Fibrous Zr-MOF Nanozyme Aerogels with Macro-Nanoporous Structure for Enhanced Catalytic Hydrolysis of Organophosphate Toxins. Adv. Mater. 2024, 36, 2300951. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Feng, Y.; Jiang, C.; Li, H.; Yu, Z.; Xiao, Y.; Hou, R.; Wan, X.; Liu, Y. A Novel Fluorescent and Photothermal Probe Based on Nanozyme-Mediated Cascade Reaction for Detecting Organophosphorus Pesticide Residues. Talanta 2024, 279, 126620. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wu, Y.; Tang, Y.; Xu, W.; Chen, Y.; Su, R.; Fan, Y.; Jiang, W.; Wen, Y.; Gu, W.; et al. In Situ Defect Engineering of Fe-MIL for Self-Enhanced Peroxidase-Like Activity. Small 2024, 20, 2403354. [Google Scholar] [CrossRef]
- Yu, X.; Wei, Y.; Qi, W.; Wang, M. Catalytic Metal–Organic Framework-Melamine Foam Composite as an Efficient Material for the Elimination of Organic Pollutants. Environ. Sci. Pollut. Res. 2023, 30, 44266–44275. [Google Scholar] [CrossRef]
- Chai, H.; Li, Y.; Yu, K.; Yuan, Z.; Guan, J.; Tan, W.; Ma, J.; Zhang, X.; Zhang, G. Two-Site Enhanced Porphyrinic Metal–Organic Framework Nanozymes and Nano-/Bioenzyme Confined Catalysis for Colorimetric/Chemiluminescent Dual-Mode Visual Biosensing. Anal. Chem. 2023, 95, 16383–16391. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Yuan, X.; Xiong, J.; Zong, M.-H.; Wu, X.; Lou, W.-Y. A Dual-Mode Sensing Platform Based on Metal–Organic Framework for Colorimetric and Ratiometric Fluorescent Detection of Organophosphorus Pesticide. Food Chem. 2024, 432, 137272. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, B.; Pan, J.; Xu, L.; Liu, J.; Hu, P.; Du, D.; Lin, Y.; Niu, X. Redox Interference-Free Bimodal Paraoxon Sensing Enabled by an Aggregation-Induced Emission Nanozyme Catalytically Hydrolyzing Phosphoesters Specifically. Biosens. Bioelectron. 2025, 267, 116756. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, Z.; Qin, X.; Wu, H.; Zhang, H.; Liu, G. Ultrasmall Au Nanoparticles Modified 2D Metalloporphyrinic Metal-Organic Framework Nanosheets with High Peroxidase-like Activity for Colorimetric Detection of Organophosphorus Pesticides. Food Chem. 2022, 376, 131906. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Lyu, Z.; Pan, J.; Ding, S.; Ruan, X.; Zhu, W.; Du, D.; Lin, Y. Metal–Organic Framework Based Nanozymes: Promising Materials for Biochemical Analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, L.; Luo, X.; Xu, W.; Wei, X.; Wang, H.; Yan, H.; Gu, W.; Xu, B.Z.; Du, D.; et al. Oxidase-Like Fe-N-C Single-Atom Nanozymes for the Detection of Acetylcholinesterase Activity. Small 2019, 15, 1903108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Gao, W.; Liu, S.G.; Zhao, Q.; Fu, Z.; Shi, X. A Smartphone-Integrated Colorimetric Sensor for Sensitive Detection of Organophosphorus Pesticides Based on Large-Scale Synthesized Fe-N/C Single-Atom Nanozymes. Sens. Actuators B Chem. 2024, 403, 135130. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Jiao, L.; Xu, W.; Wang, H.; Wei, X.; Gu, W.; Ren, G.; Zhang, N.; Zhang, Q.; et al. Cascade Reaction System Integrating Single-Atom Nanozymes with Abundant Cu Sites for Enhanced Biosensing. Anal. Chem. 2020, 92, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhang, J.; Huang, H.; Wang, X.; He, X.; Luo, Y.; Li, J.; Huang, K.; Cheng, N. Single-Atom Ce-N-C Nanozyme Bioactive Paper with a 3D-Printed Platform for Rapid Detection of Organophosphorus and Carbamate Pesticide Residues. Food Chem. 2022, 387, 132896. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wu, J.; Zhang, R.; Wang, S.; Zhu, X.; Xiang, H.; Wan, Y.; Cheng, Z.; Jin, M.; Li, X.; et al. Optimizing Single-Atom Cerium Nanozyme Activity to Function in a Sequential Catalytic System for Colorimetric Biosensing. Nano Today 2024, 56, 102236. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, X.; Shen, Z.; Gu, Y.; He, L.; Zhang, M.; Lu, N. Single-Atom Fe Nanozymes Coupling with Atomic Clusters as Superior Oxidase Mimics for Ratiometric Fluorescence Detection. Chem. Eng. J. 2023, 469, 143923. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, J.; Wang, X.; Jiao, L.; Wei, X.; Wang, H.; Li, J.; Liu, M.; Zheng, L.; Hu, L.; et al. Iron Single-Atom Catalysts Boost Photoelectrochemical Detection by Integrating Interfacial Oxygen Reduction and Enzyme-Mimicking Activity. ACS Nano 2022, 16, 2997–3007. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Tian, L.; Wang, H.; Wu, Z.; Luo, X.; Wang, X.; Jiao, L.; Wei, X.; Qin, Y.; Zheng, L.; et al. Single-Atom Nanozymes with Axial Ligand-Induced Self-Adaptive Conformation in Alkaline Medium Boost Chemiluminescence. Sci. China Chem. 2023, 66, 904–912. [Google Scholar] [CrossRef]
- Zhong, H.; Xue, Y.; Zhang, P.; Liu, B.; Zhang, X.; Chen, Z.; Li, K.; Zheng, L.; Zuo, X. Cascade Reaction System Integrating Nanozymes for Colorimetric Discrimination of Organophosphorus Pesticides. Sens. Actuators B Chem. 2022, 350, 130810. [Google Scholar] [CrossRef]
- Shen, Z.; Xu, D.; Wang, G.; Geng, L.; Xu, R.; Wang, G.; Guo, Y.; Sun, X. Novel Colorimetric Aptasensor Based on MOF-Derived Materials and Its Applications for Organophosphorus Pesticides Determination. J. Hazard. Mater. 2022, 440, 129707. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, J.; Dong, H.; Geng, L.; Sun, J.; Liu, J.; Dong, J.; Guo, Y.; Sun, X. A Dual-Mode Biosensor Featuring Single-Atom Fe Nanozyme for Multi-Pesticide Detection in Vegetables. Food Chem. 2024, 437, 137882. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Q.; Yu, J.; Sun, J.; Niu, N.; Chen, L. Lignin-Based Iron Single-Atom Nanozyme for Detection of Organophosphorus in Soil. Microchem. J. 2023, 195, 109381. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, D.; Hou, S.; Li, Y.; Liu, Y.; Zhang, M.; Zhang, G.; Xu, H. Designing Hierarchically Porous Single Atoms of Fe-N5 Catalytic Sites with High Oxidase-like Activity for Sensitive Detection of Organophosphorus Pesticides. Anal. Chem. 2022, 94, 15270–15279. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Gan, L.; Wang, J.; Dong, S. Single-Atom Nanozymes. Sci. Adv. 2019, 5, eaav5490. [Google Scholar] [CrossRef]
- Niu, X.; Shi, Q.; Zhu, W.; Liu, D.; Tian, H.; Fu, S.; Cheng, N.; Li, S.; Smith, J.N.; Du, D.; et al. Unprecedented Peroxidase-Mimicking Activity of Single-Atom Nanozyme with Atomically Dispersed Fe–Nx Moieties Hosted by MOF Derived Porous Carbon. Biosens. Bioelectron. 2019, 142, 111495. [Google Scholar] [CrossRef]
- Muhammad, P.; Hanif, S.; Li, J.; Guller, A.; Rehman, F.U.; Ismail, M.; Zhang, D.; Yan, X.; Fan, K.; Shi, B. Carbon Dots Supported Single Fe Atom Nanozyme for Drug-Resistant Glioblastoma Therapy by Activating Autophagy-Lysosome Pathway. Nano Today 2022, 45, 101530. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, C.; Jin, M.; He, J.; Zhang, H.; Ren, W.; Li, J.; Wang, D.; Li, Y. A Site Distance Effect Induced by Reactant Molecule Matchup in Single-Atom Catalysts for Fenton-Like Reactions. Angew. Chem. Int. Ed. 2022, 61, e202207268. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Zhang, H.; Chen, X.; Zhao, H. Enhanced Mimic Peroxidase Activity of Carbon Nanozyme by Simultaneous Phosphorus, Oxygen Dual-Heteroatom Doping and Nanosheet Structure Construction. Sep. Purif. Technol. 2024, 330, 125312. [Google Scholar] [CrossRef]
- Kumar, M.; Kaur, N.; Singh, N. Colorimetric Nanozyme Sensor Array Based on Metal Nanoparticle-Decorated CNTs for Quantification of Pesticides in Real Water and Soil Samples. ACS Sustain. Chem. Eng. 2024, 12, 728–736. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme Sensor Arrays Based on Heteroatom-Doped Graphene for Detecting Pesticides. Anal. Chem. 2020, 92, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Huang, W.; Shen, F.; Li, T.; Li, S.; Xu, W.; Lv, C.; Luo, Q.; Liu, J. Graphene Oxide-Based Colorimetric Detection of Organophosphorus Pesticides via a Multi-Enzyme Cascade Reaction. Nanoscale 2020, 12, 5829–5833. [Google Scholar] [CrossRef]
- Yi, G.; Tao, Z.; Fan, W.; Zhou, H.; Zhuang, Q.; Wang, Y. Copper Ion-Induced Self-Assembled Aerogels of Carbon Dots as Peroxidase-Mimicking Nanozymes for Colorimetric Biosensing of Organophosphorus Pesticide. ACS Sustain. Chem. Eng. 2024, 12, 1378–1387. [Google Scholar] [CrossRef]
- Yang, W.; Yang, X.; Zhu, L.; Chu, H.; Li, X.; Xu, W. Nanozymes: Activity Origin, Catalytic Mechanism, and Biological Application. Coord. Chem. Rev. 2021, 448, 214170. [Google Scholar] [CrossRef]
- Li, J.; Gao, M.; Xia, X.; Cen, Y.; Wei, F.; Yang, J.; Wang, L.; Hu, Q.; Xu, G. Spherical Hydrogel Sensor Based on PB@Fe-COF@Au Nanoparticles with Triplet Peroxidase-like Activity and Multiple Capture Sites for Effective Detection of Organophosphorus Pesticides. ACS Appl. Mater. Interfaces 2023, 15, 6473–6485. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.-J.; Yuan, M.-Y.; Shi, Y.-D.; Wang, M.-P.; Li, H.-H.; Zhang, L.; Qiu, J.-D. Construction of Covalent Organic Framework Nanozymes with Photo-Enhanced Hydrolase Activities for Colorimetric Sensing of Organophosphorus Nerve Agents. Anal. Chim. Acta 2023, 1278, 341706. [Google Scholar] [CrossRef]
- Wen, S.-H.; Zhang, H.; Yu, S.; Ma, J.; Zhu, J.-J.; Zhou, Y. Nanozyme Coating-Gated Multifunctional COF Composite Based Dual-Ratio Enhanced Dual-Mode Sensor for Highly Sensitive and Reliable Detection of Organophosphorus Pesticides in Real Samples. J. Hazard. Mater. 2024, 480, 135791. [Google Scholar] [CrossRef]
- Liang, L.; Jiang, Y.; Liu, F.; Wu, J.; Tian, L.; Zhao, S.; Ye, F. Smartphone Flashlight-Triggered Covalent Organic Framework Nanozyme Activity: A Universal Scheme for Visual Point-of-Care Testing. Sens. Actuators B Chem. 2023, 381, 133422. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, N.; Liu, S.; Wei, K.; Ma, C.; Pan, J.; Feng, S. Construction of Phosphatase-like COF-OMe@Valine-CeO2 Nanozymes for Ultrasensitive Electrochemical Detection of Organophosphorus Pesticides. Sens. Actuators B Chem. 2024, 417, 136068. [Google Scholar] [CrossRef]
- Fang, Q.; Zhuang, Z.; Gu, S.; Kaspar, R.B.; Zheng, J.; Wang, J.; Qiu, S.; Yan, Y. Designed Synthesis of Large-Pore Crystalline Polyimide Covalent Organic Frameworks. Nat. Commun. 2014, 5, 4503. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Wu, X.; Liu, Y.; Cui, Y. Multivariate Chiral Covalent Organic Frameworks with Controlled Crystallinity and Stability for Asymmetric Catalysis. J. Am. Chem. Soc. 2017, 139, 8277–8285. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tao, S.; Jiang, D. Proton Conduction in Crystalline and Porous Covalent Organic Frameworks. Nat. Mater. 2016, 15, 722–726. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The Atom, the Molecule, and the Covalent Organic Framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef]

| Compound | Molecular Structure | Organism | Route | LD50 (mg/kg) |
|---|---|---|---|---|
| Bromophos-ethyl | 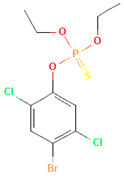 | rat | oral | 52 |
| Chlorpyrifos | 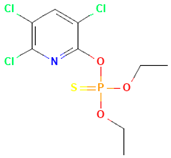 | rat | skin | 202 |
| Famphur | 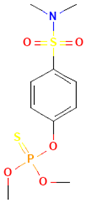 | rat | skin | 400 |
| Parathion |  | human | oral | 3 |
| Fenchlorphos |  | dog | oral | 500 |
| Sulfotep |  | dog | oral/ | 5 |
| Country | Water Body Name | Type of Organophosphorus Pesticides | Concentration (ng·L−1) | Reference | |
|---|---|---|---|---|---|
| China | North China Plain | Summer | Winter | [16] | |
| Dimethoate | ND 1 ~ 23.11 ND 1 ~ 17.91 ND 1 ~ 16.23 ND 1 ~ 15.34 | ND 1 ~ 16.23 ND 1 ~ 16.13 ND 1 ~ 15.47 ND 1 ~ 13.99 | |||
| Dichlorvos | |||||
| Methyl-parathion | |||||
| Malathion | |||||
| Yellow River | Dichlorvos | 40.7 | [17] | ||
| Dimethoate | 78.9 | ||||
| Omethoate | 90.1 | ||||
| Haihe River | Dichlorvos | 25.6 | |||
| Dimethoate | 70.8 | ||||
| Omethoate | 42.8 | ||||
| Yangtze River | Dichlorvos | 17.9 | |||
| Dimethoate | 17.5 | ||||
| Omethoate | ND 1~16.0 | ||||
| A certain river reservoir in South China | Methamidophos | 20.95~35.90 | [18] | ||
| Dichlorvos | 1.52~14.02 | ||||
| Acephate | 22.42~436.9 | ||||
| Omethoate | 10.70~55.09 | ||||
| Malathion | 14.94~33.11 | ||||
| Chlorpyrifos | 12.49~23.74 | ||||
| Quinalphos | 10.49~20.21 | ||||
| Methamidophos | 13.66~79.11 | ||||
| Triazophos | 15.47~341.9 | ||||
| Jianghan Plain | Methamidophos | 39.1 | [19] | ||
| Omethoate | 48.3 | ||||
| Dimethoate | 21.28 | ||||
| Diazinon | 47.58 | ||||
| Malaysia | Langat River | Quinalphos | 17.8 | [20] | |
| Chlorpyrifos | 20.2 | ||||
| Diazinon | 9.4 | ||||
| Chlorpyrifos | 5057 | [21] | |||
| USA | San Joaquin River | Diazinon | 100 | [22] | |
| Chlorpyrifos | 35 | ||||
| Dimethoate | 74 | ||||
| Egypt | Nile River | Chlorpyrifos | 580 | [23] | |
| Triazophos | 2600 | ||||
| Fenitrothion | 1222 | ||||
| Triazophos | 1488 | ||||
| Serial Number | Nanozyme | Target Compound | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|---|
| 1 | Cu NPs | Fingerprints were used to distinguish organophosphorus pesticides at different concentrations (1, 5, 20, 50, 100 μg mL−1). | [33] | ||
| 2 | Bi0.01Au1 | Paraoxon-ethyl | 0.8~500 ng mL−1 | 0.41 ng mL−1 | [34] |
| 3 | CoPcNS | Paraoxon | 10~2000 μg L−1 | 1.1 μg L−1 | [35] |
| 4 | Fe-PTs | Paraoxon | 1~500 ng mL−1 | 0.28 ng mL−1 | [36] |
| 5 | Fe/C/Bi2O3 | Dichlorvos | 10~100 μg L−1 | 0.6 μg L−1 | [37] |
| 6 | Fe3O4@Au-Pt | Ethephon | 0.1~500 μmol L−1 | 2.01 nmol L−1 | [38] |
| 7 | Ir NPs | Malathion | 0.1~5.0 μM | 6 nM | [39] |
| 8 | Pd@PtBi2 | Trichlorfon | 0.1~100 ng mL−1 | 0.06 ng mL−1 | [40] |
| 9 | AgNP | Chlorpyrifos | 35~210 ppm | 11.3 ppm | [41] |
| 10 | Pt-Ni NPs | Chlorpyrifos | colorimetric mode: 0.2~2.5 μg mL−1 photothermal mode: 0.005~3.0 μg mL−1 | colorimetric mode: 1.2 ng mL−1 photothermal mode: 1.66 ng mL−1 | [42] |
| 11 | PtPdNPs@g-C3N4 | Trich | colorimetric mode: 0.28~50.0 ng mL−1; fluorescence mode: 0.11~50.00 ng mL−1 | colorimetric mode: 0.083 ng mL−1; fluorescence mode: 0.033 ng mL−1 | [43] |
| Serial Number | Nanozyme | Target Compound | Linear Range | Detection Limit | Degradation Property | Reference |
|---|---|---|---|---|---|---|
| 1 | CuO NPs | Malathion | 0.1~5 mg L−1 | 0.08 mg L−1 | [52] | |
| 2 | GeO2 NPs | Paraoxon | 0.1~50 pM | 14 fM | [47] | |
| 3 | Ag2O NPs | Fenitrothion, Chlorpyrifos, Omethoate, Triazophos, Methyl parathion, Trichlorfon | Identify organophosphorus pesticides at concentrations as low as 10 ng mL−1 | [53] | ||
| 4 | γ-MnOOH NWs | Omethoate; Dichlorvos | 5~50 ng mL−1; 1~10 ng mL−1 | 0.35 ng mL−1; 0.14 ng mL−1 | [54] | |
| 5 | MO@FHO | Malathion | PEC mode: 0.0001~0.5 μmol L−1; colorimetric mode: 0.001~50 μmol L−1 | PEC mode: 0.017 ng mL−1; colorimetric mode: 0.8 nmol L−1 | [55] | |
| 6 | CeO2@NC | Paraoxon | 3.0~100.0 μM | Rapid hydrolysis was achieved at low temperature (37 °C), low dosage (0.5 mg mL−1), and short time (10 min) | [56] | |
| 7 | Au−pCeO2 | Methyl parathion | 5~200 μM | 0.5 μM | [57] | |
| 8 | CeO2@PDA@AuNCs-MIPs | Methyl parathion | 0.45~125 nM | 0.15 nM | [58] | |
| 9 | In-CeO2 | Dimethyl-p-nitrophenyl Phosphate | 75% conversion rate after 6 h | [59] | ||
| 10 | Au@MnO2-X | 0.01~50.0 ng mL−1 | 0.039 ng mL−1 | [60] |
| Serial Number | Nanozyme | Target Compound | Linear Range | Detection Limit | Degradation Property | Reference |
|---|---|---|---|---|---|---|
| 1 | Ce-MOF | p-NPP | In CHES buffer (pH 9.0), the hydrolysis rate can reach 80% after 5 min of reaction. | [61] | ||
| 2 | Cu4Co6 ZIF | Pirimiphos-methyl | 6 × 10−4~0.03 μM | 0.151 nM | [62] | |
| 3 | MIL-88B(V) | Ethion, Parathion, Dichlorvos and Paraoxon | 0.055~10 μg mL–1, 0.04~10 μg mL–1, 0.06~10 μg mL–1, and 0.08~10 μg mL–1 | 0.018, 0.01, 0.02, and 0.027 μg mL–1 | [63] | |
| 4 | Mn/Fe-MIL (53) | Methyl parathion and Chlorpyrifos | 10~120 nM; 5~50 nM | 2.8 nM; 0.95 nM | [64] | |
| 5 | Mn-ZIF-8 | Chlorpyrifos | 0.1~20 nM | 54 pM | [65] | |
| 6 | MIL-101(Fe) | Methyl parathion | 8~800 ng mL−1 | 1 ng mL−1 | [66] | |
| 7 | Pt@ZIF-8@TMS | Malathion | 0~500 ng mL−1 | 0.7 ng mL−1 | [67] | |
| 8 | MIL-888-NH2(Fe-MOF) | Dichlorvos | 0.01~10.0 ng mL−1 | 2.9 pg mL−1 | [68] | |
| 9 | Ce/Zr-MOF@FP | Dichlorvos | 0.5~500 ng mL−1 | 0.32 ng mL−1 | [69] | |
| 10 | Zr-MOF@BC | Dichlorvos | hydrolysis half-life: 1 min | [70] | ||
| 12 | ZIF-Co-Cys | Dichlorvos | fluorescence mode: 2~100 ng mL−1: photothermal mode: 10~10,000 ng mL−1 | fluorescence mode: 1.64 ng mL−1; photothermal mode: 0.084 ng mL−1 | [71] | |
| 13 | MIL-OH-D | Dichlorvos | 5~300 ng mL−1 | 2.06 ng mL−1 | [72] | |
| 14 | DDT-UiO-66-NH2@MF | Parathion | After 70 min, the hydrolysis rate reached 66.6% | [73] | ||
| 15 | VTCPP(Fe) | Chlorpyrifos | colorimetric mode: 0.61 nM; fluorescent modes: 0.13 nM | [74] | ||
| 16 | NH2-CuBDC MOF | Chlorpyrifos | colorimetric mode: 1.57 ng mL−1; fluorescent modes: 2.33 ng mL−1 | colorimetric mode: 1.57 ng mL–1; fluorescent modes: 2.33 ng mL–1 | [75] | |
| 17 | Zr-TCPE MOF | Paraoxon | colorimetric mode: 1.82~181.69 μM; fluorescence mode: 0.36~181.69 μM | colorimetric mode: 0.178 μM; fluorescence mode: 0.195 μM | [76] | |
| 18 | UsAuNPs/2D MOF | Dichlorvos | 1.7~42.4 μM | 1.7 μM | [77] |
| Serial Number | Nanozyme | Target Compound | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|---|
| 1 | Fe-N-C | Paraoxon-ethyl | 0.1~10 μg mL−1 | 0.97 ng mL−1 | [79] |
| 2 | Fe-N-C | O methoate | 1~100 nM | 0.4177 nM | [80] |
| 3 | Cu-N-C | Paraoxon-ethyl | 1~300 ng mL−1 | 0.60 ng mL−1 | [81] |
| 4 | Ce-N-C | Omethoate; Methamidophos | 100~700 μg mL−1 | 55.83 ng mL−1; 71.51 ng mL−1 | [82] |
| 5 | CeN4-SAzyme | Dichlorvos and Chlorpyrifos | 1 ng mL−1~1 μg mL−1 | 0.56 ng mL−1; 0.67 ng mL−1 | [83] |
| 6 | FeAC/FeSA-NC | 0.005~50 ng mL−1 | 1.9 pg mL−1 | [84] | |
| 7 | Fe SACs/Cu2O/Ti3C2Tx | Paraoxon-ethyl | 0.5~600 ng mL−1 | 0.08 ng/mL−1 | [85] |
| 8 | Co-N-C | 0.8 ng mL−1~500 ng mL−1 | 0.37 ng mL−1 | [86] | |
| 9 | Fe-N-C, Cu-N-C | Dichlorvos, Ethion, and Omethoate | 20 ng mL−1~100 ng mL−1 | 1.04 ng mL−1, 1.24 ng mL−1; 0.78 ng mL−1 | [87] |
| 10 | Fe-Co MNPs, Fe-N-C | Phorate, Profenofos, Isocarbophos, and Omethoate | 0.5~5000 ng mL−1, 0.5~5000 ng mL−1, 0.1~5000 ng mL−1 and 5–5000 ng mL−1 | 0.16 ng mL−1, 0.16 ng mL−1, 0.03 ng mL−1 and 1.6 ng mL | [88] |
| 11 | Fe-N-C | Ethyl parathion, Dichlorvos, and O methoate | 10−12~10−2 M | 60.97 fM, 13.62 fM and 7.54 fM | [89] |
| 12 | Fe-N-C | Chlorpyrifos | 0.05~10.0 μg mL−1 | 2.11 ng mL−1 | [90] |
| 13 | Fe SAs/N5-pC-4 | 0.001~20 μg mL−1 | 0.0006 μg mL−1 | [91] |
| Serial Number | Nanozyme | Target Compound | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|---|
| 1 | POCNS | Chlorpyrifos | 1~200 μg L−1 | 0.31 μg L−1 | [96] |
| 2 | Cu/Ni/Co@CNTs | CBZ, DTM, ISP | 1~8 μM | 10.8 nM, 28.8 nM, 16.8 nM | [97] |
| 3 | NG, NSG, GO | Lactofen, Fluoroxypyr-meptyl, Bensulfuron-methyl, Fomesafen, and Diafenthiuron | 5~500 μM | [98] | |
| 4 | GO | Omethoate, Parathion methyl, and Chlorpyrifos | 2~200, 1~50, 2~100 ng mL−1 | 2, 1, 2 ng mL−1 | [99] |
| 5 | Cu-CDs | Dichlorvos | 0.02~0.3 μM | 7.6 nM | [100] |
| Serial Number | Nanozyme | Target Compound | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|---|
| 1 | PB@Fe-COF@Au | Dichlorvos | 10~800 ng mL–1 | 0.17 mU mL–1 | [102] |
| 2 | DAFB-DCTP COF | DCNP | 0~1.308 mM | 16.8 μM | [103] |
| 3 | MB/COF@MnO2 | Dichlorvos | FL mode: 1~200 ng mL–1; EC mode: 0.25~80 ng mL–1 | FL mode: 0.083 ng mL–1; EC mode: 0.026 ng mL–1 | [104] |
| 4 | TpBTD COF | Trichlorfon | 8~2000 ng mL–1 | 1.29 ng mL–1 | [105] |
| 5 | COF-OMe@Valine-CeO2 | Methyl paraoxon | 0.034~76 μmol L–1 | 0.011 μmol L–1 | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Dong, Z.; Xu, N.; Chen, T.; Liang, J.; Xia, M.; Wang, F. A Comprehensive Review of Multifunctional Nanozymes for Degradation and Detection of Organophosphorus Pesticides in the Environment. Toxics 2024, 12, 926. https://doi.org/10.3390/toxics12120926
Liang J, Dong Z, Xu N, Chen T, Liang J, Xia M, Wang F. A Comprehensive Review of Multifunctional Nanozymes for Degradation and Detection of Organophosphorus Pesticides in the Environment. Toxics. 2024; 12(12):926. https://doi.org/10.3390/toxics12120926
Chicago/Turabian StyleLiang, Jijia, Zhongtian Dong, Ning Xu, Tao Chen, Jie Liang, Mingzhu Xia, and Fenghe Wang. 2024. "A Comprehensive Review of Multifunctional Nanozymes for Degradation and Detection of Organophosphorus Pesticides in the Environment" Toxics 12, no. 12: 926. https://doi.org/10.3390/toxics12120926
APA StyleLiang, J., Dong, Z., Xu, N., Chen, T., Liang, J., Xia, M., & Wang, F. (2024). A Comprehensive Review of Multifunctional Nanozymes for Degradation and Detection of Organophosphorus Pesticides in the Environment. Toxics, 12(12), 926. https://doi.org/10.3390/toxics12120926






