Occurrence, Bioaccumulation, and Human Exposure Risk of the Antiandrogenic Fluorescent Dye 7-(Dimethylamino)-4-methylcoumarin and 7-(Diethylamino)-4-methylcoumarin in the Dongjiang River Basin, South China
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Yeast Two-Hybrid Assay
2.3. Sample Collection
2.4. Sample Preparation
2.4.1. Surface Water
2.4.2. Sediment
2.4.3. Aquatic Biota
2.5. Instrumental Analysis
2.6. Quality Control and Quality Assurance
2.7. Bioaccumulation Capacity Estimation
2.8. Human Exposure Risk Estimation
2.9. Statistical Analysis
3. Results and Discussions
3.1. AR Antagonistic Activities of Coumarins
3.2. Coumarins in Abiotic Samples
3.2.1. Surface Water
3.2.2. Sediment
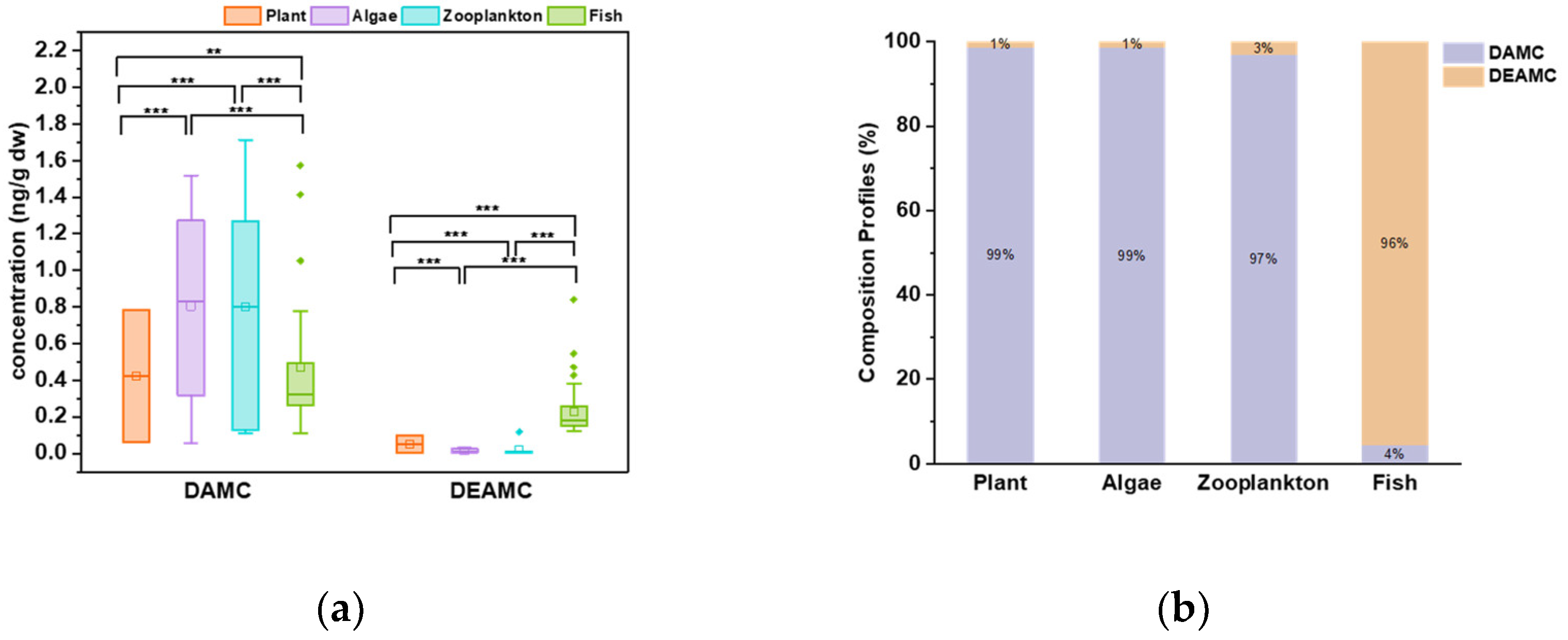
3.3. Coumarins in Aquatic Biota
3.3.1. Plants
3.3.2. Algae
3.3.3. Zooplankton
3.3.4. Fish
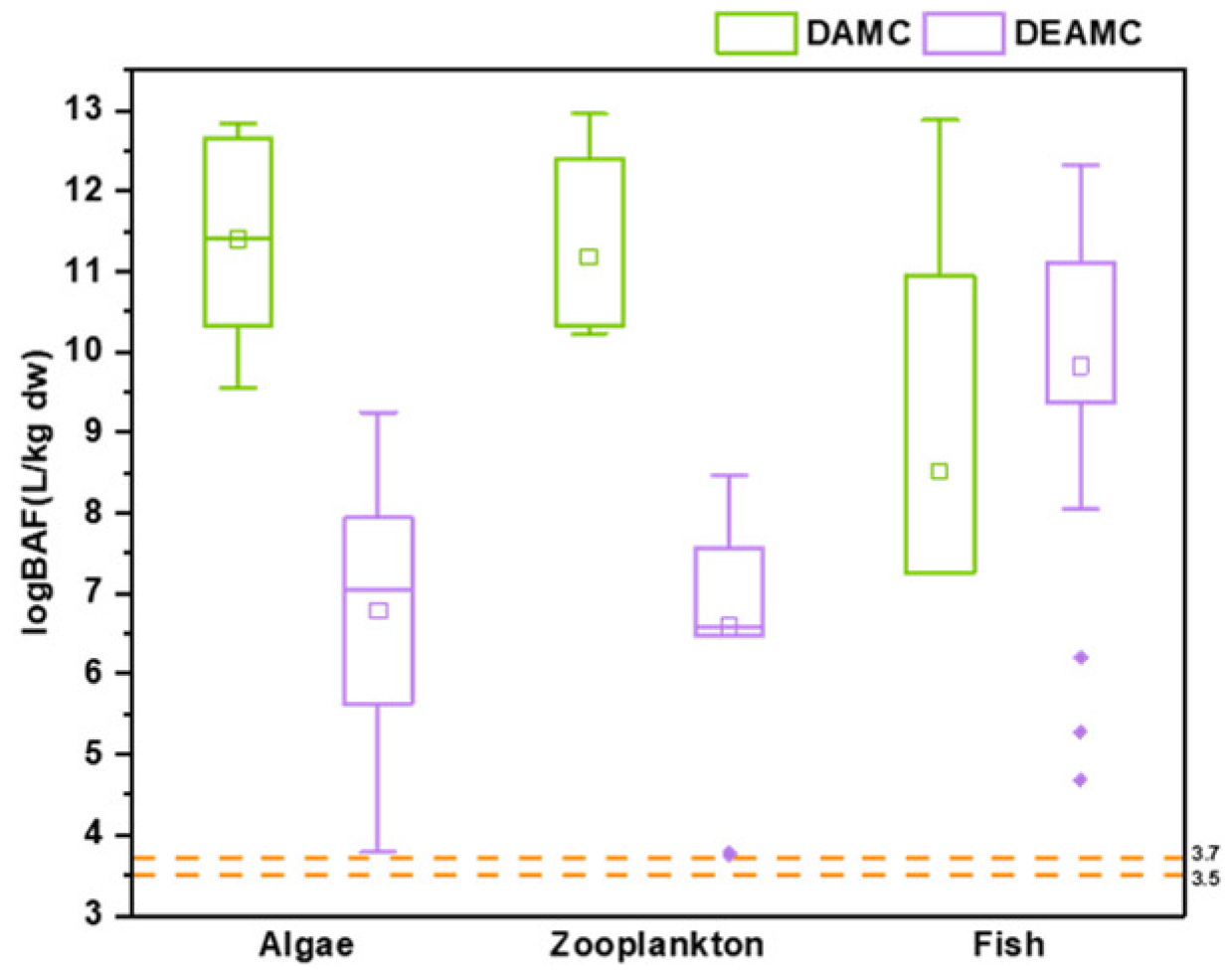
3.4. Bioaccumulation of Coumarins in Aquatic Biota
3.5. Estimated Daily Intake of Coumarins Through Fish Ingestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Muschket, M.; Brack, W.; Inostroza, P.A.; Beckers, L.; Schulze, T.; Krauss, M. Sources and Fate of the Antiandrogenic Fluorescent Dye 4-Methyl-7-Diethylaminocoumarin in Small River Systems. Environ. Toxicol. Chem. 2021, 40, 3078–3091. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Fukagawa, T.; Kohtani, S.; Kitoh, S.; Kunimoto, K.-K.; Nakagaki, R. Synthesis, Absorption, and Fluorescence Properties and Crystal Structures of 7-Aminocoumarin Derivatives. J. Photochem. Photobiol. Chem. 2007, 188, 378–386. [Google Scholar] [CrossRef]
- Di Paolo, C.; Kirchner, K.; Balk, F.G.P.; Muschket, M.; Brack, W.; Hollert, H.; Seiler, T.-B. Downscaling Procedures Reduce Chemical Use in Androgen Receptor Reporter Gene Assay. Sci. Total Environ. 2016, 571, 826–833. [Google Scholar] [CrossRef]
- Sébillot, A.; Damdimopoulou, P.; Ogino, Y.; Spirhanzlova, P.; Miyagawa, S.; Du Pasquier, D.; Mouatassim, N.; Iguchi, T.; Lemkine, G.F.; Demeneix, B.A.; et al. Rapid Fluorescent Detection of (Anti)Androgens with Spiggin-Gfp Medaka. Environ. Sci. Technol. 2014, 48, 10919–10928. [Google Scholar] [CrossRef]
- Borg, B. Androgens in Teleost Fishes. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1994, 109, 219–245. [Google Scholar] [CrossRef]
- Shi, H.; Gao, T.; Liu, Z.; Sun, L.; Jiang, X.; Chen, L.; Wang, D. Blockage of Androgen and Administration of Estrogen Induce Transdifferentiation of Testis into Ovary. J. Endocrinol. 2017, 233, 65–80. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Zheng, G.; Zhang, S.; Wan, Y.; Hu, J. Adverse Effects of Triclosan and Binary Mixtures with 17β-Estradiol on Testicular Development and Reproduction in Japanese Medaka (Oryzias latipes) at Environmentally Relevant Concentrations. Environ. Sci. Technol. Lett. 2018, 5, 136–141. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Zhao, F.; Zhang, S.; Chen, R.; Hu, J. Environmentally Relevant Concentrations of the Organophosphorus Flame Retardant Triphenyl Phosphate Impaired Testicular Development and Reproductive Behaviors in Japanese Medaka (Oryzias latipes). Environ. Sci. Technol. Lett. 2018, 5, 649–654. [Google Scholar] [CrossRef]
- Cortes, D.; Thorup, J.; Visfeldt, J. Multinucleated Spermatogonia in Cryptorchid Boys: A Possible Association with an Increased Risk of Testicular Malignancy Later in Life? APMIS 2003, 111, 25–31. [Google Scholar] [CrossRef]
- Knez, J. Endocrine-Disrupting Chemicals and Male Reproductive Health. Reprod. Biomed. Online 2013, 26, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.M. Disruption of Reproductive Development in Male Rat Offspring Following in Utero Exposure to Phthalate Esters. Int. J. Androl. 2006, 29, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.S.; Macpherson, S.; Marchetti, N.; Sharpe, R.M. Human “Testicular Dysgenesis Syndrome”: A Possible Model Using in-Utero Exposure of the Rat to Dibutyl Phthalate. Hum. Reprod. 2003, 18, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Marques-Pinto, A.; Carvalho, D. Human Infertility: Are Endocrine Disruptors to Blame? Endocr. Connect. 2013, 2, R15–R29. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.; Goh, H.; Bielanowicz, A.; Rippon, P.; Loveland, K.L.; Itman, C. Prepubertal Mouse Testis Growth and Maturation and Androgen Production Are Acutely Sensitive to Di-n-Butyl Phthalate. Endocrinology 2013, 154, 3460–3475. [Google Scholar] [CrossRef]
- Lambrot, R.; Muczynski, V.; Lecureuil, C.; Angenard, G.; Coffigny, H.; Pairault, C.; Moison, D.; Frydman, R.; Habert, R.; Rouiller-Fabre, V. Phthalates Impair Germ Cell Development in the Human Fetal Testis in Vitro without Change in Testosterone Production. Environ. Health Perspect. 2009, 117, 32–37. [Google Scholar] [CrossRef]
- Muschket, M.; Di Paolo, C.; Tindall, A.J.; Touak, G.; Phan, A.; Krauss, M.; Kirchner, K.; Seiler, T.-B.; Hollert, H.; Brack, W. Identification of Unknown Antiandrogenic Compounds in Surface Waters by Effect-Directed Analysis (EDA) Using a Parallel Fractionation Approach. Environ. Sci. Technol. 2018, 52, 288–297. [Google Scholar] [CrossRef]
- Hu, J.Y.; Aizawa, T.; Ookubo, S. Products of Aqueous Chlorination of Bisphenol a and Their Estrogenic Activity. Environ. Sci. Technol. 2002, 36, 1980–1987. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, D.; Chen, H.; Cai, Y.; Liu, Y.; Guo, F.; Li, F.; Zhang, Y.; Xu, Z.; Xue, J.; et al. Distribution, Bioaccumulation and Human Exposure Risk of Bisphenol Analogues, Bisphenol A Diglycidyl Ether and Its Derivatives in the Dongjiang River Basin, South China. Sci. Total Environ. 2024, 952, 175969. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Liu, Y.; Lan, Y.; Zhu, J.; Cai, Y.; Guo, F.; Li, F.; Zhang, Y.; Zhang, T.; et al. Evidence of Strobilurin Fungicides and Their Metabolites in Dongjiang River Ecosystem, Southern China: Bioaccumulation and Ecological Risks. Sci. Total Environ. 2024, 908, 168427. [Google Scholar] [CrossRef]
- Smalling, K.L.; Orlando, J.L.; Calhoun, D.; Battaglin, W.A.; Kuivila, K. Occurrence of Pesticides in Water and Sediment Collected from Amphibian Habitats Located throughout the United States, 2009–2010; U.S. Geological Survey: Reston, VA, USA, 2012.
- Montagner, C.C.; Vidal, C.; Acayaba, R.D.; Jardim, W.F.; Jardim, I.C.S.F.; Umbuzeiro, G.A. Trace Analysis of Pesticides and an Assessment of Their Occurrence in Surface and Drinking Waters from the State of São Paulo (Brazil). Anal. Methods 2014, 6, 6668–6677. [Google Scholar] [CrossRef]
- Liu, J.; Xia, W.; Wan, Y.; Xu, S. Azole and Strobilurin Fungicides in Source, Treated, and Tap Water from Wuhan, Central China: Assessment of Human Exposure Potential. Sci. Total Environ. 2021, 801, 149733. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Lee, S.; Moon, H.-B.; Yamashita, N.; Kannan, K. Parabens in Sediment and Sewage Sludge from the United States, Japan, and Korea: Spatial Distribution and Temporal Trends. Environ. Sci. Technol. 2013, 47, 10895–10902. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wan, Y.; Jiang, Y.; Xia, W.; He, Z.; Xu, S. Occurrence of Azole and Strobilurin Fungicides in Indoor Dust from Three Cities of China. Environ. Pollut. 2022, 304, 119168. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Xue, J.; Liu, W.; Adams, D.H.; Kannan, K. Trophic Magnification of Parabens and Their Metabolites in a Subtropical Marine Food Web. Environ. Sci. Technol. 2017, 51, 780–789. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Li, T.; Li, X.; Huang, X.; Gao, Y.; Li, B.; Lin, J.; Mu, W. Determination of Pyraclostrobin Dynamic Residual Distribution in Tilapia Tissues by UPLC-MS/MS under Acute Toxicity Conditions. Ecotoxicol. Environ. Saf. 2020, 206, 111182. [Google Scholar] [CrossRef]
- Liu, D.; Wu, S.; Xu, H.; Zhang, Q.; Zhang, S.; Shi, L.; Yao, C.; Liu, Y.; Cheng, J. Distribution and Bioaccumulation of Endocrine Disrupting Chemicals in Water, Sediment and Fishes in a Shallow Chinese Freshwater Lake: Implications for Ecological and Human Health Risks. Ecotoxicol. Environ. Saf. 2017, 140, 222–229. [Google Scholar] [CrossRef]
- Miller, M.E.; Motti, C.A.; Hamann, M.; Kroon, F.J. Assessment of Microplastic Bioconcentration, Bioaccumulation and Biomagnification in a Simple Coral Reef Food Web. Sci. Total Environ. 2023, 858, 159615. [Google Scholar] [CrossRef]
- CDC Nutrition Data Yearbook. Available online: https://www.chinanutri.cn/sjnj/ (accessed on 8 October 2024).
- Zhao, X.; Duan, X.; Wang, B.; Cao, S. Environmental Exposure Related Activity Patterns Survey of Chinese Population (Children); China Environmental Science Press: Beijing, China, 2016; Available online: https://scholar.google.com/scholar_lookup?title=Environmental%20Exposure%20Related%20Activity%20Patterns%20Survey%20of%20Chinese%20Population%20(Children)&author=X.%20Zhao&publication_year=2016 (accessed on 8 October 2024).
- Duan, X. Highlights of the Chinese Exposure Factors Handbook; Academic Press: Cambridge, MA, USA, 2015; ISBN 978-0-12-803126-1. [Google Scholar]
- Bayen, S.; Gong, Y.; Chin, H.S.; Lee, H.K.; Leong, Y.E.; Obbard, J.P. Androgenic and Estrogenic Response of Green Mussel Extracts from Singapore’s Coastal Environment Using a Human Cell-Based Bioassay. Environ. Health Perspect. 2004, 112, 1467–1471. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Temporal Trends of Parabens and Their Metabolites in Mollusks from the Chinese Bohai Sea during 2006–2015: Species-Specific Accumulation and Implications for Human Exposure. Environ. Sci. Technol. 2018, 52, 9045–9055. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) Related to Coumarin. EFSA J. 2004, 2, 104. [Google Scholar] [CrossRef]
- EFSA. Coumarin in Flavourings and Other Food Ingredients with Flavouring Properties—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). EFSA J. 2008, 6, 793. [Google Scholar] [CrossRef]
- Loos, R.; Hanke, G.; Eisenreich, S.J. Multi-Component Analysis of Polar Water Pollutants Using Sequential Solid-Phase Extraction Followed by LC-ESI-MS. J. Environ. Monit. 2003, 5, 384. [Google Scholar] [CrossRef] [PubMed]

| DR a | Median | Mean | STD b | Max | |
|---|---|---|---|---|---|
| surface water | |||||
| DAMC | -nd c | -nd | -nd | -nd | -nd |
| DEAMC | 31.40% | 0.105 | 0.12 | 0.097 | 0.309 |
| sediment | |||||
| DAMC | 37.5% | 0.189 | 0.247 | 0.202 | 0.668 |
| DEAMC | 59.4% | 0.012 | 0.038 | 0.053 | 0.173 |
| plants | |||||
| DAMC | 14.29% | 0.421 | 0.421 | 0.509 | 0.781 |
| DEAMC | 14.29% | 0.051 | 0.051 | 0.064 | 0.096 |
| algae | |||||
| DAMC | 75.00% | 0.832 | 0.804 | 0.609 | 1.517 |
| DEAMC | 62.50% | 0.009 | 0.015 | 0.012 | 0.031 |
| zooplankton | |||||
| DAMC | 58.33% | 0.798 | 0.802 | 0.620 | 1.712 |
| DEAMC | 58.33% | 0.008 | 0.023 | 0.042 | 0.118 |
| fish muscle | |||||
| DAMC | 30.30% | 0.335 | 0.467 | 0.369 | 1.574 |
| DEAMC | 50.60% | 0.181 | 0.230 | 0.135 | 0.842 |
| DAMC | DEAMC | |
|---|---|---|
| Mean Toddlers (2–5 years) | ||
| 0.10 | 0.09 | |
| Children (6–12 years) | 0.07 | 0.06 |
| Teenagers (13–17 years) | 0.05 | 0.04 |
| Adults (≥18 years) | 0.05 | 0.05 |
| 95th percentile | ||
| Toddlers (2–5 years) | 0.54 | 0.28 |
| Children (6–12 years) | 0.41 | 0.19 |
| Teenagers (13–17 years) | 0.26 | 0.14 |
| Adults (≥18 years) | 0.28 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.; Huang, Y.; Yang, D.; Xue, J.; Chen, R.; Peng, R.; Zhang, S.; Li, Y.; Yang, G.; Liu, Y. Occurrence, Bioaccumulation, and Human Exposure Risk of the Antiandrogenic Fluorescent Dye 7-(Dimethylamino)-4-methylcoumarin and 7-(Diethylamino)-4-methylcoumarin in the Dongjiang River Basin, South China. Toxics 2024, 12, 925. https://doi.org/10.3390/toxics12120925
Lai Y, Huang Y, Yang D, Xue J, Chen R, Peng R, Zhang S, Li Y, Yang G, Liu Y. Occurrence, Bioaccumulation, and Human Exposure Risk of the Antiandrogenic Fluorescent Dye 7-(Dimethylamino)-4-methylcoumarin and 7-(Diethylamino)-4-methylcoumarin in the Dongjiang River Basin, South China. Toxics. 2024; 12(12):925. https://doi.org/10.3390/toxics12120925
Chicago/Turabian StyleLai, Yufeng, Yin Huang, Danlin Yang, Jingchuan Xue, Runlin Chen, Rundong Peng, Siying Zhang, Yufei Li, Guochun Yang, and Yuxian Liu. 2024. "Occurrence, Bioaccumulation, and Human Exposure Risk of the Antiandrogenic Fluorescent Dye 7-(Dimethylamino)-4-methylcoumarin and 7-(Diethylamino)-4-methylcoumarin in the Dongjiang River Basin, South China" Toxics 12, no. 12: 925. https://doi.org/10.3390/toxics12120925
APA StyleLai, Y., Huang, Y., Yang, D., Xue, J., Chen, R., Peng, R., Zhang, S., Li, Y., Yang, G., & Liu, Y. (2024). Occurrence, Bioaccumulation, and Human Exposure Risk of the Antiandrogenic Fluorescent Dye 7-(Dimethylamino)-4-methylcoumarin and 7-(Diethylamino)-4-methylcoumarin in the Dongjiang River Basin, South China. Toxics, 12(12), 925. https://doi.org/10.3390/toxics12120925










