Toxic Effects of Lead Exposure on Freshwater Climbing Perch, Anabas testudineus, and Bioremediation Using Ocimum sanctum Leaf Powder
Abstract
1. Introduction
2. Material and Methods
2.1. Test Organisms
2.2. Test Chemical
2.3. Acute Toxicity Test
2.3.1. Experimental Set Up
2.3.2. Respiratory Rate Test
2.3.3. Test for Changes in Behaviour
2.3.4. Feeding Test
2.4. Chronic Toxicity Test
2.4.1. Experimental Set Up
- (i)
- Group 1. Fish without toxicant;
- (ii)
- Group 2. Fish with toxicant at 10% of 96 h LC50 value;
- (iii)
- Group 3. Fish with toxicant at 20% of 96 h LC50 value;
- (iv)
- Group 4. Fish with toxicant at 20% of 96 h LC50 value and 1.2 mg/L leaf powder of Ocimum sanctum/day.
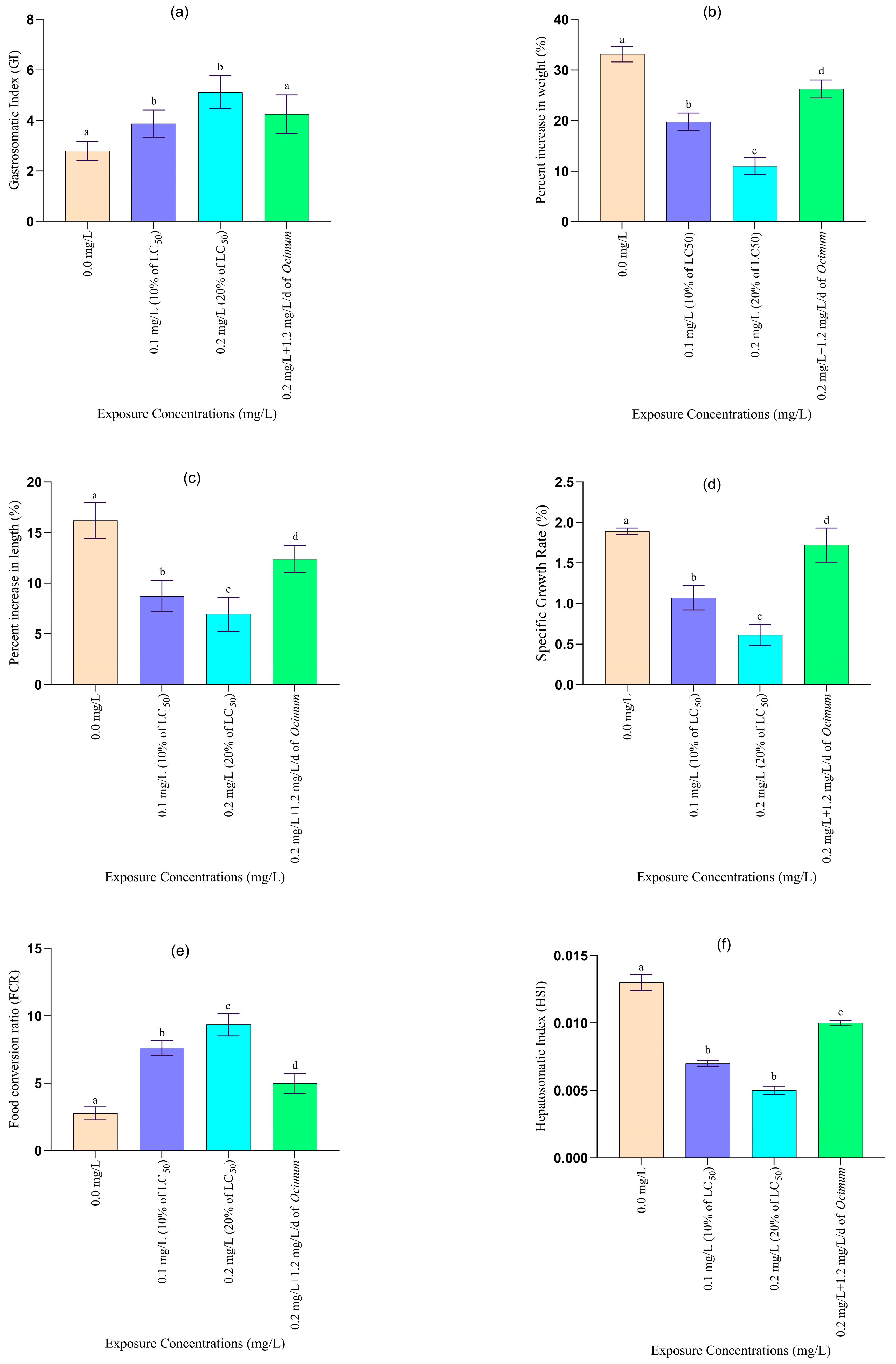
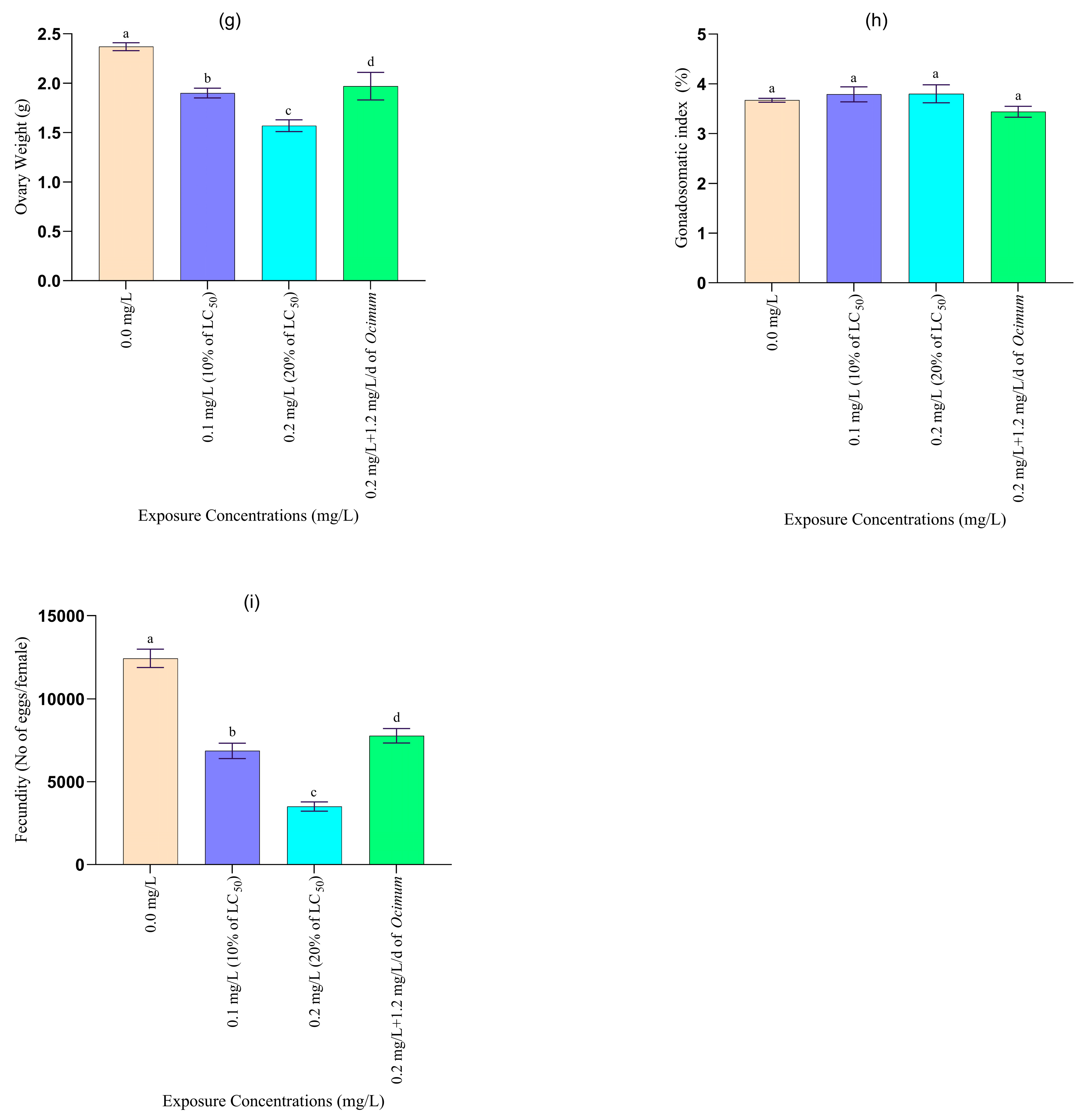
2.4.2. Growth and Organo-Somatic Indices
- (i)
- Gastrosomatic Index = (V/W) × 100,where V is the visceral weight (g) of the fish and W is the observed body weight (g) of fish;
- (ii)
- Hepatosomatic index (HSI) = [{wet weight (g) of liver without gall bladder}/wet body weight (g)] × 100;
- (iii)
- Percentage increase in weight = (W2 − W1)/W1 × 100,where W1 is the initial weight (g) of the fish and W2 is the final weight (g) of the fish;
- (iv)
- Percentage increase in length = [(L2 − L1)/L1] × 100where, L1 = initial length of fish; L2 = final length of fish;
- (v)
- Specific growth rate [34] (%/day) = {(loge W2 − loge W1)/T} × 100,where logeW1 = natural logarithm of initial body weight of fish (g), logeW2 = natural logarithm of final body weight (g) of fish and T = time interval;
- (vi)
- Food conversion ratio (FCR) = food given/weight gain,where weight gain = final weight of fish (g) − initial weight of fish (g);
- (vii)
- Gonadosomatic index of female fish = (G/W) × 100;
- (viii)
- Fecundity = total number of ripening eggs/females.
2.4.3. Haematological Biomarkers
- -
- HCT (%) = (length of packed erythrocytes ÷ total length of blood column) × 100;
- -
- TWBCC (103 mm3) = [total number of white blood cells counted in 4 squares of haemocytometer (Nwbc) × dilution factor (Df of 50)] ÷ [4 × volume factor (Vf of 0.1)];
- -
- TRBCC (106 mm3) = [total number of RBCs counted in 5 squares of haemocytometer (Nrbc) × dilution factor (Df of 200)] ÷ [5 × volume factor (Vf of 0.1)].
2.4.4. Biochemical Parameters of Blood Serum
2.5. Scanning Electron Microscopic (SEM) Study
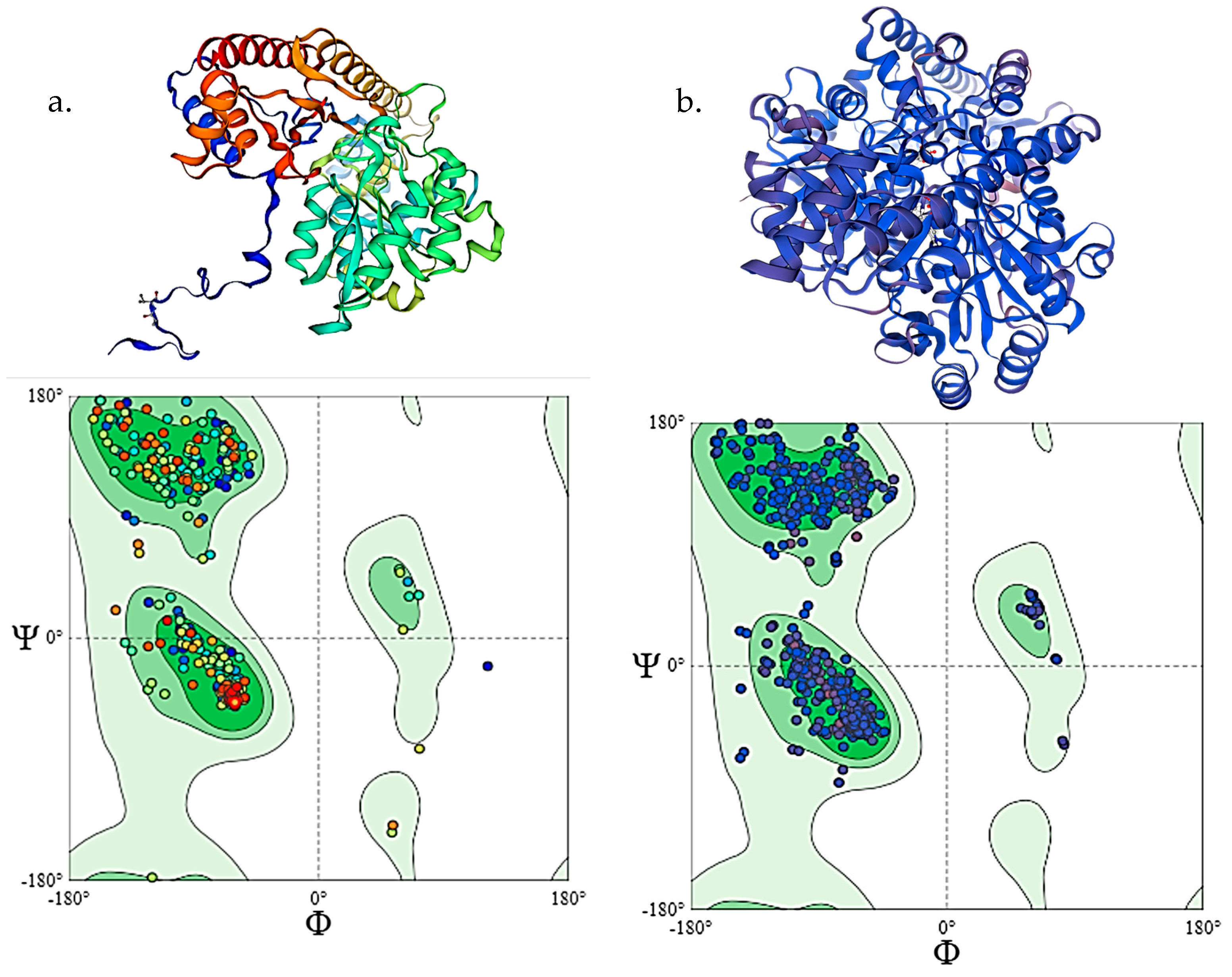
2.6. Homology Modelling and Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
3.1. Acute Toxicity Assessment
3.1.1. Effects on Mortality Rate
3.1.2. Behavioural Changes
3.1.3. Changes in Respiratory Rate
3.1.4. Changes in Feeding Rate
3.2. Chronic Toxicity Tests of Lead to Fish Anabas testudineus
3.2.1. Changes in Growth and Organo-Somatic Indices
3.2.2. Haematological Changes
3.2.3. Biochemical Changes of Blood Serum
3.2.4. Change in Blood Serum Enzymes
3.2.5. Scanning Electron Microscopic (SEM) Study on the Change in RBC of Fish
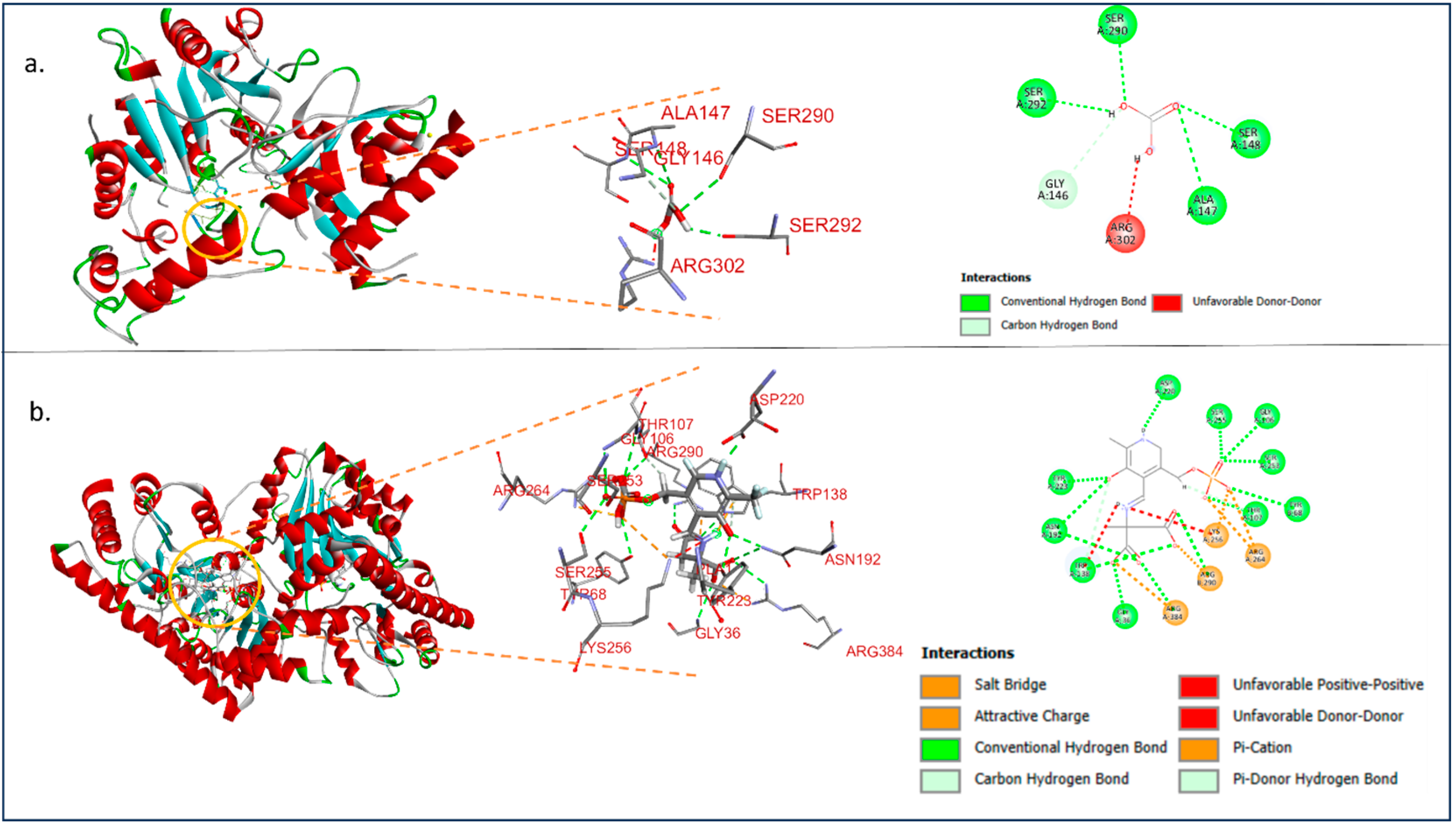
3.3. Homology Modelling and Docking Analysis of ALT and AST
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rand, G.M.; Petrocelli, S.R. Fundamentals of Aquatic Toxicology; HEMISPHERE: London, UK, 1985. [Google Scholar]
- Javed, A.; Baig, Z.; Farooqi, A.; van Geen, A. Spatial variation of arsenic in irrigation well water from three flood plains (Ravi, Chenab and Jhelum) of Punjab, Pakistan. In Environmental Arsenic in a Changing World; CRC Press: Boca Raton, FL, USA, 2019; pp. 235–236. [Google Scholar]
- Joshi, U.; Balasubramanian, R. Characteristics and environmental mobility of trace elements in urban runoff. Chemosphere 2010, 80, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sadhu, A.; Mandal, A.H.; Biswas, J.K.; Sarkar, D.; Saha, S. Copper Oxide Nanoparticles as an Emergent Threat to Aquatic Invertebrates and Photosynthetic Organisms: A Synthesis of the Known and Exploration of the Unknown. Curr. Pollut. Rep. 2024, 11, 6. [Google Scholar] [CrossRef]
- Alrabie, N.A.; Mohamat-Yusuff, F.; Rohasliney, H.; Zulkeflee, Z.; Amal, M.N.A.; Arshad, A.; Zulkifli, S.Z.; Wijaya, A.R.; Masood, N.; Sani, M.S.A. Preliminary evaluation of heavy metal contamination and source identification in Kuala Lumpur SMART Stormwater Pond Sediments using Pb isotopic signature. Sustainability 2021, 13, 9020. [Google Scholar] [CrossRef]
- Azadikhah, D.; Yalsuyi, A.M.; Saha, S.; Saha, N.C.; Faggio, C. Biochemical and Pathophysiological Responses in Capoeta capoeta under Lethal and Sub-Lethal Exposures of Silver Nanoparticles. Water 2023, 15, 585. [Google Scholar] [CrossRef]
- Dhara, K.; Chukwuka, A.V.; Saha, S.; Saha, N.C.; Faggio, C. Effects of short-term selenium exposure on respiratory activity and proximate body composition of early-life stages of Catla catla, Labeo rohita and Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2021, 90, 103805. [Google Scholar] [CrossRef]
- Dhara, K.; Saha, S.; Chukwuka, A.V.; Pal, P.; Saha, N.C.; Faggio, C. Fluoride sensitivity in freshwater snail, Bellamya bengalensis (Lamarck, 1882): An integrative biomarker response assessment of behavioral indices, oxygen consumption, haemocyte and tissue protein levels under environmentally relevant exposure concentrations. Environ. Toxicol. Pharmacol. 2021, 89, 103789. [Google Scholar] [CrossRef]
- Dhara, K.; Saha, S.; Saha, N.C. Toxicity of selenium on the freshwater tropical worm, Branchiura sowerbyi beddard, 1892. BIOINFOLET Q. J. Life Sci. 2020, 17, 346–354. [Google Scholar]
- Mandal, A.H.; Ghosh, S.; Adhurjya, D.; Chatterjee, P.; Samajdar, I.; Mukherjee, D.; Dhara, K.; Saha, N.C.; Piccione, G.; Multisanti, C.R.; et al. Exploring the impact of zinc oxide nanoparticles on fish and fish-food organisms: A review. Aquac. Rep. 2024, 36, 102038. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Marshall, C.P.; Fairbridge, R.W. Encyclopedia of Geochemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Landmeyer, J.; Bradley, P.; Bullen, T. Stable lead isotopes reveal a natural source of high lead concentrations to gasoline-contaminated groundwater. Environ. Geol. 2003, 45, 12–22. [Google Scholar] [CrossRef]
- Yurekli, Y. Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes. J. Hazard. Mater. 2016, 309, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Choi, H.; Hwang, U.-K.; Kang, J.-C.; Kang, Y.J.; Kim, K.I.; Kim, J.-H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Siddique, T.; Okeke, B.C.; Arshad, M.; Frankenberger, W.T. Biodegradation kinetics of endosulfan by Fusarium ventricosum and a Pandoraea species. J. Agric. Food Chem. 2003, 51, 8015–8019. [Google Scholar] [CrossRef]
- Ramírez-Sandoval, M.; Melchor-Partida, G.; Muñiz-Hernández, S.; Girón-Pérez, M.; Rojas-García, A.; Medina-Díaz, I.; Robledo-Marenco, M.; Velázquez-Fernández, J. Phytoremediatory effect and growth of two species of Ocimum in endosulfan polluted soil. J. Hazard. Mater. 2011, 192, 388–392. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 2. [Google Scholar]
- Kaviraj, A.; Bhunia, F.; Saha, N. Toxicity of methanol to fish, crustacean, oligochaete worm, and aquatic ecosystem. Int. J. Toxicol. 2004, 23, 55–63. [Google Scholar] [CrossRef]
- Saha, S.; Saha, N.C.; Mukherjee, D. Acute Toxicity and Behavioral Alterations of Oligochaete Worm, Branchiura sowerbyi Exposed to Diazinon. Res. Rev. J. Life Sci. 2018, 8, 1–5. [Google Scholar]
- Saha, S.; Mukherjee, D.; Saha, N.C. Evaluation of acute toxicity and behavioral responses of Heteropneustes fossilis (Linn.) exposed to Captan. Int. J. Life Sci. 2018, 6, 205–208. [Google Scholar]
- Saha, S.; Mukherjee, D.; Dhara, K.; Saha, N.C. Acute Toxicity Bioassay of a Pyrethroid Pesticide Bifenthrin to the Asian Stinging Catfish, Heteropneustes Fossilis (Bloch). Curr. World Environ. 2021, 16, 250–258. [Google Scholar] [CrossRef]
- Saha, S.; Chukwuka, A.V.; Mukherjee, D.; Dhara, K.; Adeogun, A.O.; Saha, N.C. Effects of short-term sub-lethal diazinon® exposure on behavioural patterns and respiratory function in Clarias batrachus: Inferences for adaptive capacity in the wild. Chem. Ecol. 2022, 38, 180–194. [Google Scholar] [CrossRef]
- Saha, S.; Banerjee, P.; Saha, N.C.; Chukwuka, A.V. Triazophos-induced Respiratory and Behavioral Effects and Development of Adverse Outcome Pathway (AOP) for short-term Exposed Freshwater Snail, Bellamya Bengalensis. Bull. Environ. Contam. Toxicol. 2023, 110, 94. [Google Scholar] [CrossRef] [PubMed]
- Chukwuka, A.V.; Saha, S.; Mukherjee, D.; Banerjee, P.; Dhara, K.; Saha, N.C. Deltamethrin-Induced Respiratory and Behavioral Effects and Adverse Outcome Pathways (AOP) in Short-Term Exposed Mozambique Tilapia, Oreochromis mossambicus. Toxics 2022, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Dhara, K.; Saha, S.; Saha, N.C. Sensitivity of Common Carp, Cyprinus carpio (Linnaeus, 1758) to the Grey List Metal, Zinc under Laboratory Condition. Asian J. Biol. Life Sci. 2021, 10, 133. [Google Scholar] [CrossRef]
- Dhara, K.; Shubhajit, S.; Chukwuka, A.V.; Saha, N.C. Behavioural toxicity and respiratory distress in early life and adult stage of walking catfish Clarias batrachus (Linnaeus) under acute fluoride exposures. Toxicol. Environ. Health Sci. 2021, 14, 33–46. [Google Scholar] [CrossRef]
- Dhara, K.; Saha, S.; Mukherjee, D.; Saha, N.C. Comparative acute toxicity of mercury to air breathing fish, Channa gachua (Ham.) and non-air breathing fish Cyprinus carpio (Linn.): Ethological and Haematological Consideration. Indian J. Ecol. 2021, 48, 1243–1253. [Google Scholar]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Saha, S.; Chukwuka, A.V.; Mukherjee, D.; Dhara, K.; Pal, P.; Saha, N.C. Physiological (haematological, growth and endocrine) and biochemical biomarker responses in air-breathing catfish, Clarias batrachus under long-term Captan® pesticide exposures. Environ. Toxicol. Pharmacol. 2022, 90, 103815. [Google Scholar] [CrossRef]
- Bourlière, F.; Brown, M.E. (Eds.) The Physiology of Fishes; Academic Press: New York, NY, USA, 1957; Volume 2. [Google Scholar]
- Magnhagen, C.; Braithwaite, V.; Forsgren, E.; Kapoor, B. Fish Behaviour; Science Publishers: Enfield, NH, USA, 2008. [Google Scholar]
- Dacie, J.V.; Lewis, S.M. Practical haematology. In Practical Hæmatology; Elsevier: Amsterdam, The Netherlands, 1995; p. 609. [Google Scholar]
- Sahli, T. Text Book of Clinical Pathology; Williams and Willams, Co.: Baltimore, MD, USA, 1962; 35p. [Google Scholar]
- Poet, T.S.; Kousba, A.A.; Dennison, S.L.; Timchalk, C. Physiologically based pharmacokinetic/pharmacodynamic model for the organophosphorus pesticide diazinon. Neurotoxicology 2004, 25, 1013–1030. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Kattermann, R.; Jaworek, D.; Möller, G. Multicentre study of a new enzymatic method of cholesterol determination. Clin. Chem. Lab. Med. 1984, 22, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Bowers, L.D. Kinetic serum creatinine assays I. The role of various factors in determining specificity. Clin. Chem. 1980, 26, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Studer, G.; Tauriello, G.; Bienert, S.; Biasini, M.; Johner, N.; Schwede, T. ProMod3—A versatile homology modelling toolbox. PLoS Comput. Biol. 2021, 17, e1008667. [Google Scholar] [CrossRef]
- Muddagoni, N.; Bathula, R.; Dasari, M.; Potlapally, S.R. Homology modeling, virtual screening, prime-MMGBSA, AutoDock-identification of inhibitors of FGR protein. Biointerface Res. Appl. Chem. 2021, 11, 11088–11103. [Google Scholar]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Chem. Biol. Methods Protoc. 2015, 1263, 243–250. [Google Scholar]
- Studio, D.J.A. Discovery Studio; Accelrys: San Diego, CA, USA, 2008; p. 420. [Google Scholar]
- Finney, D. Statistical logic in the monitoring of reactions to therapeutic drugs. Methods Inf. Med. 1971, 10, 237–245. [Google Scholar] [CrossRef]
- Ullah, S.; Li, Z.; Hasan, Z.; Khan, S.U.; Fahad, S.J.E.; Safety, E. Malathion induced oxidative stress leads to histopathological and biochemical toxicity in the liver of rohu (Labeo rohita, Hamilton) at acute concentration. Ecotoxicol. Environ. Saf. 2018, 161, 270–280. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, P.K.; Kumar, D.; Tripathi, D.K.; Chauhan, D.K.; Prasad, S.M. Morpho-anatomical and biochemical adapting strategies of maize (Zea mays L.) seedlings against lead and chromium stresses. Biocatal. Agric. Biotechnol. 2015, 4, 286–295. [Google Scholar] [CrossRef]
- Murugan, S.S.; Karuppasamy, R.; Poongodi, K.; Puvaneswari, S. Bioaccumulation pattern of zinc in freshwater fish Channa punctatus (Bloch.) after chronic exposure. Turk. J. Fish. Aquat. Sci. 2008, 8, 55–59. [Google Scholar]
- Hesni, M.A.; Dadolahi-Sohrab, A.; Savari, A.; Mortazavi, M.S. Study the acute toxicity of lead nitrate metal salt on behavioral changes of the milkfish (Chanos chanos). World J. Fish Mar. Sci. 2011, 3, 496–501. [Google Scholar]
- Nekoubin, H.; Gharedaashi, E.; Hatefi, S.; Sudagar, M.; Shahriari, R.; Asgharimoghadam, A. Determination of LC50 of copper sulfate and lead (II) nitrate and behavioral responses of grass carp (Ctenopharyngodon idella). Walailak J. Sci. Technol. 2012, 9, 333–340. [Google Scholar]
- Nuraini, N.; Tanjung, A.; Warningsih, T.; Muchlisin, Z.A. Induced spawning of siban fish Cyclocheilichthys apogon using Ovaprim. F1000 Res. 2017, 6, 1855. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; El-Sayed, G.O.; Shady, S.H. Growth, biochemical variables, and zinc bioaccumulation in Nile tilapia, Oreochromis niloticus (L.) as affected by water-born zinc toxicity and exposure period. Int. Aquat. Res. 2016, 8, 197–206. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Dauble, D.D.; Barraclough, S.A.; Bean, R.M.; Fallon, W.E. Chronic Effects of Coal-Liquid Dispersions on Fathead Minnows and Rainbow Trout. Trans. Am. Fish. Soc. 1983, 112, 712–719. [Google Scholar] [CrossRef]
- Shukla, J.; Pandey, K. Effect of a sublethal concentration of zinc sulphate on the growth rate of fingerlings of Channa punctatus (Bloch), a freshwater murrel. Acta Hydrochim. Hydrobiol. 1986, 14, 677–680. [Google Scholar] [CrossRef]
- Bekmezci, H.D.; Nevin, Ü. Aşaği Seyhan Ovasi Drenaj Sistemlerindeki Kirlilik Etmenlerinin Clarias gariepinus’ da Toksik Etkileri. Ç.Ü Fen ve Mühendislik Bilimleri 2010, 28, 11–20. [Google Scholar]
- Ahmed, A.S.; Sultana, S.; Habib, A.; Ullah, H.; Musa, N.; Hossain, M.B.; Rahman, M.M.; Sarker, M.S.I. Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PLoS ONE 2019, 14, e0219336. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mousa, M.A.; Ahmad, M.H.; Sakr, S.F. The use of calcium pre-exposure as a protective agent against environmental copper toxicity for juvenile Nile tilapia, Oreochromis niloticus (L.). Aquaculture 2007, 264, 236–246. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef] [PubMed]
- Dewi, N.K.; Prabowo, R. Determination of liver somatic index (LSI) and gonadosomatic index (GSI) value of crap (Cyprinus carpio) and Nile tilapia (Perca fluviatilis). Int. J. Sci. Res. Publ. 2017, 7, 220–223. [Google Scholar]
- Bain, P.; Harr, K.E. Hematology of amphibians. Schalm’s Vet. Hematol. 2022, 1228–1232. [Google Scholar] [CrossRef]
- Nussey, G.; Van Vuren, J.; Du Preez, H. Effect of copper on blood coagulation of Oreochromis mossambicus (Cichlidae). Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1995, 111, 359–367. [Google Scholar] [CrossRef]
- Nakagawa, H.; Nakagawa, K.; Sato, T. Evaluation of erythrocyte 5-aminolevulinic acid dehydratase activity in the blood of carp Cyprinus carpio as an indicator in fish with water lead pollution. Fish. Sci. 1995, 61, 91–95. [Google Scholar] [CrossRef]
- Petřivalský, M.; Machala, M.; Nezveda, K.; Piačka, V.; Svobodová, Z.; Drábek, P. Glutathione-dependent detoxifying enzymes in rainbow trout liver: Search for specific biochemical markers of chemical stress. Environ. Toxicol. Chem. 1997, 16, 1417–1421. [Google Scholar]
- Dick, P.; Dixon, D.G. Changes in circulating blood cell levels of rainbow trout, Salmo gairdneri Richardson, following acute and chronic exposure to copper. J. Fish Biol. 1985, 26, 475–481. [Google Scholar] [CrossRef]
- Allen, P. Changes in the haematological profile of the cichlid Oreochromis aureus (Steindachner) during acute inorganic mercury intoxication. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1994, 108, 117–121. [Google Scholar] [CrossRef]
- Maheshwari, S.; Dua, A. Structural analysis of the erythrocytes of Channa punctatus (Bloch) exposed to mercuric chloride using scanning electron microscopy. Turk. J. Fish. Aquat. Sci. 2016, 16, 865–871. [Google Scholar] [CrossRef]
- Abdel-Hameid, N. A protective effect of calcium carbonate against arsenic toxicity on the Nile catfish, larias gariepinus. Turk. J. Fish. Aquat. Sci. 2008, 12, 143–163. [Google Scholar] [CrossRef][Green Version]
- Livingstone, D. The fate of organic xenobiotics in aquatic ecosystems: Quantitative and qualitative differences in biotransformation by invertebrates and fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, C.; Malarvizhi, A.; Kumaran, S.S.; Ramesh, M. Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem. Toxicol. 2010, 48, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Syversen, T.L. Effects of methyl mercury on protein synthesis in vitro. Acta Pharmacol. Toxicol. 1981, 49, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Nanda, P.; Behera, M.K. Nickel induced changes in some hemato-biochemical parameters of a catfish Heteropneustes fossilis (Bloch). Environ. Ecol. 1996, 14, 82–85. [Google Scholar]
- Kumar, R.; Banerjee, T.K. Arsenic induced hematological and biochemical responses in nutritionally important catfish Clarias batrachus (L.). Toxicol. Rep. 2016, 3, 148–152. [Google Scholar] [CrossRef][Green Version]
- Canli, M. Effects of mercury, chromium and nickel on some blood parameters in the carp Cyprinus carpio. Turk. J. Zool. 1995, 19, 305–312. [Google Scholar]
- Yang, J.-L.; Chen, H.-C. Effects of gallium on common carp (Cyprinus carpio): Acute test, serum biochemistry, and erythrocyte morphology. Chemosphere 2003, 53, 877–882. [Google Scholar] [CrossRef]
- Parvathi, K.; Palanivel, S.; Mathan, R.; Sarasu, R. Sublethal effects of chromium on some biochemical profiles of the fresh water teleost, Cyprinus carpio. Portal Reg. Da BVS 2011, 2, 295–300. [Google Scholar]
- Abdel-Tawwab, M.; El-Sayed, G.O.; Shady, S.H. Effects of dietary protein levels and environmental zinc exposure on the growth, feed utilization, and biochemical variables of Nile tilapia, Oreochromis niloticus (L.). Toxicol. Environ. Chem. 2012, 94, 1368–1382. [Google Scholar] [CrossRef]
- Roy, S.; Bhattacharya, S. Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicol. Environ. Saf. 2006, 65, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Onah, C.E.; Meludu, S.C.; Dioka, C.E.; Onuegbu, A.J.; Onah, C.F.; Ajaghaku, D.L.; Nnodim, J.K.; Ejeatuluchukwu, O. Amelioratory effect of methanolic leaf extract of Moringa oleifera on some liver and kidney function and oxidative stress markers in lead-intoxicated rats. Eur. J. Med. Plants 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Sharma, K.; Upreti, N.; Sharma, S.; Sharma, S. Protective effect of Spirulina and tamarind fruit pulp diet supplement in fish (Gambusia affinis Baird & Girard) exposed to sublethal concentration of fluoride, aluminum and aluminum fluoride. NIScPR Online Period. Repos. 2012, 50, 897–903. [Google Scholar]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Ugurlu, P. Scanning electron microscopy study of the gills, scales and erythrocytes of Anabas testudineus upon exposure to chlorpyrifos. Toxicol. Environ. Chem. 2015, 97, 208–220. [Google Scholar] [CrossRef]
- Sawhney, A.; Johal, M.S. Erythrocyte alterations induced by malathion in Channa punctatus (Bloch). Bull. Environ. Contam. Toxicol. 2000, 64, 398–405. [Google Scholar] [CrossRef]
- Massar, B.; Dey, S.; Barua, R.; Dutta, K. Microscopy and microanalysis of hematological parameters in common carp, Cyprinus carpio, inhabiting a polluted lake in North East India. Microsc. Microanal. 2012, 18, 1077–1087. [Google Scholar] [CrossRef]
- Isomaa, B.; Hägerstrand, H.; Paatero, G.; Engblom, A.C. Permeability alterations and antihaemolysis induced by amphiphiles in human erythrocytes. Biochim. Biophys. Acta (BBA)-Biomembr. 1986, 860, 510–524. [Google Scholar] [CrossRef]
- Naskar, R.; Veenapani, S.M.; Kumari, K.; Sen, N.S.; Ahmad, M.F. Surface ultrastructural changes in the gills of an indian stenohaline catfish, Clarias batrachus (Linn.) under acute acid and aluminium stress. Ecoscan 2009, 3, 221–226. [Google Scholar]
- Gosto, R. Assessment of accuracies of protein 3-dimensional prediction software. Southeast Eur. J. Soft Comput. 2018, 7, 83–85. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Ramachandran, S.; Kota, P.; Ding, F.; Dokholyan, N.V. Automated minimization of steric clashes in protein structures. Proteins Struct. Funct. Bioinform. 2011, 79, 261–270. [Google Scholar] [CrossRef]
- Sievers, C.K.; Shanle, E.K.; Bradfield, C.A.; Xu, W. Differential action of monohydroxylated polycyclic aromatic hydrocarbons with estrogen receptors α and β. Toxicol. Sci. 2013, 132, 359–367. [Google Scholar] [CrossRef]


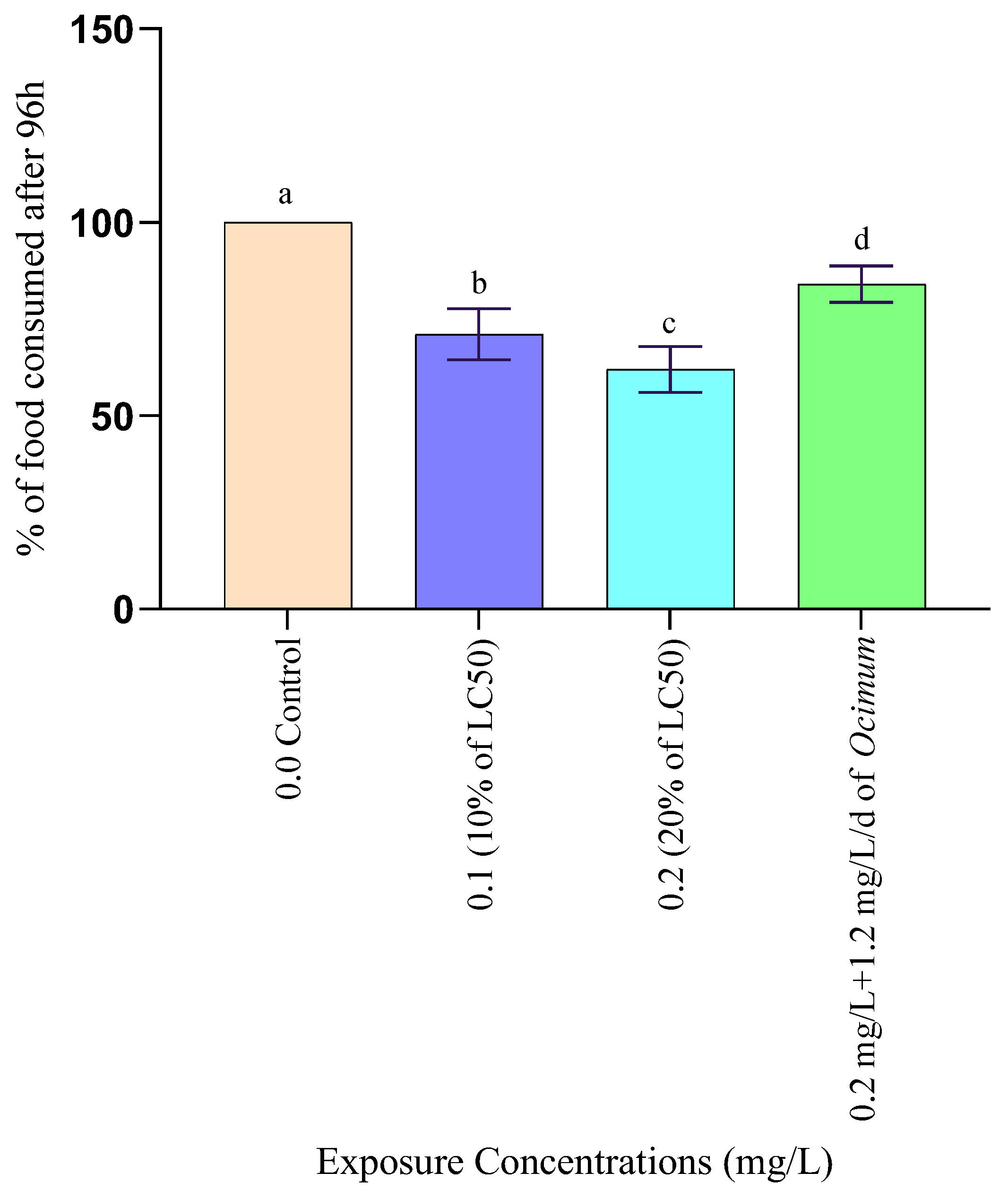


| Time of Exposure | LC50 (mg/L) | 95% Fiducial Limits of LC50 | Probit Regression Equation Y = ax + b | R2 Value | r Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| 24 h | 1.47 ± 0.22 | 1.28 | 1.70 | y = 4.41x + 4.25 | 0.9 | 0.9 |
| 48 h | 1.41 ± 0.28 | 1.19 | 1.69 | y = 3.54x + 4.45 | 0.9 | 0.9 |
| 72 h | 1.15 ± 0.28 | 0.96 | 1.37 | y = 3.53x + 4.78 | 0.9 | 0.9 |
| 96 h | 1.08 ± 0.34 | 0.86 | 1.36 | y = 2.91x + 4.90 | 0.8 | 0.8 |
| Dose (mg/L) | MS | HE | LE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | 24 h | 48 h | 72 h | 96 h | |
| 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| 0.5 | - | - | - | + | - | - | - | + | - | - | + | + |
| 0.9 | - | + | + | + | + | + | + | + | - | - | + | ++ |
| 1.1 | - | + | ++ | +++ | + | + | ++ | +++ | - | + | ++ | +++ |
| 1.5 | - | + | +++ | +++ | + | + | +++ | +++ | - | + | +++ | +++ |
| 1.9 | + | ++ | +++ | +++ | + | ++ | +++ | +++ | + | +++ | +++ | +++ |
| Hematological Parameter | Exposure Time (d) | Concentration of F (mg/L) | |||
|---|---|---|---|---|---|
| 0.0 mg/L (Control) | 0.1 mg/L (10% of LC50) | 0.2 mg/L (20% of LC50) | 0.2 mg/L (20% of LC50) + 1.2 mg/L/d (Ocimum sanctum Leaf Powder) | ||
| Total RBC (106/mm3) | 1 | 2.83 ± 0.07 am | 2.84 ± 0.09 am | 2.84 ± 0.12 am | 2.83 ± 0.08 am |
| 15 | 2.85 ± 0.16 am | 2.71 ± 0.07 bn | 2.65 ± 0.17 bo | 2.76 ± 0.2 bp | |
| 30 | 2.84 ± 0.16 am | 2.62 ± 0.16 bn | 2.53 ± 0.13 co | 2.78± 0.25 cp | |
| 45 | 2.83 ± 0.15 am | 2.51 ± 0.05 dn | 2.42 ± 0.16 do | 2.80 ± 0.15 dp | |
| Total WBC (103/mm3) | 1 | 9.72 ± 0.34 am | 9.71 ± 0.45 am | 9.72 ± 0.44 am | 9.73 ± 0.57 am |
| 15 | 9.69 ± 0.43 am | 9.14 ± 0.34 bn | 9.01 ± 0.58 bo | 9.38± 0.37 bp | |
| 30 | 9.71 ± 0.22 am | 8.87 ± 0.43 cn | 8.53 ± 0.57 co | 8.59 ± 0.57 bp | |
| 45 | 9.72 ± 0.41 am | 8.58 ± 0.22 dn | 7.17 ± 0.82 do | 9.23 ± 0.48 cp | |
| Haemoglobin (g/dL) | 1 | 14.92 ± 0.52 am | 14.91 ± 0.51 am | 14.91 ± 0.47 am | 14.92 ± 0.68 am |
| 15 | 14.90 ± 0.43 am | 13.12 ± 0.29 bn | 12.02 ± 0.17 bo | 12.58 ± 0.47 bp | |
| 30 | 14.93 ± 0.24 am | 12.76 ± 0.58 cn | 11.38 ± 0.37 co | 13.44 ± 0.73 cp | |
| 45 | 14.92 ± 0.44 am | 11.69 ± 0.57 dn | 9.57 ± 0.36 co | 13.81 ± 0.27 dp | |
| Hct (%) | 1 | 24.47 ± 1.35 am | 24.46 ± 0.96 am | 24.45 ± 1.35 am | 24.46 ± 0.99 am |
| 15 | 24.44 ± 1.46 am | 21.52 ± 1.25 am | 20.37 ± 1.34 bn | 22.43 ± 1.38 bo | |
| 30 | 24.43 ± 1.57 am | 20.39 ± 0.94 bn | 19.53 ± 1.52 co | 22.87 ±1.96 bp | |
| 45 | 24.46 ± 1.39 am | 18.65 ± 0.93 cn | 17.21 ± 0.81 co | 23.15 ± 1.01 cm | |
| Parameter | Exposure Time (days) | Concentration of Pb (mg/L) | |||
|---|---|---|---|---|---|
| 0.0 mg/L (Control) | 0.1 mg/L (10% of LC50) | 0.2 mg/L (20% of LC50) | 0.2 mg/L (20% of LC50) + 1.2 mg/L/d (Ocimum sanctum Leaf Powder) | ||
| Plasma glucose (mg/dL) | 1 | 60.23 ± 0.97 am | 60.47 ± 0.74 am | 60.35 ± 1.03 am | 60.27 ± 1.02 am |
| 15 | 61.14 ± 0.30 am | 62.32 ± 0.85 bn | 63.17 ± 0.68 bm | 62.15 ± 0.77 bo | |
| 30 | 61.03 ± 0.98 am | 65.83 ± 0.36 cn | 67.29 ± 0.77 co | 63.12 ± 1.26 cp | |
| 45 | 60.67 ± 0.69 am | 68.17 ± 0.51 dn | 70.18 ± 0.75 do | 61.74 ± 0.94 am | |
| Plasma protein (mg/dL) | 1 | 3.21 ± 0.18 am | 3.24 ± 0.13 am | 3.23 ± 0.04 am | 3.22 ± 0.02 am |
| 15 | 3.20 ± 0.16 am | 2.91 ± 0.06 bn | 2.62 ± 0.13 bo | 2.93 ± 0.15 bp | |
| 30 | 3.19 ± 0.16 am | 2.73 ± 0.11 cn | 2.43 ± 0.12 co | 2.95 ± 0.18 cp | |
| 45 | 3.20 ± 0.17 am | 2.62 ± 0.11 dn | 2.19 ± 0.11 do | 2.99 ± 0.17 dp | |
| Cholesterol (mg/dL) | 1 | 192.25 ± 1.07 am | 191.78 ± 1.22 am | 192.63 ± 1.84 am | 192.12 ± 1.36 am |
| 15 | 191.79 ± 1.09 am | 187.34± 1.43 bn | 182.13 ± 1.38 bo | 188.19 ± 0.96 bp | |
| 30 | 192.13 ± 1.27 am | 181.57 ± 1.36 cn | 176.70 ± 1.57 co | 183.22 ± 1.65 bm | |
| 45 | 192.37 ± 1.58 am | 177.72 ± 1.55 dn | 167.58 ± 1.26 do | 189.18 ± 1.25 cm | |
| Creatinine (mg/dL) | 1 | 0.43 ± 0.049 am | 0.49 ± 0.074 am | 0.49 ± 0.046 am | 0.46 ± 0.063 am |
| 15 | 0.49 ± 0.067 am | 0.69 ± 0.053 bn | 1.06 ± 0.055 bo | 0.88 ± 0.043 bp | |
| 30 | 0.46 ± 0.054 am | 0.88 ± 0.045 cn | 1.17 ± 0.064 co | 0.79 ± 0.062 cp | |
| 45 | 0.49 ± 0.036 am | 1.19 ± 0.036 dn | 1.28 ± 0.042 do | 0.53 ± 0.032 dm | |
| Sl. No. | Proteins | Binding Affinities (kcal/mol) |
|---|---|---|
| 1. | Serum alanine aminotransferase (ALT) | −4.0 |
| 2. | Serum aspartate aminotransferase (AST) | −3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, N.C.; Chatterjee, A.; Banerjee, P.; Bhattacharya, R.; Sadhu, A.; Pastorino, P.; Saha, S. Toxic Effects of Lead Exposure on Freshwater Climbing Perch, Anabas testudineus, and Bioremediation Using Ocimum sanctum Leaf Powder. Toxics 2024, 12, 927. https://doi.org/10.3390/toxics12120927
Saha NC, Chatterjee A, Banerjee P, Bhattacharya R, Sadhu A, Pastorino P, Saha S. Toxic Effects of Lead Exposure on Freshwater Climbing Perch, Anabas testudineus, and Bioremediation Using Ocimum sanctum Leaf Powder. Toxics. 2024; 12(12):927. https://doi.org/10.3390/toxics12120927
Chicago/Turabian StyleSaha, Nimai Chandra, Arnab Chatterjee, Priyajit Banerjee, Ritwick Bhattacharya, Auroshree Sadhu, Paolo Pastorino, and Shubhajit Saha. 2024. "Toxic Effects of Lead Exposure on Freshwater Climbing Perch, Anabas testudineus, and Bioremediation Using Ocimum sanctum Leaf Powder" Toxics 12, no. 12: 927. https://doi.org/10.3390/toxics12120927
APA StyleSaha, N. C., Chatterjee, A., Banerjee, P., Bhattacharya, R., Sadhu, A., Pastorino, P., & Saha, S. (2024). Toxic Effects of Lead Exposure on Freshwater Climbing Perch, Anabas testudineus, and Bioremediation Using Ocimum sanctum Leaf Powder. Toxics, 12(12), 927. https://doi.org/10.3390/toxics12120927








