The Perils of Methanol Exposure: Insights into Toxicity and Clinical Management
Abstract
:1. Introduction
2. Pathophysiology
3. Toxicokinetic
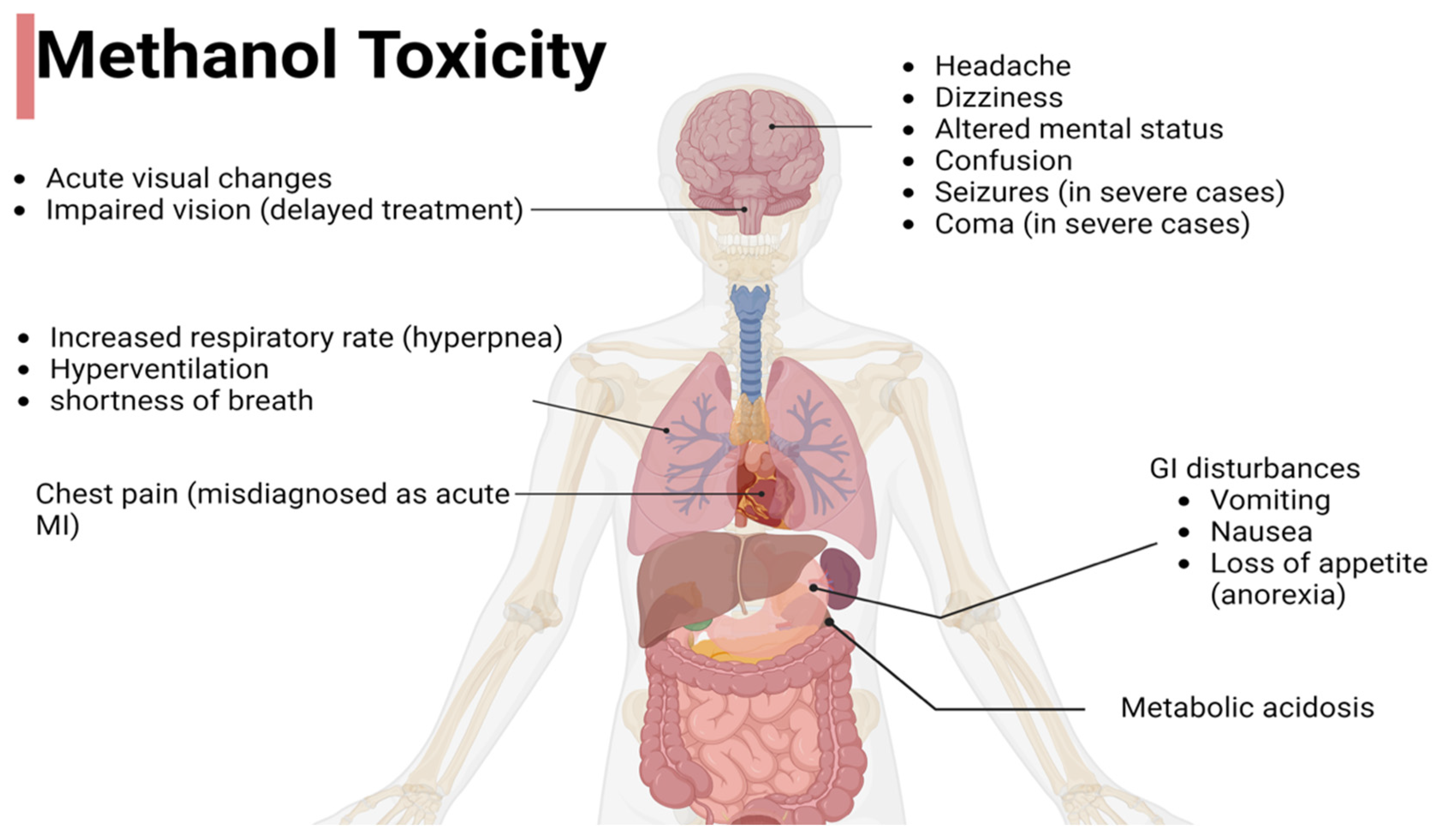
4. Clinical Presentation
5. Evaluation and Diagnostic Testing
6. Diagnostic Testing
7. Treatment
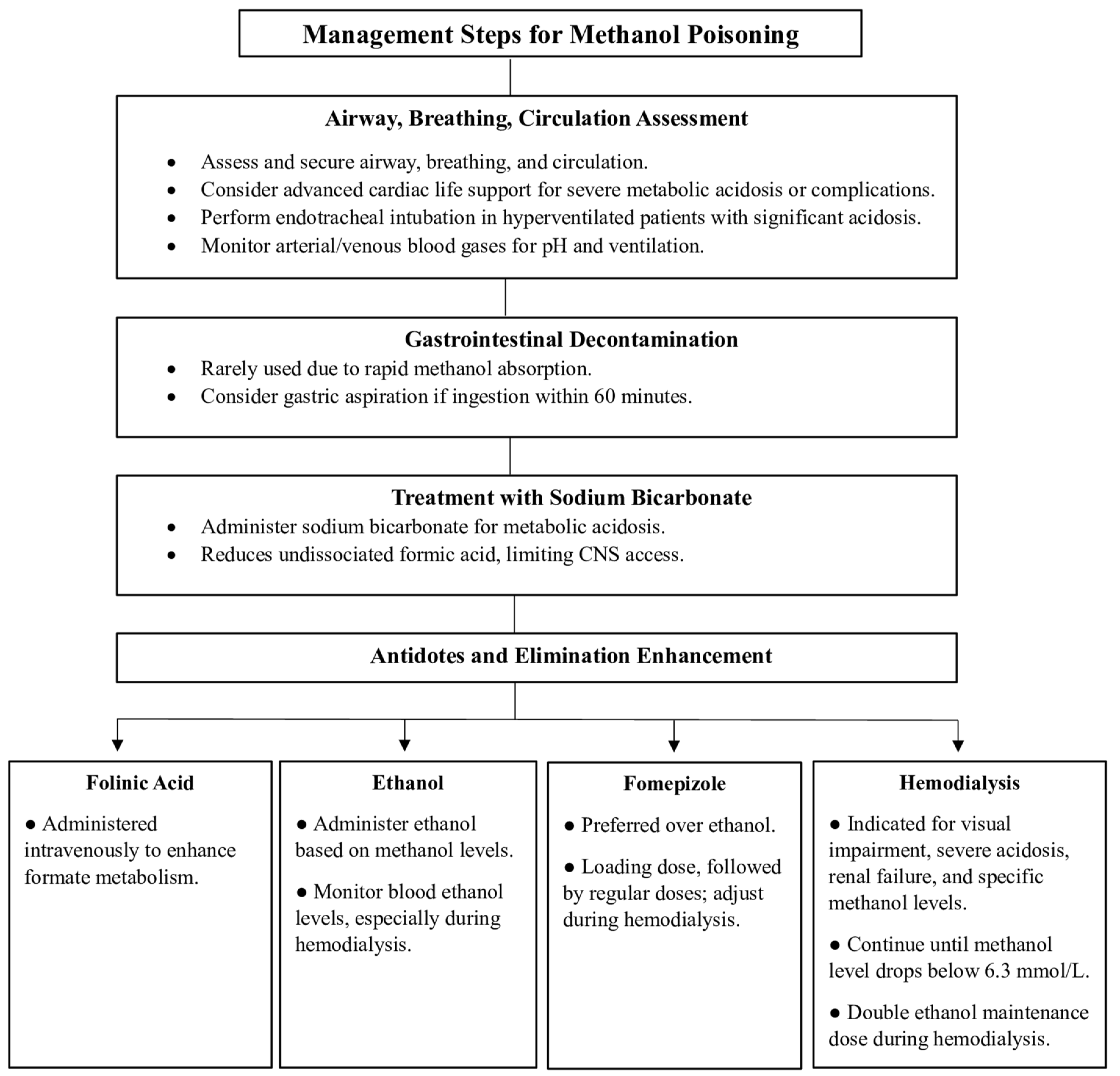
7.1. Airways, Breathing, and Circulation
7.2. Gastrointestinal Decontamination
7.3. Treatment with Sodium Bicarbonate
7.4. Antidotes and Elimination Enhancement
7.4.1. Folinic Acid
7.4.2. Ethanol
7.4.3. Fomepizole
7.4.4. Hemodialysis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ashurst, J.V.; Nappe, T.M. Methanol Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2023; StatPearls Web Site. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482121/ (accessed on 2 July 2024).
- World Health Organization (WHO). Methanol Poisoning Outbreaks; World Health Organization (WHO): Geneva, Switzerland, 2014; Available online: https://www.methanol.org/wp-content/uploads/2016/06/WHO-Methanol-Poisoning-Fact-Sheet.pdf (accessed on 2 July 2024).
- Loza, R.; Rodriguez, D. A case of methanol poisoning in a child. Case Rep. Nephrol. 2014, 2014, 652129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nekoukar, Z.; Zakariaei, Z.; Taghizadeh, F.; Musavi, F.; Banimostafavi, E.S.; Sharifpour, A.; Ebrahim Ghuchi, N.; Fakhar, M.; Tabaripour, R.; Safanavaei, S. Methanol poisoning as a new world challenge: A review. Ann. Med. Surg. 2021, 66, 102445. [Google Scholar] [CrossRef]
- Bateman, N.; Jefferson, R.; Thomas, S.; Thompson, J.; Vale, A. (Eds.) Common complications of poisoning. In Oxford Desk Reference: Toxicology; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Hantson, P.E. Acute methanol intoxication: Physiopathology, prognosis and treatment. Bull. Mem. Acad. R. Med. Belg. 2005, 160, 294–300. [Google Scholar]
- Moon, C.S. Estimations of the lethal and exposure doses for representative methanol symptoms in humans. Ann. Occup. Environ. Med. 2017, 29, 44. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Shindyapina, A.V.; Sheshukova, E.V.; Komarova, T.V. Metabolic methanol: Molecular pathways and physiological roles. Physiol. Rev. 2015, 95, 603–644. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Quiroz Torres, J.; Royer, S.; Bellat, J.-P.; Giraudon, J.-M.; Lamonier, J.-F. Formaldehyde: Catalytic Oxidation as a Promising Soft Way of Elimination. ChemSusChem 2013, 6, 578–592. [Google Scholar] [CrossRef]
- Eells, J.T.; Makar, A.B.; Noker, P.E.; Tephly, T.R. Methanol poisoning and formate oxidation in nitrous oxide-treated rats. J. Pharmacol. Exp. Ther. 1981, 217, 57–61. [Google Scholar] [PubMed]
- Baumbach, G.L.; Cancilla, P.A.; Martin-Amat, G.; Tephly, T.R.; McMartin, K.E.; Makar, A.B.; Hayreh, M.S.; Hayreh, S.S. Methyl alcohol poisoning. IV. Alterations of the morphological findings of the retina and optic nerve. Arch. Ophthalmol. 1977, 95, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Pietzke, M.; Meiser, J.; Vazquez, A. Formate metabolism in health and disease. Mol. Metab. 2020, 33, 23–37. [Google Scholar] [CrossRef]
- Jacobsen, D.; Webb, R.; Collins, T.D.; McMartin, K.E. Methanol and formate kinetics in late diagnosed methanol intoxication. Med. Toxicol. Adverse. Drug Exp. 1988, 3, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewska, E. Toxicological and metabolic consequences of methanol poisoning. Toxicol. Mech. Methods 2003, 13, 277–293. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Graw, M.; Haffner, H.T.; Althaus, L.; Besserer, K.; Voges, S. Invasion and distribution of methanol. Arch. Toxicol. 2000, 74, 313–321. [Google Scholar] [CrossRef]
- Haffner, H.T.; Wehner, H.D.; Scheytt, K.D.; Besserer, K. The elimination kinetics of methanol and the influence of ethanol. Int. J. Legal Med. 1992, 105, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Methanol: Systemic Agent. Available online: https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750029.html (accessed on 27 January 2024).
- Bennett, I.L., Jr.; Cary, F.H.; Mitchell, G.L., Jr.; Cooper, M.N. Acute methyl alcohol poisoning: A review based on experiences in an outbreak of 323 cases. Medicine 1953, 32, 431–463. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.A.; Warren, A.K.; Baumal, C.R.; Hedges, T.R. Optical coherence tomography findings in methanol toxicity. Int. J. Retin. Vitr. 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Liberski, S.; Kaluzny, B.J.; Kocięcki, J. Methanol-induced optic neuropathy: A still-present problem. Arch. Toxicol. 2022, 96, 431–451. [Google Scholar] [CrossRef]
- Aabakken, L.; Johansen, K.S.; Rydningen, E.B.; Bredesen, J.E.; Ovrebø, S.; Jacobsen, D. Osmolal and anion gaps in patients admitted to an emergency medical department. Hum. Exp. Toxicol. 1994, 13, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.D.; Richardson, K.J.; Purssell, R.A.; Abu-Laban, R.B.; Brubacher, J.R.; Lepik, K.J.; Sivilotti, M.L. An evaluation of the osmole gap as a screening test for toxic alcohol poisoning. BMC Emerg. Med. 2008, 8, 5. [Google Scholar] [CrossRef]
- Kleiman, R.; Nickle, R.; Schwartz, M. Medical toxicology and public health--update on research and activities at the Centers for Disease Control and Prevention, and the Agency for Toxic Substances and Disease Registry inhalational methanol toxicity. J. Med. Toxicol. 2009, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G.; Bond, G.R.; Krenzelok, E.P.; Cooper, H.; Vale, J.A. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J. Toxicol. Clin. Toxicol. 2002, 40, 415–446. [Google Scholar] [CrossRef]
- Driscoll, D.; Bleecker, G.; Francis, J.; Jaberi, A. Acute Hemodialysis for Treatment of Severe Ethanol Intoxication. Kidney Med. 2020, 2, 793–796. [Google Scholar] [CrossRef]
- Gallagher, N.; Edwards, F.J. The Diagnosis and Management of Toxic Alcohol Poisoning in the Emergency Department: A Review Article. Adv. J. Emerg. Med. 2019, 3, e28. [Google Scholar] [CrossRef]
- Mégarbane, B. Treatment of patients with ethylene glycol or methanol poisoning: Focus on fomepizole. Open Access Emerg. Med. 2010, 2, 67–75. [Google Scholar] [CrossRef]
- Shannon, M.W.; Borron, S.W.; Burns, M.J. (Eds.) Emergency Management of Poisoning. In Haddad and Winchester’s Clinical Management of Poisoning and Drug Overdose, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 13–61. [Google Scholar]
- Beatty, L.; Green, R.; Magee, K.; Zed, P. A Systematic Review of Ethanol and Fomepizole Use in Toxic Alcohol Ingestions. Emerg. Med. Int. 2013, 2013, 638057. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashed, M.; Aldeghaither, N.S.; Almutairi, S.Y.; Almutairi, M.; Alghamdi, A.; Alqahtani, T.; Almojathel, G.H.; Alnassar, N.A.; Alghadeer, S.M.; Alshehri, A.; et al. The Perils of Methanol Exposure: Insights into Toxicity and Clinical Management. Toxics 2024, 12, 924. https://doi.org/10.3390/toxics12120924
Alrashed M, Aldeghaither NS, Almutairi SY, Almutairi M, Alghamdi A, Alqahtani T, Almojathel GH, Alnassar NA, Alghadeer SM, Alshehri A, et al. The Perils of Methanol Exposure: Insights into Toxicity and Clinical Management. Toxics. 2024; 12(12):924. https://doi.org/10.3390/toxics12120924
Chicago/Turabian StyleAlrashed, Mohammed, Norah S. Aldeghaither, Shatha Y. Almutairi, Meshari Almutairi, Abdulrhman Alghamdi, Tariq Alqahtani, Ghada H. Almojathel, Nada A. Alnassar, Sultan M. Alghadeer, Abdulmajeed Alshehri, and et al. 2024. "The Perils of Methanol Exposure: Insights into Toxicity and Clinical Management" Toxics 12, no. 12: 924. https://doi.org/10.3390/toxics12120924
APA StyleAlrashed, M., Aldeghaither, N. S., Almutairi, S. Y., Almutairi, M., Alghamdi, A., Alqahtani, T., Almojathel, G. H., Alnassar, N. A., Alghadeer, S. M., Alshehri, A., Alnuhait, M., & Almohammed, O. A. (2024). The Perils of Methanol Exposure: Insights into Toxicity and Clinical Management. Toxics, 12(12), 924. https://doi.org/10.3390/toxics12120924







