Timed Up and Go and Six-Minute Walking Tests with Wearable Inertial Sensor: One Step Further for the Prediction of the Risk of Fall in Elderly Nursing Home People
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
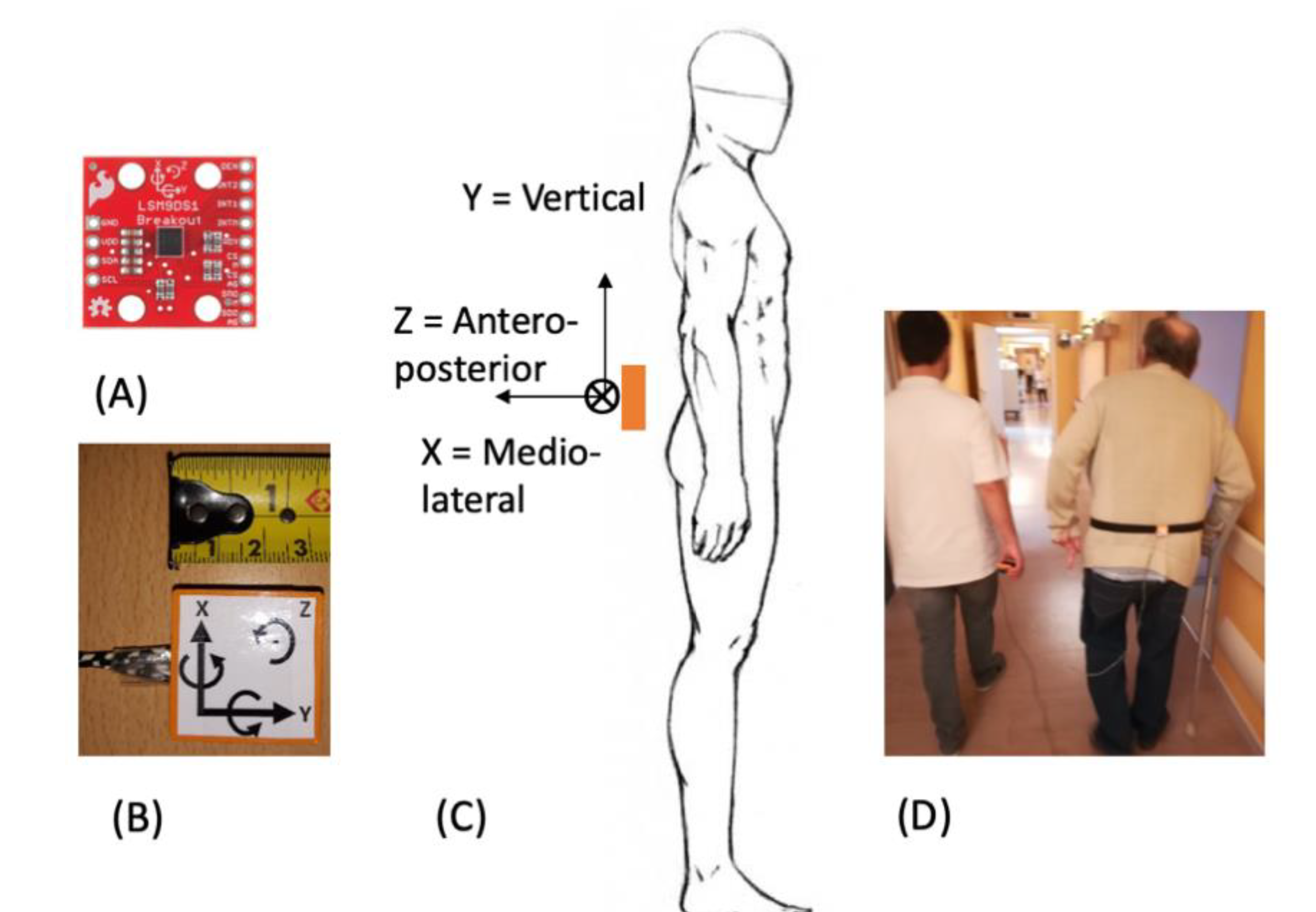
2.2. Protocol
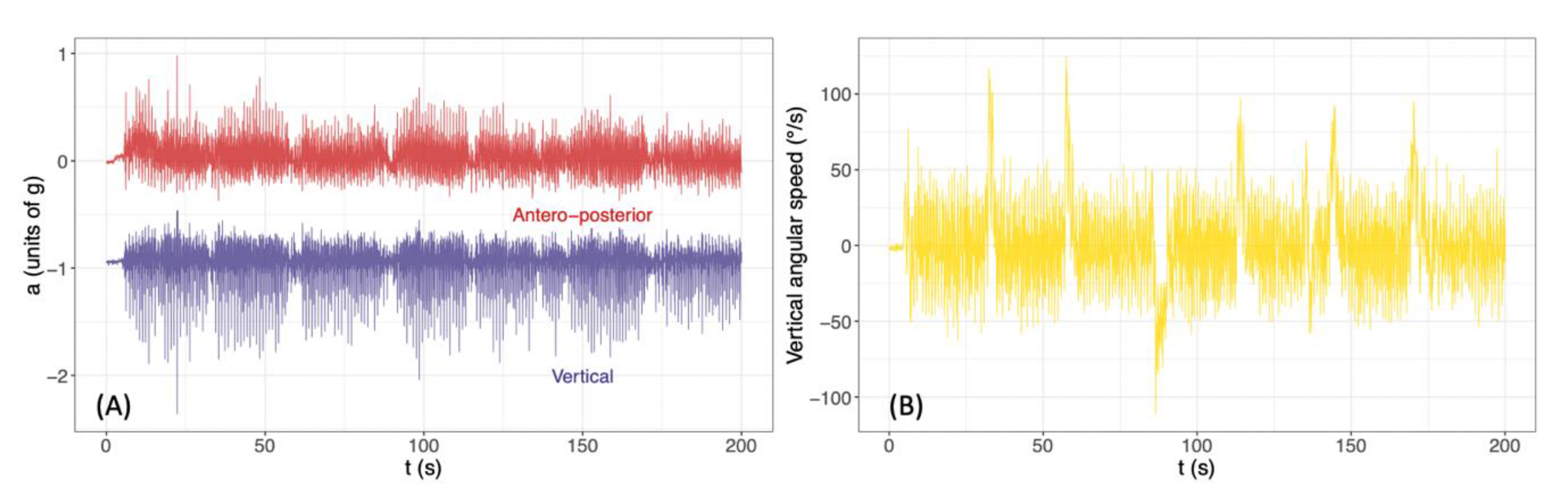
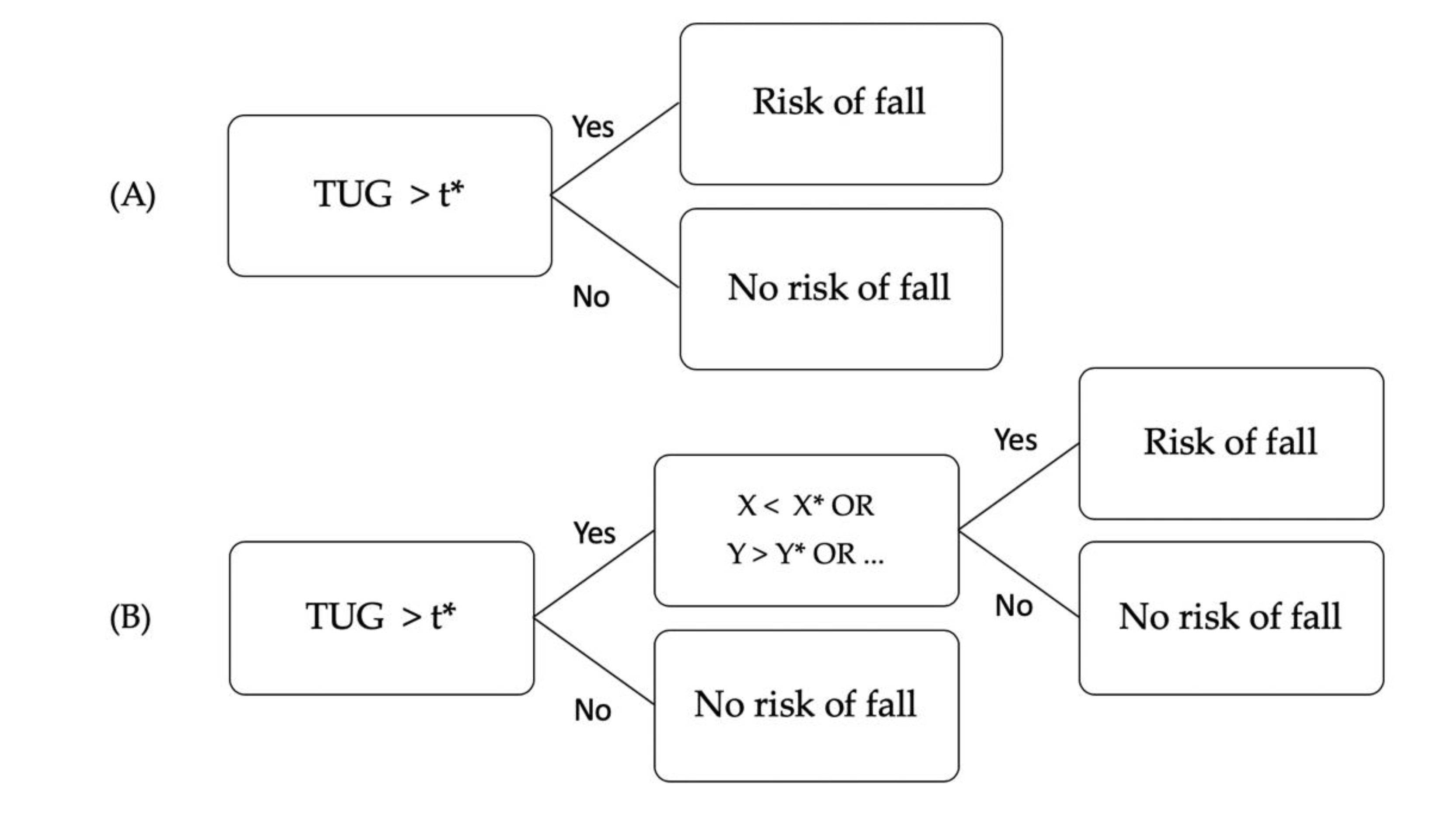
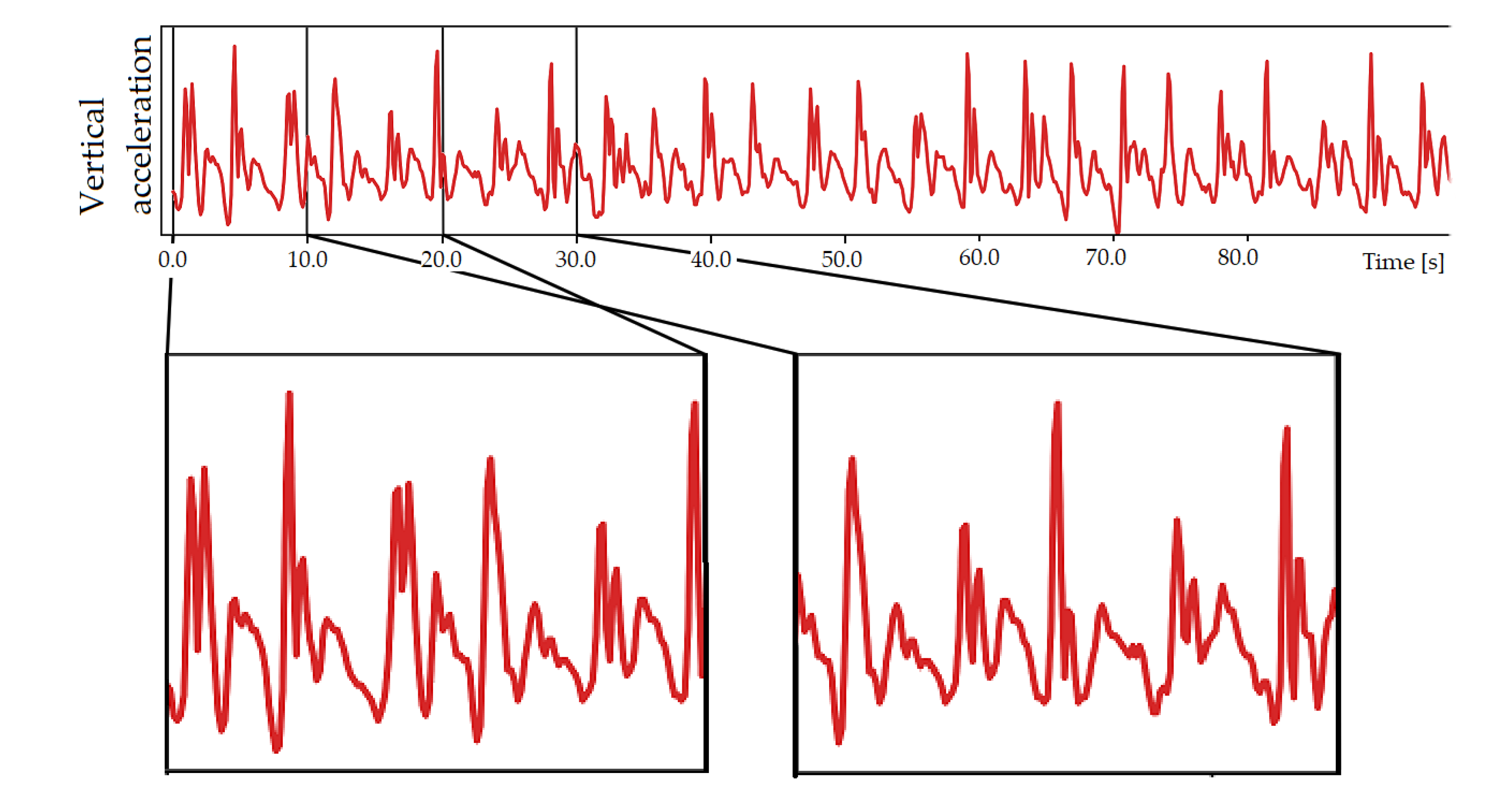
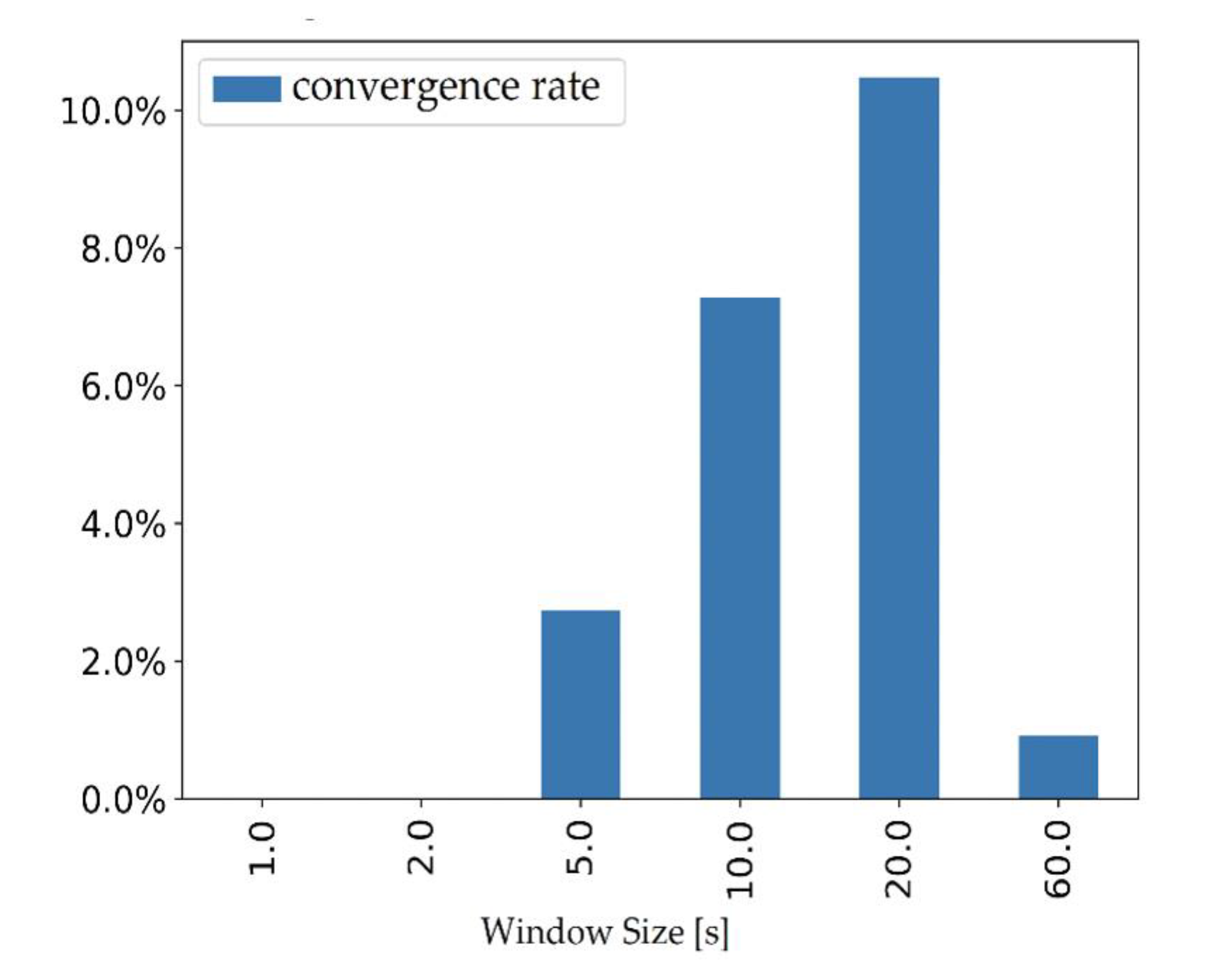
2.3. Data Analysis
3. Results
3.1. Variability Indices
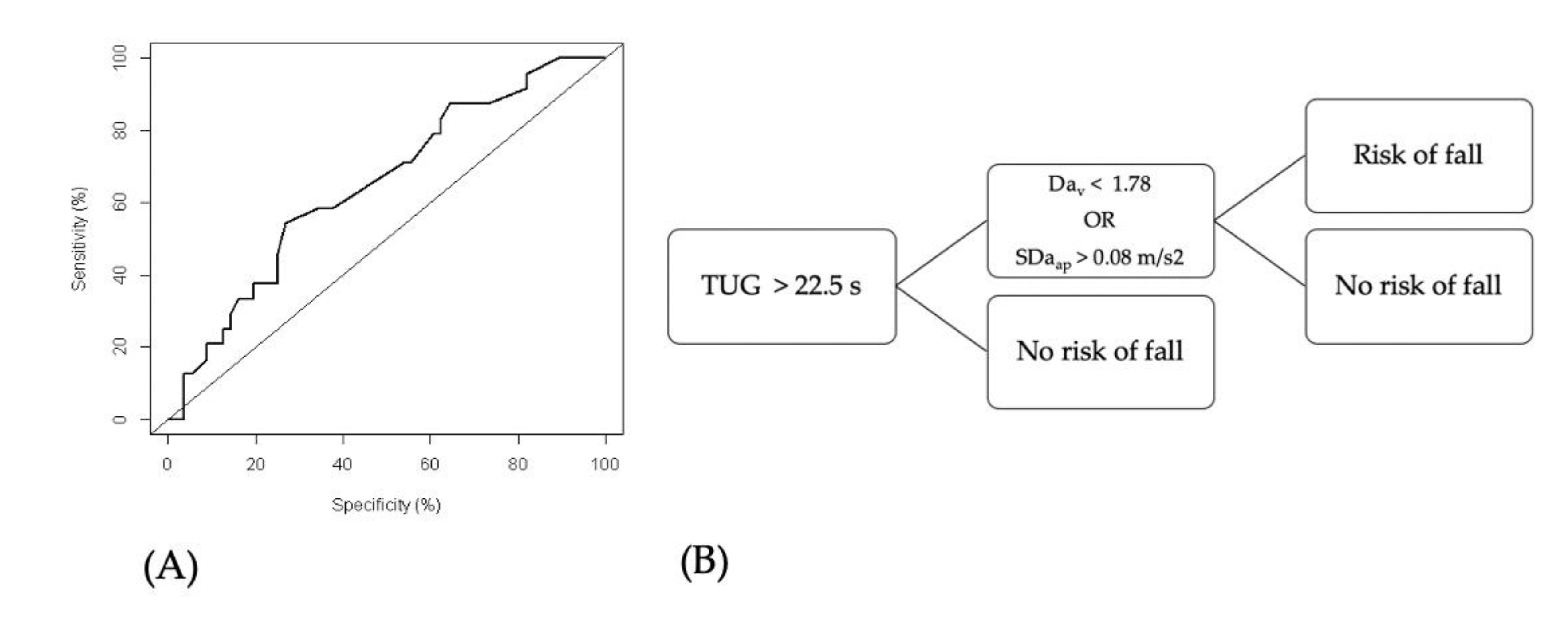
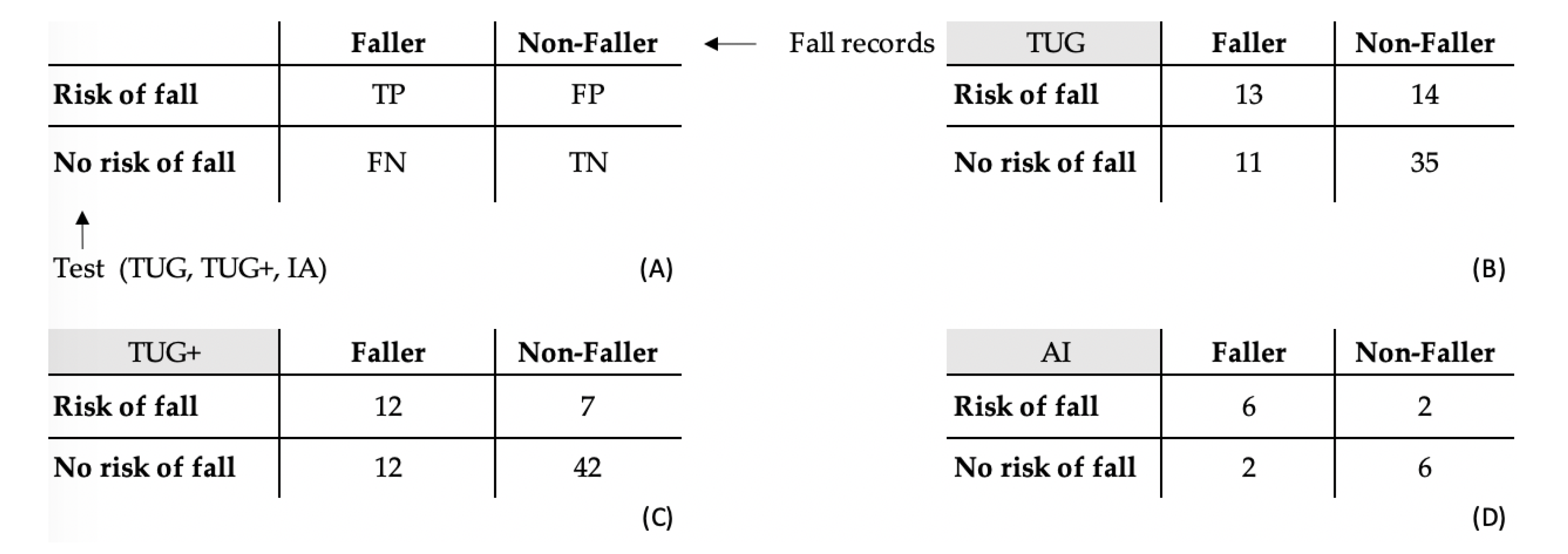
3.2. TUG and TUG+ Tests
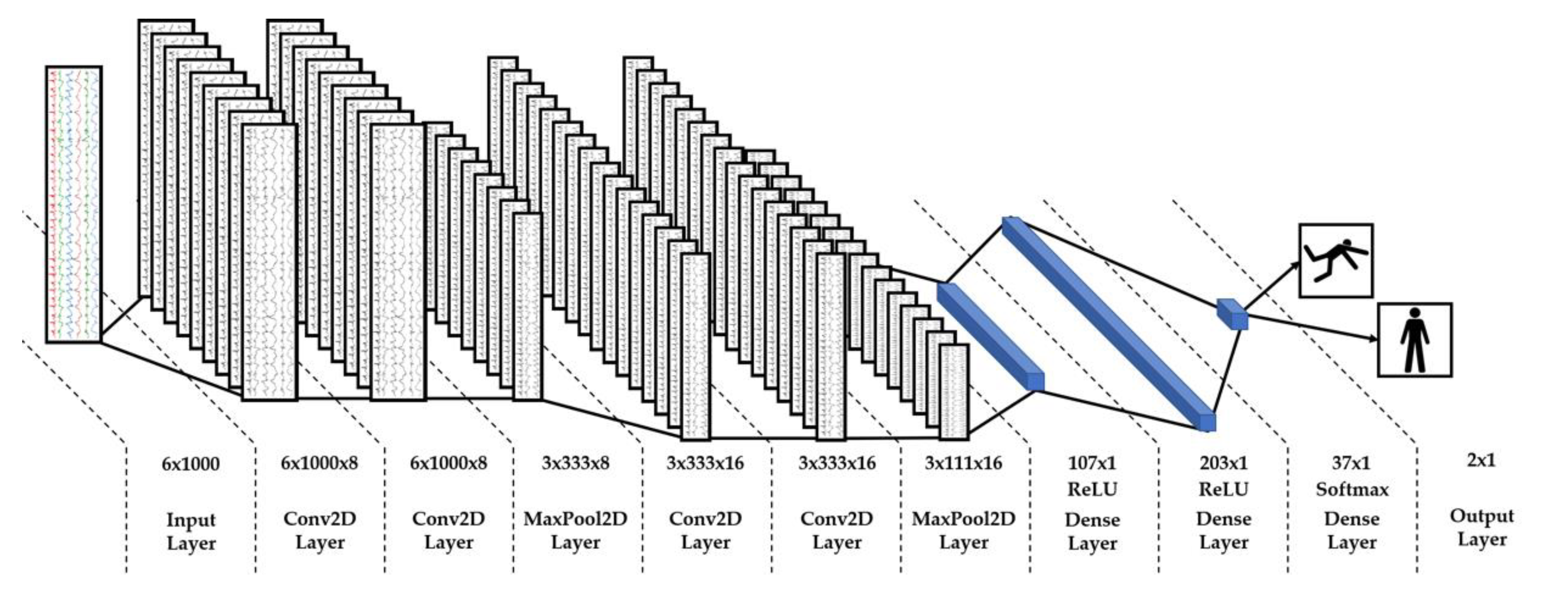
3.3. AI Classification
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. TUG and TUG+ Tests with Logistic Regressions
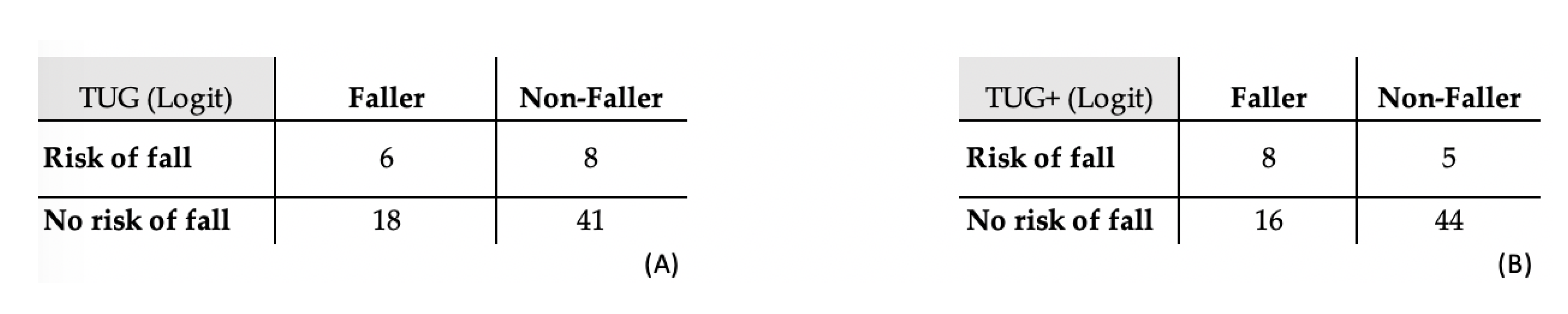
| TUG (Logit) | TUG+ (Logit) | |
|---|---|---|
| Se | 0.837 | 0.898 |
| Sp | 0.250 | 0.333 |
| LR+ | 1.12 | 1.35 |
| LR− | 0.652 | 0.306 |
| PPV | 0.695 | 0.733 |
| NPV | 0.439 | 0.615 |
| Acc | 0.644 | 0.712 |
References
- Fatal Falls: WHO, Mortality Database 2010–2012. Available online: http://www.who.int/healthinfo/mortality_data/en/ (accessed on 12 February 2020).
- Hartholt, K. Falls and Drugs in Older Population: Medical and Societal Consequences; Erasmus University: Rotterdam, The Netherlands, 2011. [Google Scholar]
- Moreland, J.D.; Richardson, J.A.; Goldsmith, C.H.; Clase, C.M. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2004, 52, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Rossat, A.; Fantino, B.; Nitenberg, C.; Annweiler, C.; Poujol, L.; Herrmann, F.R.; Beauchet, O. Risk factors for falling in community-dwelling older adults: Which of them are associated with the recurrence of falls? J. Nutr. Health Aging 2010, 14, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, A.F.; Paul, G.; Hausdorff, J.M. Risk factors for falls among older adults: A review of the literature. Maturitas 2013, 75, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Posdiadlo, D.; Richardson, S. The time “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ‘timed up and go’ test: More than meets the eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Panel on Prevention of Falls in Older Persons; American Geriatrics Society; British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J. Am. Geriatr. Soc. 2011, 14, 148–157. [Google Scholar]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef]
- Weiss, A.; Herman, T.; Plotnik, M.; Brozgol, M.; Giladi, N.; Hausdorff, J.M. An instrumented timed up and go: The added value of an accelerometer for identifying fall risk in idiopathic fallers. Physiol. Meas. 2011, 32, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar]
- Jehn, M.; Schmidt-Trucksäess, M.D.; Schuster, T.; Hanssen, H.; Weis, M.; Halle, M.; Koehler, F. Accelerometer-Based Quantification of 6-Minute Walk Test Performance in Patients with Chronic Heart Failure: Applicability in Telemedicine. J. Card. Fail. 2009, 15, 334–340. [Google Scholar] [CrossRef]
- Annegarn, J.; Spruit, M.A.; Savelberg, H.H.C.M.; Willems, P.J.B.; van de Bool, C.; Schols, A.M.W.J.; Wouters, E.F.M.; Meijer, K. Differences in Walking Pattern during 6-Min Walk Test between Patients with COPD and Healthy Subjects. PLoS ONE 2012, 7, e37329. [Google Scholar] [CrossRef]
- Hage, R.; Detrembleur, C.; Dierick, F.; Pitance, L.; Jojczyk, L.; Estievenart, W.; Buisseret, F. DYSKIMOT: An Ultra-Low-Cost Inertial Sensor to Assess Head’s Rotational Kinematics in Adults during the Didren-Laser Test. Sensors 2020, 20, 833. [Google Scholar] [CrossRef] [PubMed]
- Oks, A.; Katashev, A.; Zadinans, M.; Rancans, M.; Litvak, J. Development of Smart Sock System for Gate Analysis and Foot Pressure Control. In XIV Mediterranean Conference on Medical and Biological Engineering and Computing; Kyriacou, E., Christofides, S., Pattichis, C.S., Eds.; Springer International Publishing: Paphos, Cyprus, 2016; pp. 466–469. [Google Scholar]
- Esfahani, M.I.M.; Nussbaum, M.A.; Kong, Z. Using a smart textile system for classifying occupational manual material handling tasks: Evidence from lab-based simulations. Ergonomics 2019, 62, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, R.; Litvan, I.; Jung, T.-P. Fall Prediction and Prevention Systems: Recent Trends, Challenges, and Future Research Directions. Sensors 2017, 17, 2509. [Google Scholar] [CrossRef] [PubMed]
- Moulias, S.; Peigne, V.; Guérin, O.; Daire, R. Gériatrie, Cahier des EC, 3rd ed; Elsevier Masson: Paris, France, 2015; p. 95. [Google Scholar]
- Dierick, F.; Nivard, A.-L.; White, O.; Buisseret, F. Fractal analyses reveal independent complexity and predictability of gait. PLoS ONE 2017, 12, e0188711. [Google Scholar] [CrossRef]
- Kantz, H.; Schreiber, T. Nonlinear Time Series Analysis; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Hausdorff, J.M.; Peng, C.K.; Ladin, Z.; Wei, J.Y.; Goldberger, A.L. Is walking a random walk? Evidence for long-range correlations in stride interval of human gait. J. Appl. Physiol. 1995, 78, 349–358. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Bergstra, J.; Bengio, Y. Random Search for Hyper-Parameter Optimization. J. Mach. Learn. Res. 2012, 13, 281. [Google Scholar]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the Probability for Falls in Community-Dwelling Older Adults Using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Schoene, D.; Wu, S.M.-S.; Mikolaizak, A.S.; Menant, J.C.; Smith, S.T.; Delbaere, K.; Lord, S.R. Discriminative ability and predictive validity of the timed up and go test in identifying older people who fall: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2013, 61, 202–208. [Google Scholar] [CrossRef]
- Viccaro, L.J.; Perera, S.; Studenski, S.A. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J. Am. Geriatr. Soc. 2001, 59, 887–892. [Google Scholar] [CrossRef]
- Preece, S.J.; Kenney, L.P.; Major, M.J.; Dias, T.; Lay, E.; Fernandes, B.T. Automatic identification of gait events using an instrumented sock. J. Neuroeng. Rehabil. 2011, 8, 32. [Google Scholar] [CrossRef]
- Tirosh, O.; Begg, R.; Passmore, E.; Knopp-Steinberg, N. Wearable textile sensor sock for gait analysis. In Proceedings of the Seventh International Conference on Sensing Technology, Wellington, New Zealand, 3–5 December 2013. [Google Scholar]
- Bergmann, J.H.M.; Chandaria, V.; McGregor, A. Wearable and implantable sensors: The patient’s perspective. Sensors 2012, 12, 16695–16709. [Google Scholar] [CrossRef]
- Kavanagh, J.J.; Barrett, R.S.; Morrison, S. Upper body accelerations during walking in healthy young and elderly men. Gait Posture 2004, 20, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.N.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.-K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Natl. Acad. Sci. USA 2002, 99 (Suppl. 1), 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Kobsar, D.; Olson, C.; Paranjape, R.; Hadjistavropoulos, T.; Barden, J.M. Evaluation of age-related differences in the stride-to-stride fluctuations, regularity and symmetry of gait using a waist-mounted tri-axial accelerometer. Gait Posture 2014, 39, 553–557. [Google Scholar] [CrossRef]
- Sciurba, F.; Criner, G.J.; Lee, S.M.; Mohsenifar, Z.; Shade, D.; Slivka, W.; Wise, R.A. National Emphysema Treatment Trial Research Group. Six-minute Walk Distance in Chronic Obstructive Pulmonary Disease: Reproducibility and Effect of Walking Course Layout and Length. Am. J. Respir. Crit. Care Med. 2003, 167, 1522–1527. [Google Scholar] [CrossRef]
- Sun, R.; Sosnoff, J.J. Novel sensing technology in fall risk assessment in older adults: A systematic review. BMC Geriatr. 2014, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Howcroft, J.; Lemaire, E.D.; Kofman, J. Wearable-Sensor-Based Classification Models of Faller Status in Older Adults. PLoS ONE 2016, 11, e0153240. [Google Scholar] [CrossRef]
- Wall, J.C.; Bell, C.; Campbell, S.; Davis, J. The Timed Get-up-and-Go test revisited: Measurement of the component tasks. J. Rehabil. Res. Dev. 2000, 37, 109–113. [Google Scholar]
- Greene, B.R.; Doheny, E.P.; Kenny, R.A.; Caulfield, B. Classification of frailty and falls history using a combination of sensor-based mobility assessments. Physiol. Meas. 2014, 35, 2053–2066. [Google Scholar] [CrossRef]
- Greene, B.; Redmond, S.; Caulfield, B. Fall Risk Assessment through Automatic Combination of Clinical Fall Risk Factors and Body-Worn Sensor Data. IEEE J. Biomed. Health Inform. 2016, 21, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Luna-Perejón, F.; Domínguez-Morales, M.J.; Civit-Balcells, A. Wearable Fall Detector Using Recurrent Neural Networks. Sensors 2019, 19, 4885. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.L.; Endo, P.T.; Monteiro, K.H.C.; Rocha, E.S.; Silva, I.; Lynn, T. Accelerometer-Based Human Fall Detection Using Convolutional Neural Networks. Sensors 2019, 19, 1644. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Wang, Y. Human Fall Detection Based on Body Posture Spatio-Temporal Evolution. Sensors 2020, 20, 946. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.H.M.; McGregor, A.H. Body-worn sensor design: What do patients and clinicians want? Ann. Biomed. Eng. 2011, 39, 2299–2312. [Google Scholar] [CrossRef]
- Mokhlespour Esfahani, M.I.; Nussbaum, M.A. Classifying Diverse Physical Activities Using “Smart Garments”. Sensors 2019, 19, 3133. [Google Scholar] [CrossRef]
| t1 | t2 | |
|---|---|---|
| N | 80 | 73 |
| Age (years) | 83.2 ± 8.2 | 83.0 ± 8.3 |
| Male/Female | 28/52 | 28/45 |
| Walking aid required | 49 | 52 |
| Hypertension (%) | 44 | 42 |
| Number of medications | 4 [2–5] | 4 [2–5] |
| Cerebrovascular accident (%) | 10 | 10 |
| Dementia (%) | 14 | 16 |
| Previous heart surgery (%) | 21 | 23 |
| Diabetes (%) | 16 | 15 |
| Hip or knee replacement (%) | 16 | 16 |
| Fallers | 23 | |
| TUG (s) | 20 [17–27] | 17 [14–23] |
| Fallers | Nonfallers | p | |
|---|---|---|---|
| SDav (m/s2) | 0.0949 [0.0810–0.149] | 0.101 [0.0868–0.130] | 0.245 |
| SDaml (m/s2) | 0.0864 [0.0752–0.109] | 0.0950 [0.0747–0.109] | 0.891 |
| SDaap (m/s2) | 0.120 [0.0901–0.173] | 0.0900 [0.0753–0.120] | 0.010 |
| SDωv (°/s) | 17.4 [15.5–20.2] | 18.4 [15.0–21.9] | 0.957 |
| SDωml (°/s) | 15.8 [12.3–20.3] | 13.7 [11.1–19.2] | 0.480 |
| SDωap (°/s) | 8.29 [6.77–11.6] | 8.79 [7.44–12.6] | 0.487 |
| Dav | 1.78 [1.73–1.82] | 1.81 [1.77–1.85] | 0.044 |
| Daml | 1.78 [1.66–1.81] | 1.81 [1.77–1.83] | 0.088 |
| Daap | 1.73 [1.68–1.80] | 1.79 [1.73–1.83] | 0.072 |
| Dωv | 1.71 [1.67–1.76] | 1.74 [1.69–1.76] | 0.376 |
| Dωml | 1.74 [1.71–1.78] | 1.78 [1.72–1.82] | 0.098 |
| Dωap | 1.81 [1.75–1.83] | 1.82 [1.78–1.85] | 0.149 |
| TUG (s) | 23 [19–31] | 19 [16–25] | 0.035 |
| TUG | TUG+ | AI | |
|---|---|---|---|
| Se | 0.714 | 0.857 | 0.750 |
| Sp | 0.541 | 0.500 | 0.750 |
| LR+ | 1.56 | 1.71 | 3.00 |
| LR− | 0.529 | 0.286 | 0.333 |
| PPV | 0.481 | 0.778 | 0.750 |
| NPV | 0.761 | 0.632 | 0.750 |
| Acc | 0.657 | 0.739 | 0.750 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buisseret, F.; Catinus, L.; Grenard, R.; Jojczyk, L.; Fievez, D.; Barvaux, V.; Dierick, F. Timed Up and Go and Six-Minute Walking Tests with Wearable Inertial Sensor: One Step Further for the Prediction of the Risk of Fall in Elderly Nursing Home People. Sensors 2020, 20, 3207. https://doi.org/10.3390/s20113207
Buisseret F, Catinus L, Grenard R, Jojczyk L, Fievez D, Barvaux V, Dierick F. Timed Up and Go and Six-Minute Walking Tests with Wearable Inertial Sensor: One Step Further for the Prediction of the Risk of Fall in Elderly Nursing Home People. Sensors. 2020; 20(11):3207. https://doi.org/10.3390/s20113207
Chicago/Turabian StyleBuisseret, Fabien, Louis Catinus, Rémi Grenard, Laurent Jojczyk, Dylan Fievez, Vincent Barvaux, and Frédéric Dierick. 2020. "Timed Up and Go and Six-Minute Walking Tests with Wearable Inertial Sensor: One Step Further for the Prediction of the Risk of Fall in Elderly Nursing Home People" Sensors 20, no. 11: 3207. https://doi.org/10.3390/s20113207
APA StyleBuisseret, F., Catinus, L., Grenard, R., Jojczyk, L., Fievez, D., Barvaux, V., & Dierick, F. (2020). Timed Up and Go and Six-Minute Walking Tests with Wearable Inertial Sensor: One Step Further for the Prediction of the Risk of Fall in Elderly Nursing Home People. Sensors, 20(11), 3207. https://doi.org/10.3390/s20113207







