Validation of a Single RGB-D Camera for Gait Assessment of Polyneuropathy Patients
Abstract
:1. Introduction
2. Background and Related Work
3. Materials and Methods
3.1. Participants
3.2. Experimental Setup and Protocol
3.3. Data Acquisition and Pre-Processing
3.4. Gait Cycle Phases Detection
3.5. Gait Parameter Computation
3.6. Validation of the Kinect v2
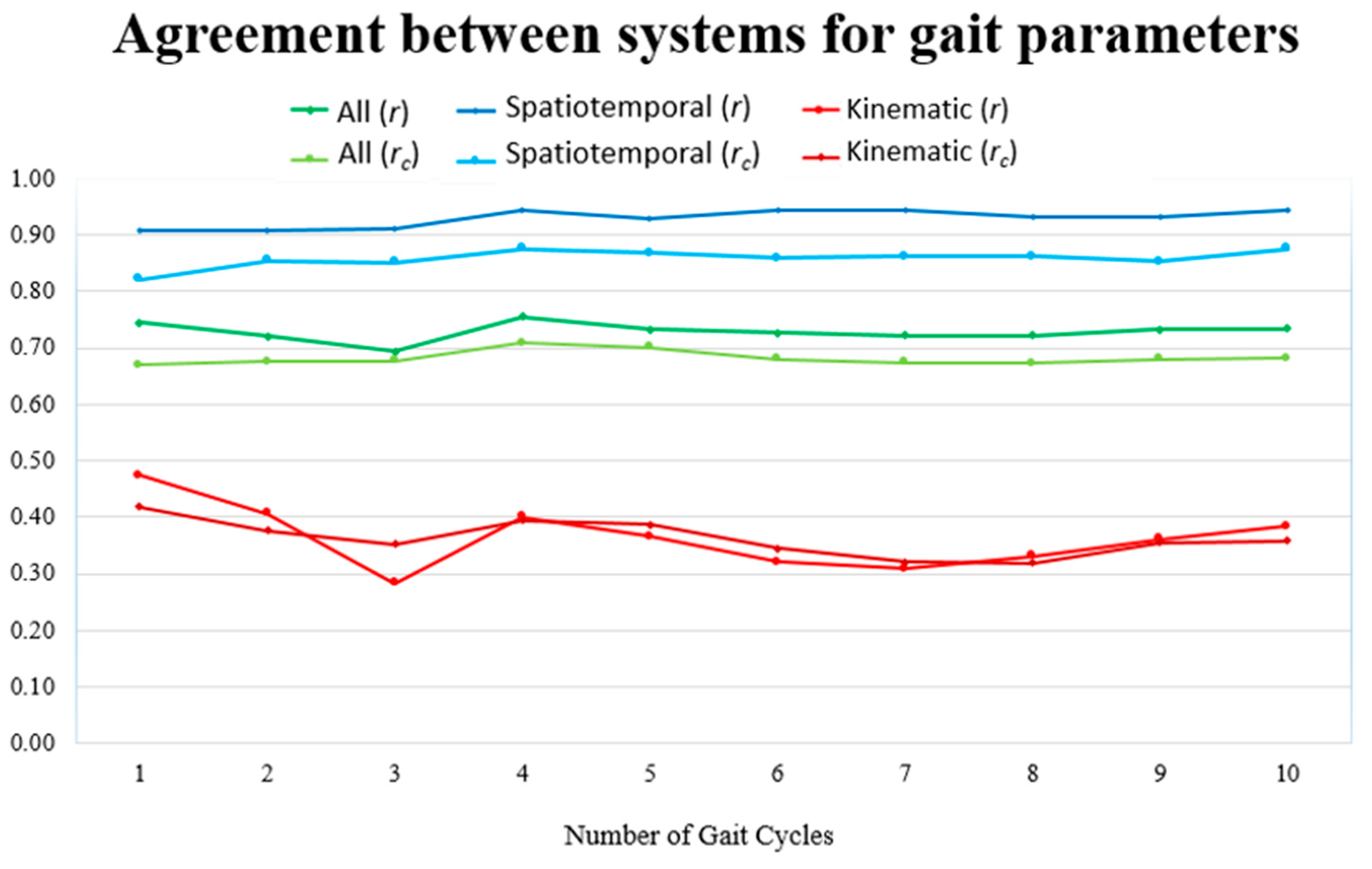
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plante-Bordeneuve, V. Transthyretin familial amyloid polyneuropathy: An update. J. Neurol. 2018, 265, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, P.; Silva, A.L.d.; Lopes, J.; Resende, P.-E.; Silva, A.M.d.; Resende, L.A.L.; Silva, A.M.d.; Lima, J.L.; Barbosa, A.R. Forty Years of Experience with Type 1 Amyloid Neuropathy: Review of 483 Cases; Excerpta Medica: Amsterdam, The Netherland, 1980. [Google Scholar]
- Adams, D.; Coelho, T.; Obici, L.; Merlini, G.; Mincheva, Z.; Suanprasert, N.; Bettencourt, B.R.; Gollob, J.A.; Gandhi, P.J.; Litchy, W.J.; et al. Rapid progression of familial amyloidotic polyneuropathy. Neurology 2015, 85, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Plante-Bordeneuve, V.; Gorram, F.; Salhi, H.; Nordine, T.; Ayache, S.S.; Le Corvoisier, P.; Azoulay, D.; Feray, C.; Damy, T.; Lefaucheur, J.P. Long-term treatment of transthyretin familial amyloid polyneuropathy with tafamidis: A clinical and neurophysiological study. J. Neurol. 2017, 264, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Buades, J.; Suhr, O.; Obici, L.; Coelho, T. Preliminary assessment of neuropathy progression in patients with hereditary ATTR amyloidosis after orthotopic liver transplantation (OLT). Orphanet J. Rare Dis. 2015, 10, P19. [Google Scholar] [CrossRef]
- Simon, S.R. Quantification of human motion: Gait analysis-benefits and limitations to its application to clinical problems. J. Biomech. 2004, 37, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, M.C.; Rocha, A.P.; Choupina, H.M.P.; Fernandes, J.M.; Coelho, T.; Cunha, J.P.S. The first Transthyretin Familial Amyloid Polyneuropathy gait quantification study-preliminary results. In Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE, Ed.; IEEE: Seogwipo, Korea, 2017; pp. 1368–1371. [Google Scholar]
- Rocha, A.P.; Choupina, H.M.P.; Fernandes, J.M.; Rosas, M.J.; Vaz, R.; Cunha, J.P.S. Kinect v2 Based System for Parkinson’s Disease Assessment. In 7th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE, Ed.; IEEE: Milan, Italy, 2015; pp. 1279–1282. [Google Scholar]
- Vilas-Boas, M.d.C.; Choupina, H.M.P.; Rocha, A.P.; Fernandes, J.M.; Cunha, J.P.S. Full-body motion assessment: Concurrent validation of two body tracking depth sensors versus a gold standard system during gait. J. Biomech. 2019, 87, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.P.; Choupina, H.M.P.; Vilas-Boas, M.C.; Fernandes, J.M.; Cunha, J.P.S. System for automatic gait analysis based on a single RGB-D camera. PLoS ONE 2018, 13, e0201728. [Google Scholar] [CrossRef] [PubMed]
- Castelli, A.; Paolini, G.; Cereatti, A.; Croce, U.D. A 2D markerless gait analysis methodology: Validation on healthy subjects. Comput. Math. Methods Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, M.C.; Cunha, J.P.S. Movement quantification in neurological diseases: Methods and applications. IEEE Rev. Biomed. Eng. 2016, 9, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Mündermann, L.; Corazza, S.; Chaudhari, A.M.; Andriacchi, T.P.; Sundaresan, A.; Chellappa, R. Measuring human movement for biomechanical applications using markerless motion capture. In SPIE 6056, Three-Dimensional Image Capture and Applications, 7th ed.; Corner, B.D., Li, P., Tocheri, M., Eds.; The International Society for Optical Engineering: San Jose, CA, USA, 2006; Volume 6056, p. 60560R. [Google Scholar]
- Clark, R.A.; Mentiplay, B.F.; Hough, E.; Pua, Y.H. Three-dimensional cameras and skeleton pose tracking for physical function assessment: A review of uses, validity, current developments and Kinect alternatives. Gait Posture 2018, 68, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Yogev Seligmann, G. Validity of the Kinect for gait assessment: A focused review. Sensors 2016, 16, 194. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; McGorry, R.W. The validity of the first and second generation Microsoft Kinect for identifying joint center locations during static postures. Appl. Ergon. 2015, 49, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Pua, Y.H.; Oliveira, C.C.; Bower, K.J.; Thilarajah, S.; McGaw, R.; Hasanki, K.; Mentiplay, B.F. Reliability and concurrent validity of the Microsoft Xbox One Kinect for assessment of standing balance and postural control. Gait Posture 2015, 42, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Bower, K.J.; Mentiplay, B.F.; Paterson, K.; Pua, Y.H. Concurrent validity of the Microsoft Kinect for assessment of spatiotemporal gait variables. J. Biomech. 2013, 46, 2722–2725. [Google Scholar] [CrossRef] [PubMed]
- Mentiplay, B.F.; Perraton, L.G.; Bower, K.J.; Pua, Y.H.; McGaw, R.; Heywood, S.; Clark, R.A. Gait assessment using the Microsoft Xbox One Kinect: Concurrent validity and inter-day reliability of spatiotemporal and kinematic variables. J. Biomech. 2015, 48, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Pfister, A.; West, A.M.; Bronner, S.; Noah, J.A. Comparative abilities of Microsoft Kinect and Vicon 3D motion capture for gait analysis. J. Med. Eng. Technol. 2014, 38, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Behrens, J.; Pfüller, C.; Mansow-Model, S.; Otte, K.; Paul, F.; Brandt, A.U. Using perceptive computing in multiple sclerosis the Short Maximum Speed Walk test. J. Neuroeng. Rehabil. 2014, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Behrens, J.R.; Mertens, S.; Kruger, T.; Grobelny, A.; Otte, K.; Mansow-Model, S.; Gusho, E.; Paul, F.; Brandt, A.U.; Schmitz-Hubsch, T. Validity of visual perceptive computing for static posturography in patients with multiple sclerosis. Mult. Scler. 2016, 22, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Grobelny, A.; Behrens, J.R.; Mertens, S.; Otte, K.; Mansow-Model, S.; Kruger, T.; Gusho, E.; Bellmann-Strobl, J.; Paul, F.; Brandt, A.U.; et al. Maximum walking speed in multiple sclerosis assessed with visual perceptive computing. PLoS ONE 2017, 12, e0189281. [Google Scholar] [CrossRef] [PubMed]
- Galna, B.; Barry, G.; Jackson, D.; Mhiripiri, D.; Olivier, P.; Rochester, L. Accuracy of the Microsoft Kinect sensor for measuring movement in people with Parkinson’s disease. Gait Posture 2014, 39, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.P.; Choupina, H.M.; Rocha, A.P.; Fernandes, J.M.; Achilles, F.; Loesch, A.M.; Vollmar, C.; Hartl, E.; Noachtar, S. NeuroKinect: A Novel Low-Cost 3D video-EEG System for Epileptic Seizure Motion Quantification. PLoS ONE 2016, 11, e0145669. [Google Scholar] [CrossRef] [PubMed]
- Kinect for Windows SDK 2.0-Reference-CameraSpacePoint Structure. Available online: https://docs.microsoft.com/en-us/previous-versions/windows/kinect/dn758354(v%3Dieb.10) (accessed on 23 October 2019).
- O’Connor, C.M.; Thorpe, S.K.; O’Malley, M.J.; Vaughan, C.L. Automatic detection of gait events using kinematic data. Gait Posture 2007, 25, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Yeung, L.F.; Cheng, K.C.; Fong, C.H.; Lee, W.C.; Tong, K.Y. Evaluation of the Microsoft Kinect as a clinical assessment tool of body sway. Gait Posture 2014, 40, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- King, T.S.; Chinchilli, V.M.; Carrasco, J.L. A repeated measures concordance correlation coefficient. Stat. Med. 2007, 26, 3095–3113. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.L.; Phillips, B.R.; Puig-Martinez, J.; King, T.S.; Chinchilli, V.M. Estimation of the concordance correlation coefficient for repeated measures using SAS and R. Comput. Methods Programs. Biomed. 2013, 109, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd Revised ed.; F.A. Davis Company: Philadelphia, PA, USA, 2015; p. 912. [Google Scholar]
- Ludbrook, J. Confidence in Altman-Bland plots: A critical review of the method of differences. Clin. Exp. Pharm. Physiol. 2010, 37, 143–149. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Carrasco, J.L.; Martinez, J.P. cccrm: Concordance Correlation Coefficient for Repeated (and Non-Repeated) Measures. 2015. Available online: https://CRAN.R-project.org/package=cccrm (accessed on 15 September 2019).
- Microsoft Azure Kinect DK. Available online: https://azure.microsoft.com/en-in/services/kinect-dk/ (accessed on 15 September 2019).
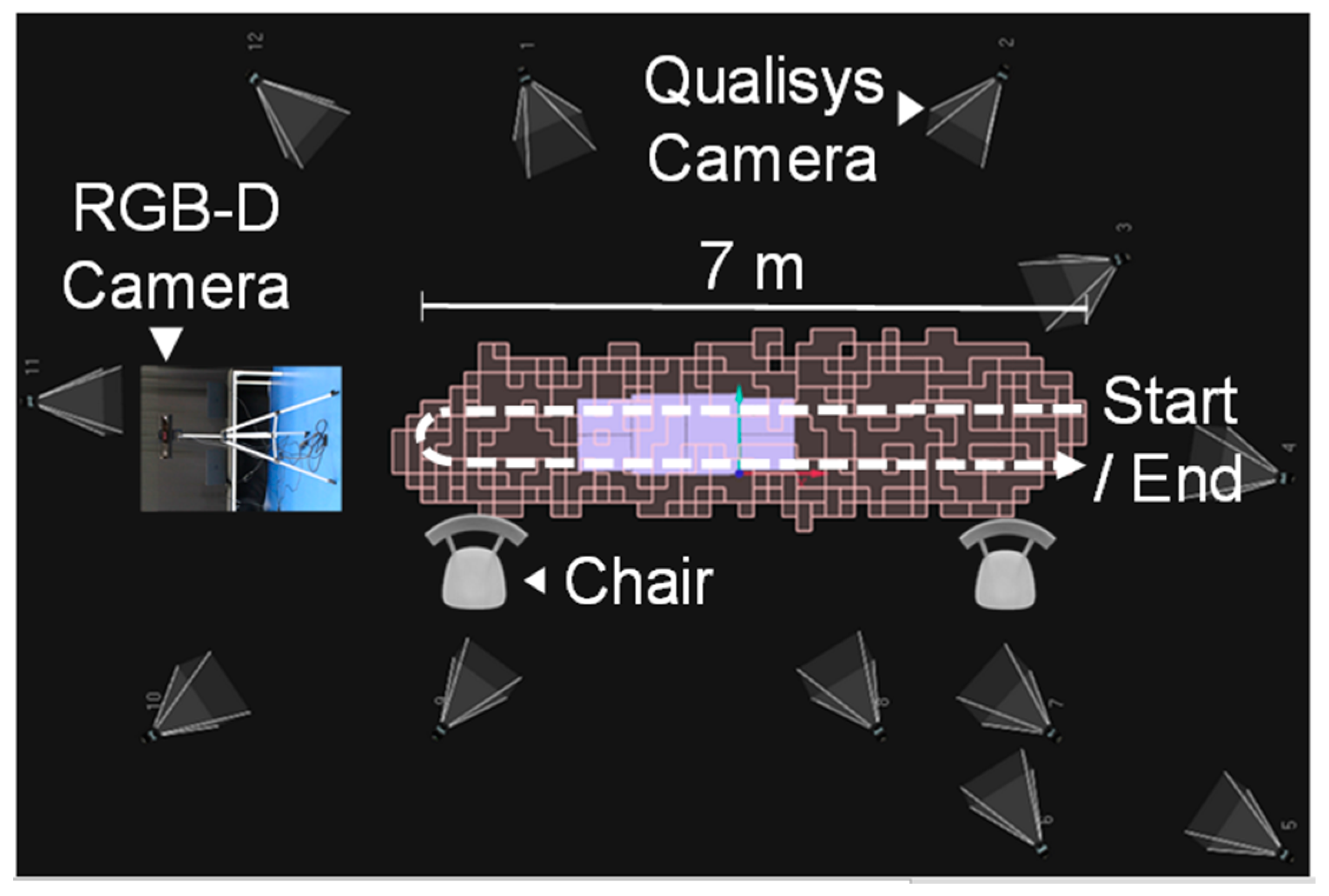

| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Age (years) | 46 ± 7 | 33 | 59 |
| Height (m) | 1.70 ± 0.1 | 1.48 | 1.82 |
| Weight (kg) | 66.0 ± 14.9 | 49.0 | 99.3 |
| Gait Parameter | Definition |
|---|---|
| Stride duration | Duration of the gait cycle or stride |
| Step duration | Duration of the left/right step, when considering a left/right gait cycle |
| Stance duration | Duration of the stance phase |
| Swing duration | Duration of the swing phase |
| Single support duration | Duration of the single-limb support phase |
| Double support duration | Duration of the double-limb support phase |
| Stride length | Distance (in the xz-plane) between the position of the left/right ankle at the beginning and end of the left/right gait cycle |
| Step length | Distance (in the xz-plane) between the position of the left/right ankle at the instant during the swing phase when the ankle distance is minimum, and at the instant corresponding to the end of the left/right gait cycle |
| Step width | Minimum value of the distance between ankles (in the xz-plane) during the swing phase |
| Gait speed | Mean value of the trunk velocity (mean of velocity of the head, neck, spine shoulder, spine middle, and spine base joints) |
| Gait speed variability | Standard deviation of the trunk velocity |
| Foot swing velocity | Maximum value of the left/right ankle joint velocity during the swing phase, when considering a left/right gait cycle |
| Arm swing velocity | Maximum value of the left/right hand velocity (mean of velocity of the left/right wrist and hand joints) during the stance phase, when considering a left/right gait cycle |
| TBCM sway x/y-component | Measure of the body sway computed as the mean distance (in the x/y-axis) of the total body center of mass (TBCM), in relation to the RGB-D sensor’s coordinate system, for all gait cycle frames. For each frame, TBCM’s position is the mean position of all body segments’ CM, which was obtained according to [28] using the parameters indicated by Dempster in [29]. |
| Spine shoulder angle | Mean value of the angle at spine shoulder (defined by head, spine shoulder, and spine base joints), considering the whole gait cycle |
| Spine middle angle | Mean value of the angle at spine middle (defined by head, spine middle, and spine base joints), considering the whole gait cycle |
| Maximum elbow angle | Maximum value of angle at the left/right elbow (defined by left/right shoulder, elbow and wrist joints) during the stance phase, when considering a left/right gait cycle |
| Minimum elbow angle | Minimum value of angle at the left/right elbow during the stance phase, when considering a left/right gait cycle |
| Maximum knee angle | Maximum value of angle at the left/right knee (defined by left/right hip, knee, and ankle joints) during the stance phase, when considering a left/right gait cycle |
| Minimum knee angle | Minimum value of angle at the left/right knee during the swing phase, when considering a left/right gait cycle |
| Hip angle range | Difference between the maximum and minimum value for the left/right hip angle (defined by left/right knee joint, hip joint, and point with knee y-coordinate and hip x- and z-coordinates) during the stance and swing phases respectively, when considering a left/right gait cycle |
| Ankle angle range | Difference between the maximum and minimum value for left/right ankle (defined by left/right knee, ankle, and foot joints) during the swing and stance phases respectively, when considering a left/right gait cycle |
| Gait Parameter | Mean ± SD | Mean Diff | 95% LoA | r (95% CI) | rc (95% CI) | |
|---|---|---|---|---|---|---|
| Kinect | Qualisys | |||||
| Stride duration (s) | 1.284 ± 0.202 | 1.289 ± 0.206 | −0.005 | −0.053 to 0.042 | 0.99 * (0.97 to 1.00) | 0.99 (0.97 to 1.00) |
| Step duration (s) | 0.644 ± 0.111 | 0.651 ± 0.112 | −0.007 | −0.050 to 0.035 | 0.98 * (0.92 to 1.00) | 0.98 (0.93 to 0.99) |
| Stance duration (s) | 0.838 ± 0.143 | 0.890 ± 0.167 | −0.052 ~ | −0.126 to 0.022 | 0.98 * (0.92 to 1.00) | 0.92 (0.78 to 0.97) |
| Swing duration (s) | 0.446 ± 0.072 | 0.399 ± 0.054 | 0.047 ~ | −0.021 to 0.115 | 0.89 * (0.59 to 0.97) | 0.68 (0.31 to 0.87) |
| Single support duration (s) | 0.885 ± 0.126 | 0.789 ± 0.093 | 0.096 ~ | −0.051 to 0.242 | 0.81 * (0.37 to 0.95) | 0.57 (0.17 to 0.81) |
| Double support duration (s) | 0.399 ± 0.104 | 0.500 ± 0.149 | −0.101 ~ | −0.258 to 0.056 | 0.86 * (0.50 to 0.97) | 0.62 (0.23 to 0.84) |
| Stride length (cm) | 94.2 ± 14.3 | 94.0 ± 14.1 | 0.2 | −1.6 to 2.1 | 1.00 * (0.99 to 1.00) | 1.00 (0.99 to 1.00) |
| Step length (cm) | 43.6 ± 6.2 | 44.3 ± 5.4 | −0.7 | −5.3 to 3.8 | 0.93 * (0.72 to 0.98) | 0.92 (0.73 to 0.98) |
| Step width (cm) | 14.8 ± 2.8 | 14.6 ± 3.1 | 0.2 | −1.7 to 2.1 | 0.95 * (0.81 to 0.99) | 0.95 (0.82 to 0.99) |
| Gait speed (m/s) | 0.788 ± 0.200 | 0.773 ± 0.200 | 0.015 ~ | 0.001 to 0.029 | 1.00 * (1.00 to 1.00) | 1.00 (0.99 to 1.00) |
| Gait speed variability (m/s) | 0.118 ± 0.027 | 0.113 ± 0.027 | 0.005 | −0.014 to 0.025 | 0.93 * (0.74 to 0.98) | 0.92 (0.74 to 0.98) |
| Foot swing velocity (m/s) | 3.019 ± 0.479 | 2.934 ± 0.621 | 0.085 ^ | −0.323 to 0.493 | 0.96 * (0.84 to 0.99) | 0.93 (0.75 to 0.98) |
| Arm swing velocity (m/s) | 1.613 ± 0.387 | 1.608 ± 0.404 | 0.005 | −0.109 to 0.119 | 0.99 * (0.96 to 1.00) | 0.99 (0.96 to 1.00) |
| TBCM a sway x-component (mm2) | 0.471 ± 0.275 | 0.442 ± 0.304 | 0.029 | −0.172 to 0.230 | 0.94 * (0.77 to 0.99) | 0.94 (0.79 to 0.98) |
| TBCM a sway y-component (mm2) | 0.101 ± 0.053 | 0.146 ± 0.089 | −0.044 ~,^ | −0.122 to 0.033 | 0.97 * (0.87 to 0.99) | 0.73 (0.37 to 0.90) |
| Spine shoulder angle (deg.) | 173 ± 4 | 170 ± 6 | 3 ~,^ | −3 to 9 | 0.90 * (0.62 to 0.98) | 0.69 (0.30 to 0.88) |
| Spine middle angle (deg.) | 176 ± 2 | 162 ± 8 | 14 ~,^ | −5 to 33 | −0.51 (−0.86 to 0.18) | 0.00 (0.00 to 0.00) |
| Maximum elbow angle (deg.) | 155 ± 6 | 155 ± 4 | 0 | −9 to 9 | 0.67 * (0.06 to 0.91) | 0.60 (0.04 to 0.87) |
| Minimum elbow angle (deg.) | 137 ± 10 | 134 ± 9 | 2 | −7 to 11 | 0.90 * (0.62 to 0.98) | 0.88 (0.62 to 0.97) |
| Maximum knee angle (deg.) | 174 ± 2 | 174 ± 3 | 0 | −8 to 8 | −0.21 (−0.74 to 0.48) | 0.00 (0.00 to 0.00) |
| Minimum knee angle (deg.) | 130 ± 4 | 123 ± 7 | 8 ~,^ | −2 to 17 | 0.76 * (0.26 to 0.94) | 0.34 (0.00 to 0.62) |
| Hip angle range (deg.) | 14 ± 4 | 14 ± 5 | 0 | −11 to 10 | 0.30 (−0.40 to 0.78) | 0.30 (−0.33 to 0.75) |
| Ankle angle range (deg.) | 29 ± 7 | 32 ± 12 | −2 | −24 to 20 | 0.39 (−0.32 to 0.82) | 0.35 (−0.28 to 0.77) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, M.d.C.; Rocha, A.P.; Choupina, H.M.P.; Cardoso, M.N.; Fernandes, J.M.; Coelho, T.; Cunha, J.P.S. Validation of a Single RGB-D Camera for Gait Assessment of Polyneuropathy Patients. Sensors 2019, 19, 4929. https://doi.org/10.3390/s19224929
Vilas-Boas MdC, Rocha AP, Choupina HMP, Cardoso MN, Fernandes JM, Coelho T, Cunha JPS. Validation of a Single RGB-D Camera for Gait Assessment of Polyneuropathy Patients. Sensors. 2019; 19(22):4929. https://doi.org/10.3390/s19224929
Chicago/Turabian StyleVilas-Boas, Maria do Carmo, Ana Patrícia Rocha, Hugo Miguel Pereira Choupina, Márcio Neves Cardoso, José Maria Fernandes, Teresa Coelho, and João Paulo Silva Cunha. 2019. "Validation of a Single RGB-D Camera for Gait Assessment of Polyneuropathy Patients" Sensors 19, no. 22: 4929. https://doi.org/10.3390/s19224929
APA StyleVilas-Boas, M. d. C., Rocha, A. P., Choupina, H. M. P., Cardoso, M. N., Fernandes, J. M., Coelho, T., & Cunha, J. P. S. (2019). Validation of a Single RGB-D Camera for Gait Assessment of Polyneuropathy Patients. Sensors, 19(22), 4929. https://doi.org/10.3390/s19224929





