In Situ Crystalline Growth ZnS Nanoparticles on Conjugated Polymer for Enhancement of the Photocatalytic Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
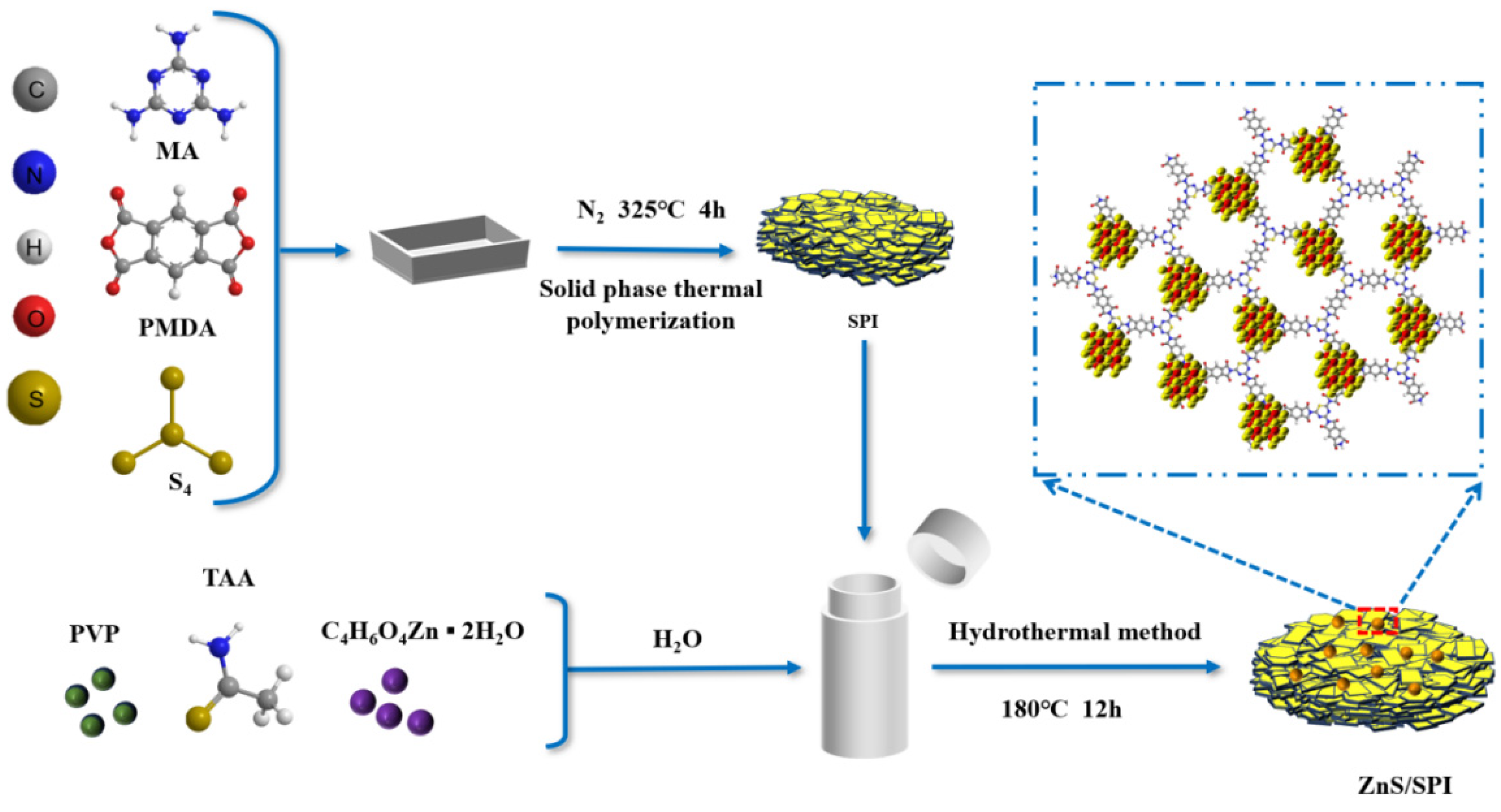
2.2. Synthesis of the Photocatalyst
2.3. Catalyst Characterization
2.4. Electrochemical Tests
2.5. Photocatalytic Performance Test
3. Results and Discussion
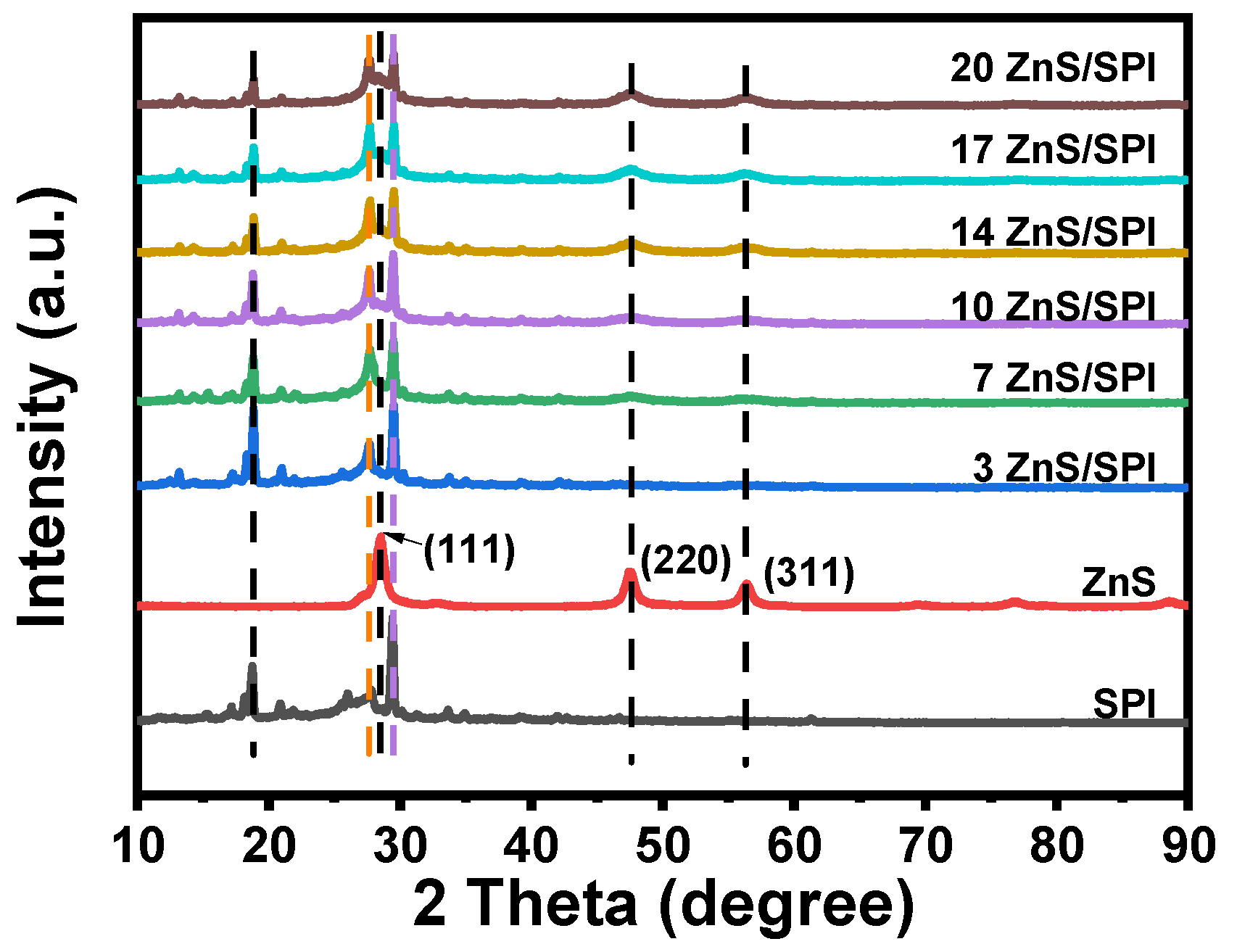
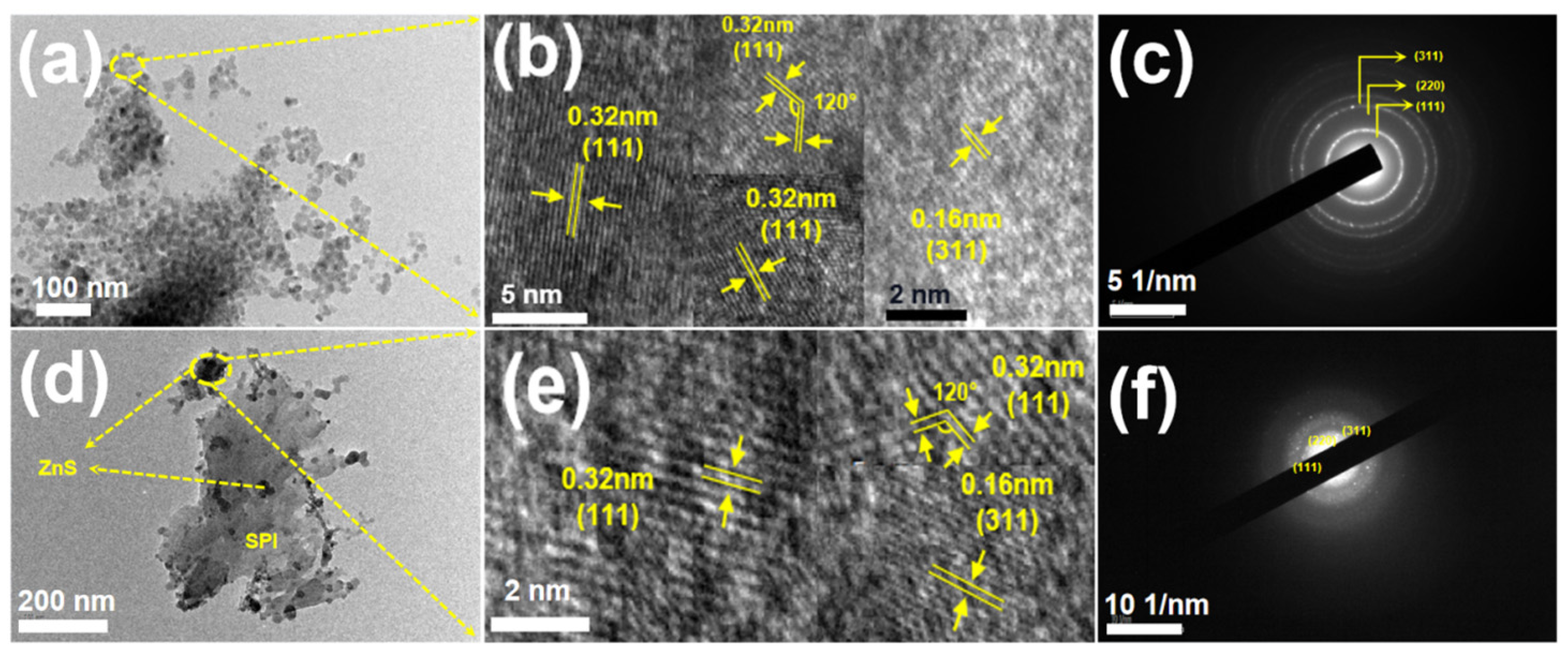
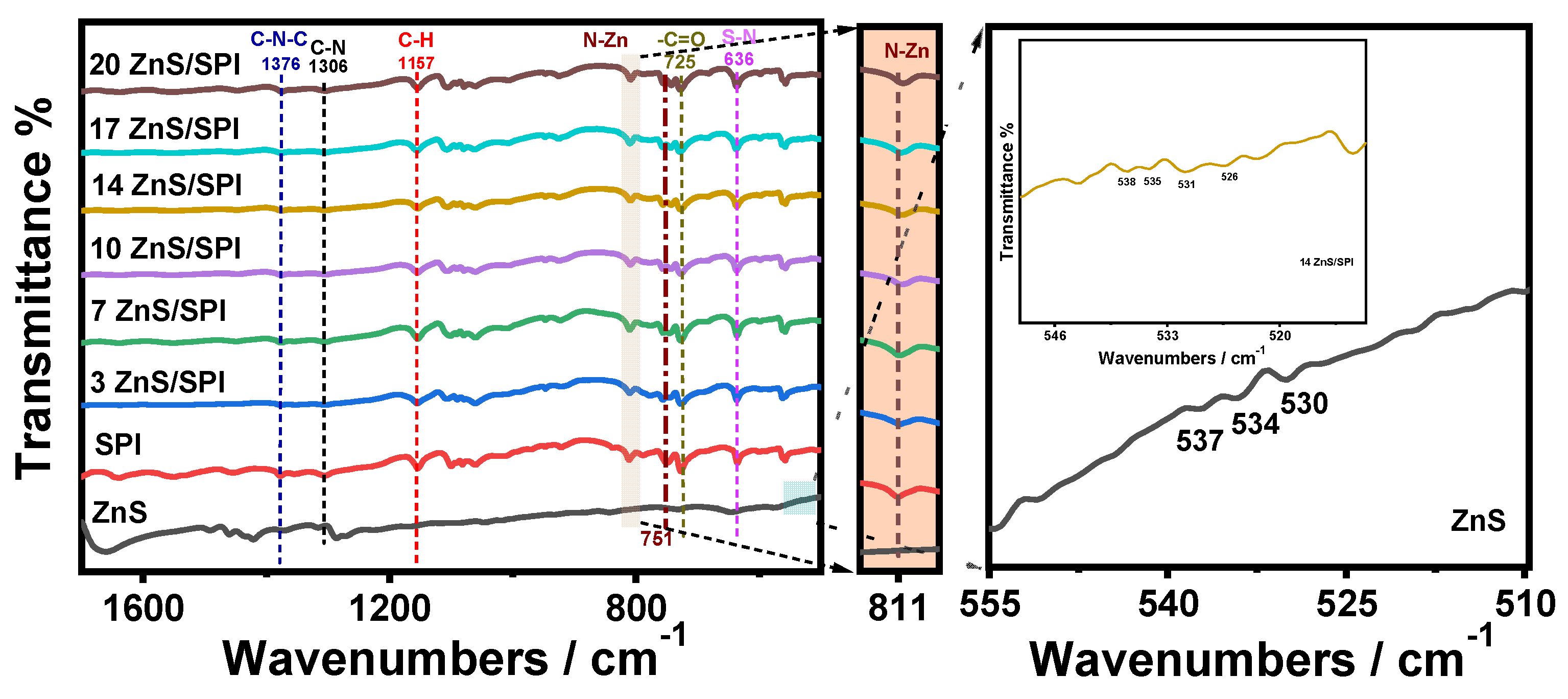
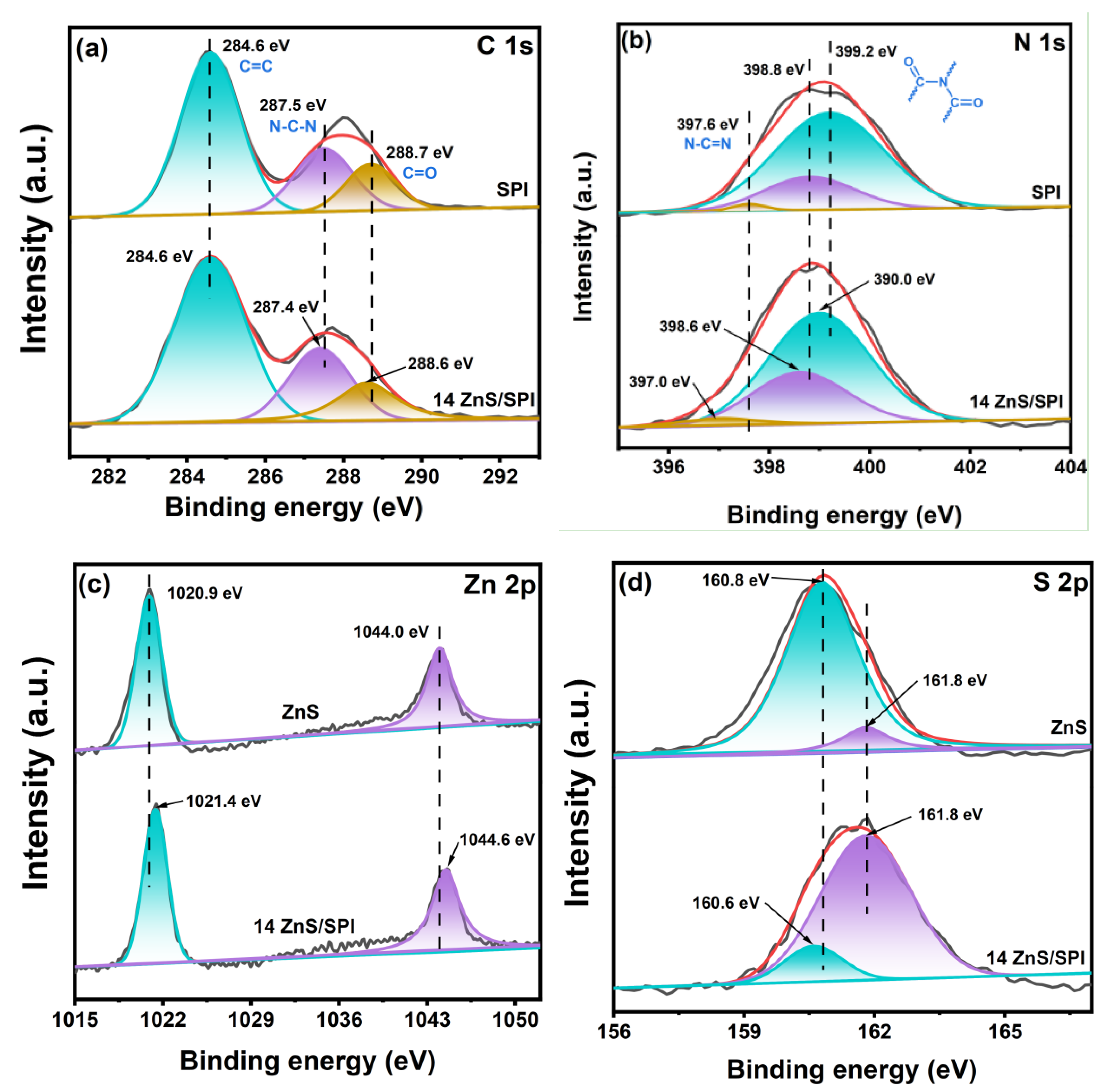
3.1. Structure and Morphology Analysis
3.2. Optical and Electronic Properties
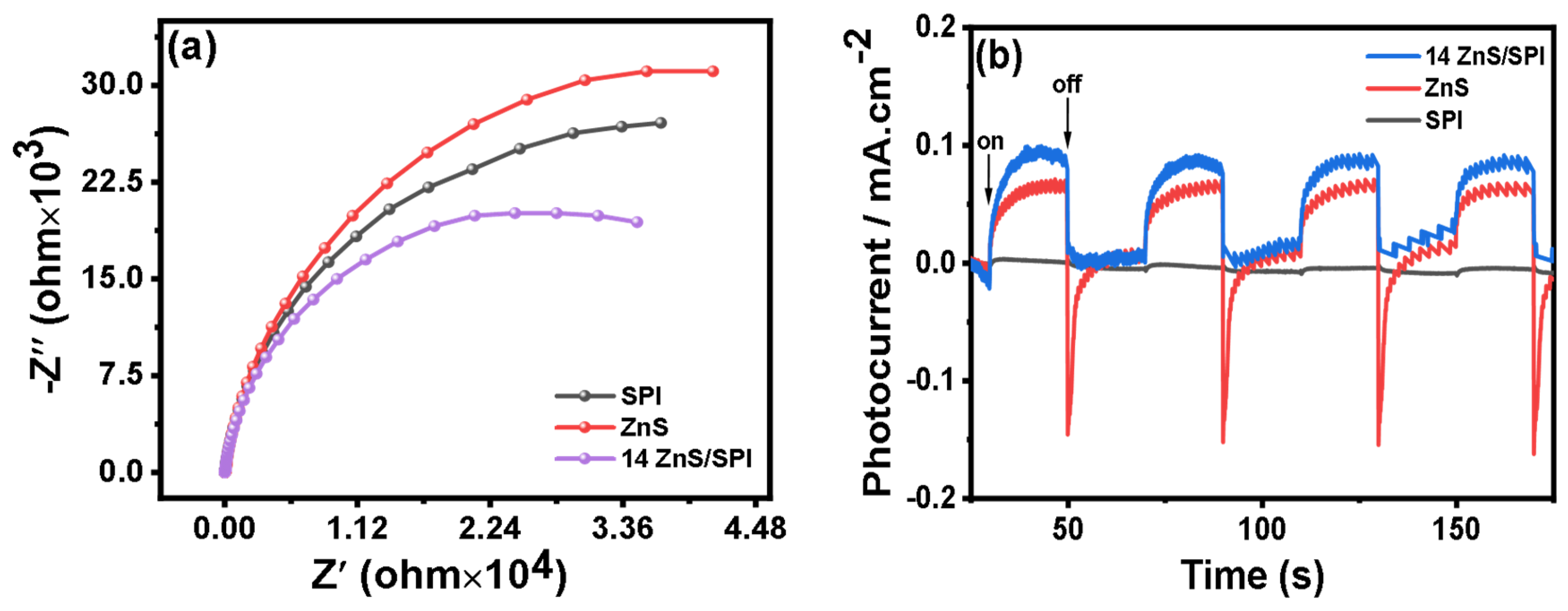
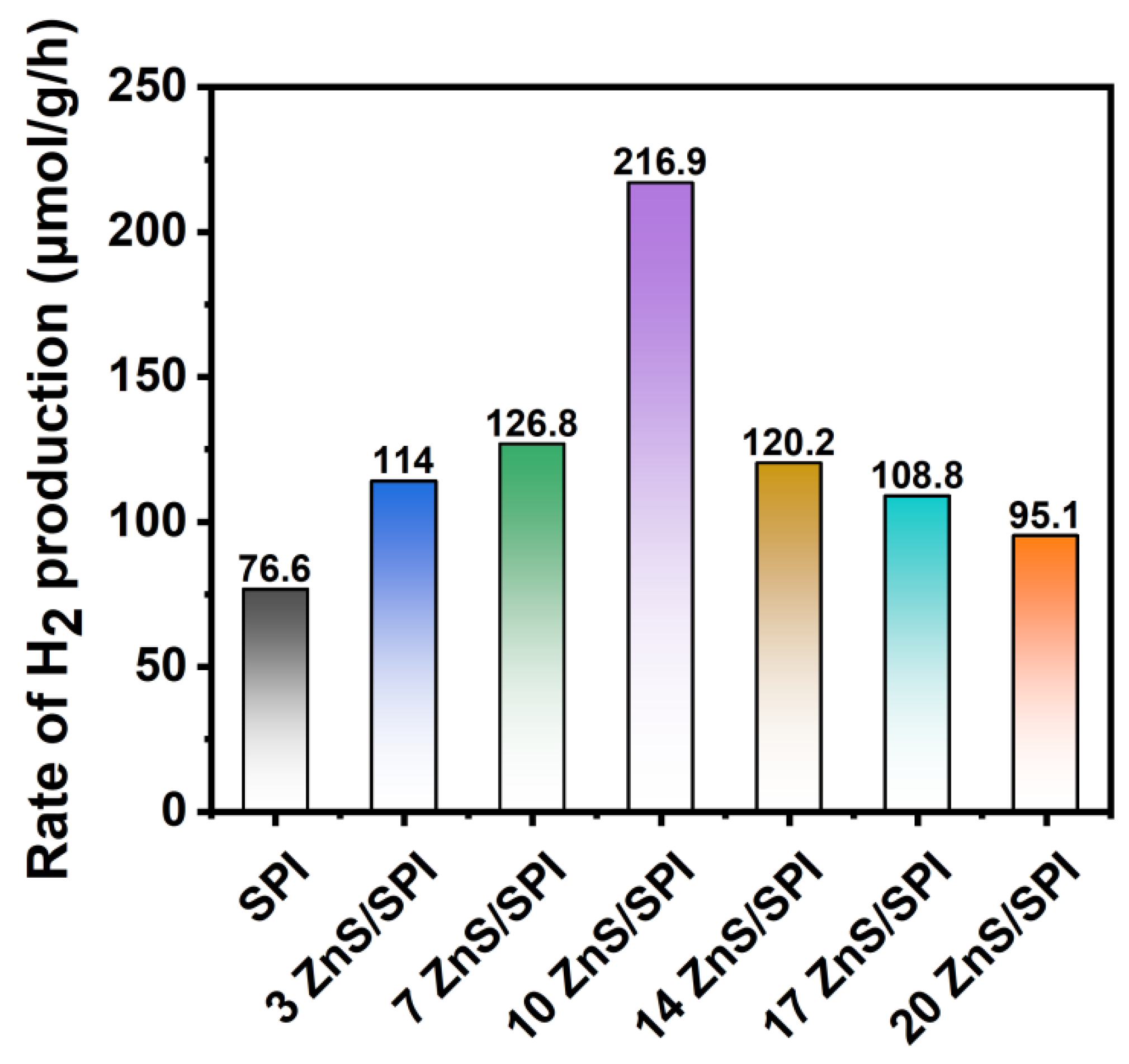
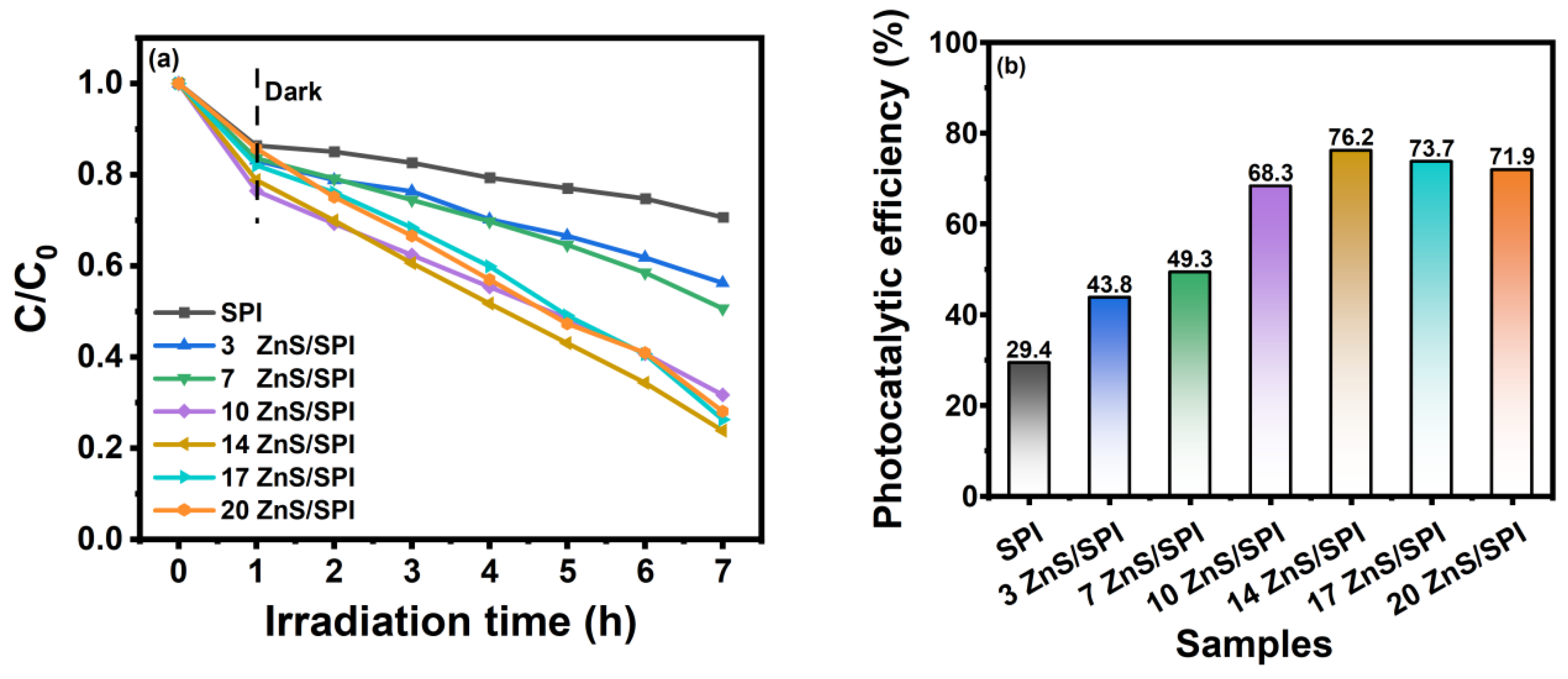
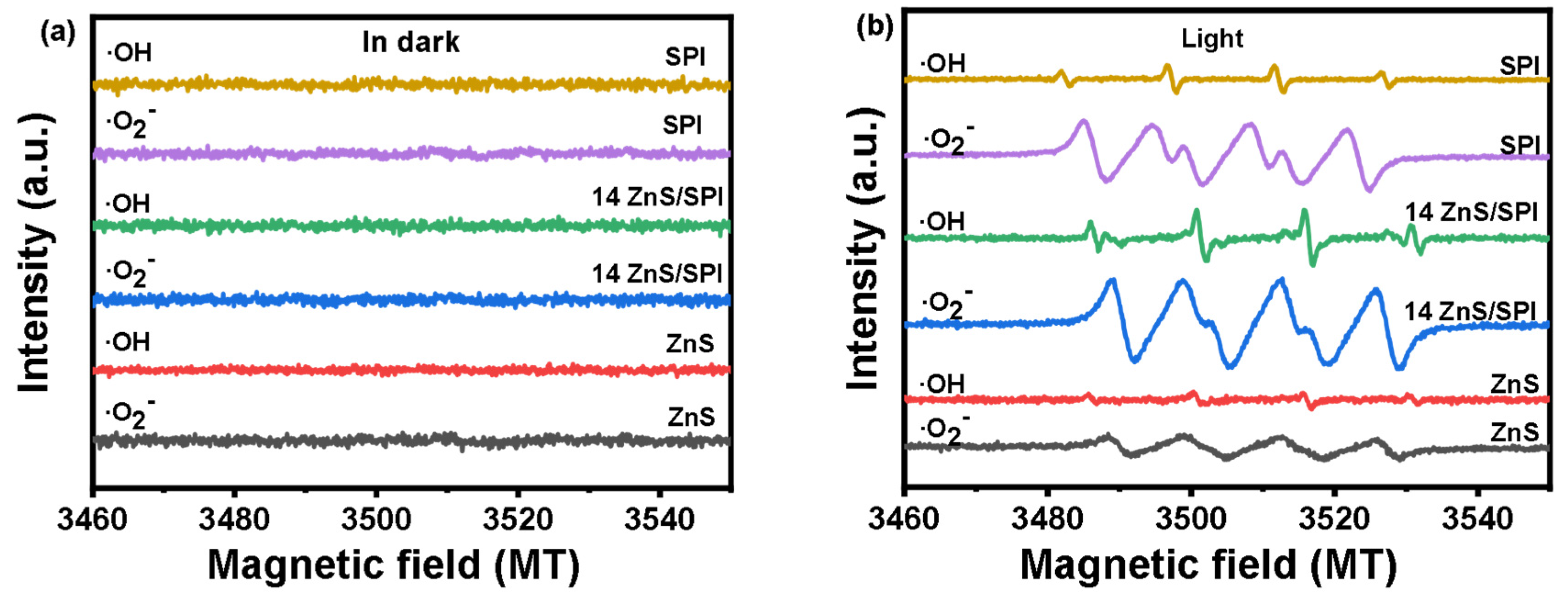
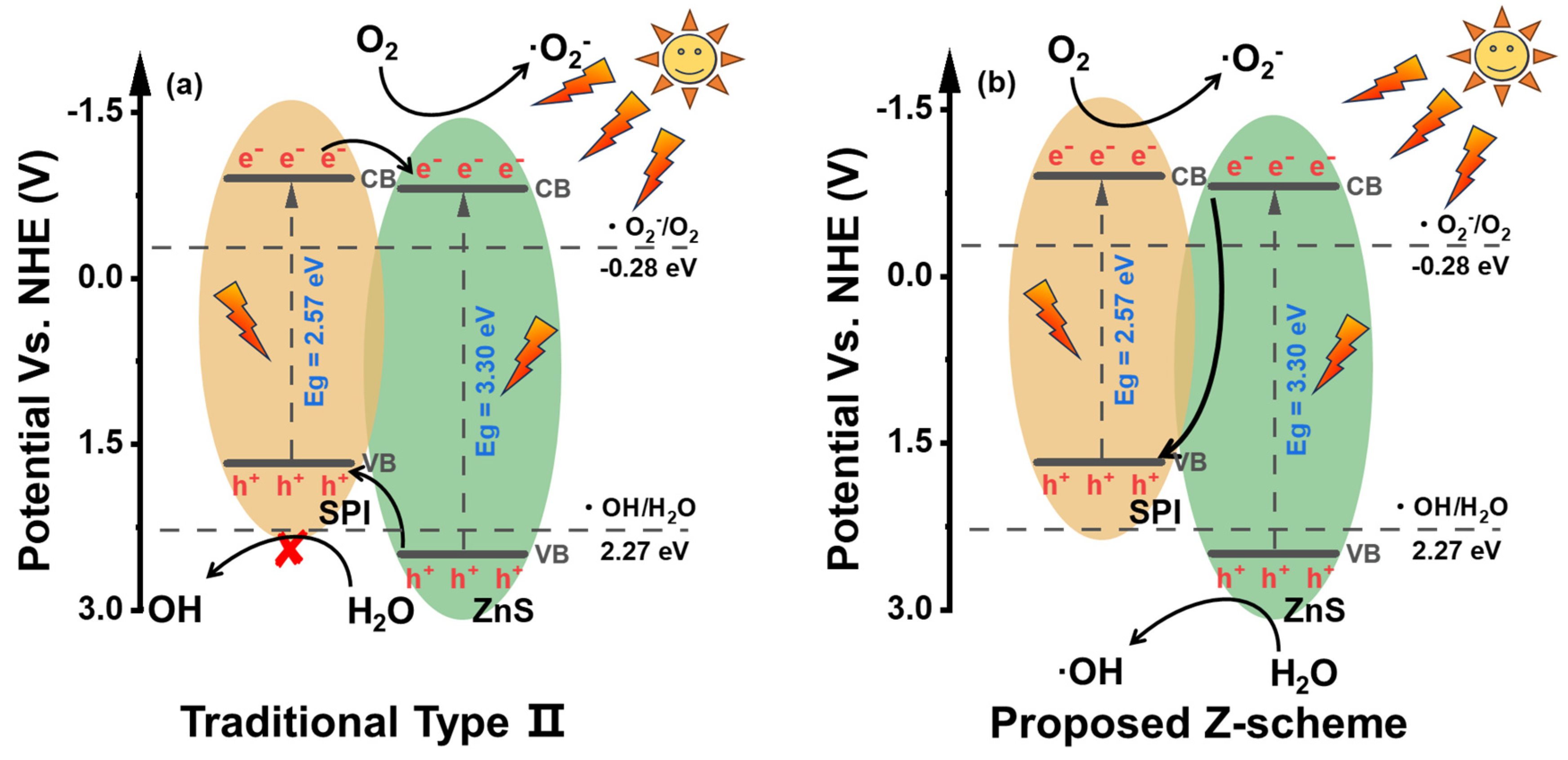
3.3. Photocatalytic Activity and Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Takata, T.; Domen, K. Particulate Photocatalysts for Overall Water Splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Xue, L.; Li, M.; Liu, L.; Hu, Y.; Cui, B.; Du, Y. Construction of the Novel Polyimide/Bi2MoO6-OVs p-n Type Heterojunction Aerogel Photocatalysts to Enhance the Photodegradation on Organic Pollutants Driven by the Internal Electric Field. J. Alloys Compd. 2022, 919, 165848. [Google Scholar] [CrossRef]
- Wei, G.; Niu, F.; Wang, Z.; Liu, X.; Feng, S.; Hu, K.; Gong, X.; Hua, J. Enhanced Photocatalytic H2 Evolution Based on a Polymer/TiO2 Film Heterojunction. Mater. Today Chem. 2022, 26, 101075. [Google Scholar] [CrossRef]
- Mannepalli, K.; Vinoth, K.; Mohapatra, S.K.; Rahul, R.; Gangodkar, D.P.; Madduri, A.; Ravichandran, M.; Sathyamurthy, R.; Mohanavel, V. Allocation of Optimal Energy from Storage Systems Using Solar Energy. Energy Rep. 2022, 8, 836–846. [Google Scholar] [CrossRef]
- Kumar, C.M.S.; Singh, S.; Gupta, M.K.; Nimdeo, Y.M.; Raushan, R.; Deorankar, A.V.; Kumar, T.M.A.; Rout, P.K.; Chanotiya, C.S.; Pakhale, V.D.; et al. Solar Energy: A Promising Renewable Source for Meeting Energy Demand in Indian Agriculture Applications. Sustain. Energy Technol. Assess. 2023, 55, 102905. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Yue, C.; He, L.; Li, H.; Zhang, H.; Yang, S.; Ma, T. Photocatalytic Degradation by TiO2-Conjugated/Coordination Polymer Heterojunction: Preparation, Mechanisms, and Prospects. Appl. Catal. B 2024, 344, 123605. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Tang, H.; Liu, Q.; Bahadur, I.; Karlapudi, S.; Jiang, Y. A Latest Overview on Photocatalytic Application of G-C3N4 Based Nanostructured Materials for Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 337–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Ding, Y.; Liu, B.; Zhao, L.; Zhang, S. Research Progress on g–C3N4–Based Photocatalysts for Organic Pollutants Degradation in Wastewater: From Exciton and Carrier Perspectives. Ceram. Int. 2021, 47, 31005–31030. [Google Scholar] [CrossRef]
- Xu, Q.; Xia, Z.; Zhang, J.; Wei, Z.; Guo, Q.; Jin, H.; Tang, H.; Li, S.; Pan, X.; Su, Z.; et al. Recent Advances in Solar-Driven CO2 Reduction over g-C3N4-Based Photocatalysts. Carbon Energy 2023, 5, e205. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Y.; Yang, Y.; Chu, S.; Zhou, C.; Wang, Y.; Zou, Z. Sulfur-Doped Polyimide Photocatalyst with Enhanced Photocatalytic Activity under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2014, 6, 4321–4328. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Sohail, M.; Anwar, U.; Taha, T.A.; Qazi, H.I.A.; Amina; Ajmal, Z.; Al-Sehemi, A.G.; Algarni, H.; Al-Ghamdi, A.A.; et al. A Targeted Review of Current Progress, Challenges and Future Perspective of g-C3N4 Based Hybrid Photocatalyst Toward Multidimensional Applications. Chem. Rec. 2023, 23, e202200143. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, D.; Ma, C.; Zhu, M.; Li, B.; Song, L.; Yang, S. Nanoarchitecture of a Two-Dimensional Few-Layer Graphene Oxide/π-Conjugated Polyimide Composite for Enhanced Photocatalytic Performance. ACS Omega 2022, 8, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jiang, M.; Yang, C.; Yang, Z.; Meng, W.; Zhou, L.; Sun, C.; Chen, W. Construction of α-Fe2O3/Sulfur-Doped Polyimide Direct Z-Scheme Photocatalyst with Enhanced Solar Light Photocatalytic Activity. ACS Omega 2022, 7, 11371–11381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, M.; Lang, Z.; Liu, J.; Liu, M.; Chang, J.; Li, L.; Shang, L.; Wang, M.; Li, S.; et al. Semiconductor/Covalent-Organic-Framework Z-Scheme Heterojunctions for Artificial Photosynthesis. Angew. Chem. 2020, 132, 6562–6568. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Chen, H.; Weng, Y.X.; Tang, H.; Chen, Z.; Zhu, W.; She, Y.; Xia, J.; Li, H. Unique Z-Scheme Carbonized Polymer Dots/Bi4O5Br2 Hybrids for Efficiently Boosting Photocatalytic CO2 Reduction. Appl. Catal. B 2021, 293, 120182. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. Zinc Vacancy-Promoted Photocatalytic Activity and Photostability of ZnS for Efficient Visible-Light-Driven Hydrogen Evolution. Appl. Catal. B 2018, 221, 302–311. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, D.; Ong, W.J. Interfacial Engineering of Graphitic Carbon Nitride (g-C3N4)-Based Metal Sulfide Heterojunction Photocatalysts for Energy Conversion: A Review. Cuihua Xuebao/Chin. J. Catal. 2019, 40, 289–319. [Google Scholar] [CrossRef]
- Lee, G.J.; Wu, J.J. Recent Developments in ZnS Photocatalysts from Synthesis to Photocatalytic Applications—A Review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review. Top. Curr. Chem. 2023, 381, 31. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Szabó-Bárdos, E.; Őze, C.; Juzsakova, T.; Rácz, K.; Németh, M.; Horváth, O. G-C3N4 Modified with Metal Sulfides for Visible-Light-Driven Photocatalytic Degradation of Organic Pollutants. Molecules 2025, 30, 253. [Google Scholar] [CrossRef]
- Hao, X.; Zhou, J.; Cui, Z.; Wang, Y.; Wang, Y.; Zou, Z. Zn-Vacancy Mediated Electron-Hole Separation in ZnS/g-C3N4 Heterojunction for Efficient Visible-Light Photocatalytic Hydrogen Production. Appl. Catal. B 2018, 229, 41–51. [Google Scholar] [CrossRef]
- Danish, M.; Muneer, M. Excellent Visible-Light-Driven Ni-ZnS/g-C3N4 Photocatalyst for Enhanced Pollutants Degradation Performance: Insight into the Photocatalytic Mechanism and Adsorption Isotherm. Appl. Surf. Sci. 2021, 563, 150262. [Google Scholar] [CrossRef]
- Zhu, B.; Zhou, J.; Ni, L.; Diao, G. Synthesis and Characterization of P-Doped g-C3N4 Nanosheet Hybridized ZnS Nanospheres with Enhanced Visible-Light Photocatalytic Activity. J. Solid State Chem. 2022, 305, 122703. [Google Scholar] [CrossRef]
- Chopan, N.A.; Chishti, H.T.N. Polypyrrole-Decorated ZnO/g-C3N4 S-Scheme Photocatalyst for Rhodamine B Dye Degradation: Mechanism and Antibacterial Activity. Mater. Today Chem. 2023, 32, 101643. [Google Scholar] [CrossRef]
- Wei, B.; Liang, H.; Wang, R.; Zhang, D.; Qi, Z.; Wang, Z. One-Step Synthesis of Graphitic-C3N4/ZnS Composites for Enhanced Supercapacitor Performance. J. Energy Chem. 2018, 27, 472–477. [Google Scholar] [CrossRef]
- Palanisamy, G.; Bhuvaneswari, K.; Pazhanivel, T.; Shankar, R.; Khadijah, K.M.; Alsalari, N.S.; Ouladsmane, M. ZnS Quantum Dots and Bi Metals Embedded with Two Dimensional β-Bi2O4 Nanosheets for Efficient UV-Visible Light Driven Photocatalysis. Mater. Res. Bull. 2021, 142, 111387. [Google Scholar] [CrossRef]
- Alafif, Z.O.; Anjum, M.; Ansari, M.O.; Kumar, R.; Rashid, J.; Madkour, M.; Barakat, M.A. Synthesis and Characterization of S-Doped-RGO/ZnS Nanocomposite for the Photocatalytic Degradation of 2-Chlorophenol and Disinfection of Real Dairy Wastewater. J. Photochem. Photobiol. A Chem. 2019, 377, 190–197. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, H.; Zhou, J.; Cui, Z.; Liu, T.; Wang, Y.; Wang, Y.; Zou, Z. Confinement Effect of Monolayer MoS2 Quantum Dots on Conjugated Polyimide and Promotion of Solar-Driven Photocatalytic Hydrogen Generation. Dalton Trans. 2017, 46, 3877–3886. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, P.; Zhang, G.; Hu, L.; Wang, P. Visible-Light Responsive g-C3N4 Coupled with ZnS Nanoparticles via a Rapid Microwave Route: Characterization and Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2019, 488, 360–369. [Google Scholar] [CrossRef]
- Yang, C.; Ma, C.; Zhang, D.; Luo, Z.; Zhu, M.; Li, B.; Zhang, Y.; Wang, J. Construction of Direct Z−Scheme SnS2 Quantum Dots/Conjugated Polyimide with Superior Photocarrier Separation for Enhanced Photocatalytic Performances. Polymers 2022, 14, 5483. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Li, K.; Jin, F.L. Synthesis and Characterization of Hyper-Branched Polyimides from 2,4,6-Triaminopyrimidine and Dianhydrides System. Mater. Chem. Phys. 2008, 108, 214–219. [Google Scholar] [CrossRef]
- Manecke, V.G.; Wöhrle, D. Synthese Und Halbleitereigenschaften Einiger Komplexe Und Der Aus Ihnen Hergestellten Polymeren. Teil 2. Polymere Mit Phthalocyaninartiger Und Triazinartiger Stuktur. Die Makromol. Chem. 1968, 120, 176–191. [Google Scholar] [CrossRef]
- Song, Y.; Gu, J.; Xia, K.; Yi, J.; Chen, H.; She, X.; Chen, Z.; Ding, C.; Li, H.; Xu, H. Construction of 2D SnS2/g-C3N4 Z-Scheme Composite with Superior Visible-Light Photocatalytic Performance. Appl. Surf. Sci. 2019, 467–468, 56–64. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Wohlgemuth, S.A.; Thomas, A.; Antonietti, M. Ionothermal Route to Layered Two-Dimensional Polymer-Frameworks Based on Heptazine Linkers. Macromolecules 2010, 43, 6639–6645. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Cai, J.; Zhong, L.; Lang, Y.; Ma, Q. Z-Scheme g-C3N4/ZnS Heterojunction Photocatalyst: One-Pot Synthesis, Interfacial Structure Regulation, and Improved Photocatalysis Activity for Bisphenol A. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130027. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, M.; Wang, C.; Liu, E.; Hu, X.; Fan, J. Defected ZnS/Bulk g–C3N4 Heterojunction with Enhanced Photocatalytic Activity for Dyes Oxidation and Cr (VI) Reduction. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123861. [Google Scholar] [CrossRef]
- Lan, H.; Li, L.; An, X.; Liu, F.; Chen, C.; Liu, H.; Qu, J. Microstructure of Carbon Nitride Affecting Synergetic Photocatalytic Activity: Hydrogen Bonds vs. Structural Defects. Appl. Catal. B 2017, 204, 49–57. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Huang, Z.H.; Kang, F.; Yang, Q.H. Holey Graphitic Carbon Nitride Nanosheets with Carbon Vacancies for Highly Improved Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Li, Z.; Nan, Y.; Ding, F.; Shen, Y.; Jiang, Z. Thylakoid-Inspired Multishell g-C3N4 Nanocapsules with Enhanced Visible-Light Harvesting and Electron Transfer Properties for High-Efficiency Photocatalysis. ACS Nano 2017, 11, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, W.; Xu, R.; Shi, Y.; Zhang, B. Synthesis of Ultrathin CdS Nanosheets as Efficient Visible-Light-Driven Water Splitting Photocatalysts for Hydrogen Evolution. Chem. Commun. 2013, 49, 9803–9805. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ye, P.; Cao, C.; Jin, Q.; Xu, G.S.; Shen, Y.H.; Yuan, Y.P. Rapid Microwave-Assisted Green Production of a Crystalline Polyimide for Enhanced Visible-Light-Induced Photocatalytic Hydrogen Production. J. Mater. Chem. A Mater. 2015, 3, 10205–10208. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Yang, L.; Zhang, M.; Zhu, H.; Wang, F.; Yin, J. Co3O4 Imbedded G-C3N4 Heterojunction Photocatalysts for Visible-Light-Driven Hydrogen Evolution. Renew. Energy 2020, 145, 691–698. [Google Scholar] [CrossRef]
- Suyana, P.; Ganguly, P.; Nair, B.N.; Mohamed, A.P.; Warrier, K.G.K.; Hareesh, U.S. Co3O4–C3N4 p–n Nano-Heterojunctions for the Simultaneous Degradation of a Mixture of Pollutants under Solar Irradiation. Environ. Sci. Nano 2017, 4, 212–221. [Google Scholar] [CrossRef]
- Zheng, J.; Lei, Z. Incorporation of CoO Nanoparticles in 3D Marigold Flower-like Hierarchical Architecture MnCo2O4 for Highly Boosting Solar Light Photo-Oxidation and Reduction Ability. Appl. Catal. B 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, Y.; Guo, Y.; Zhang, S.; Song, H.; Li, X.; Gao, Q.; Shang, W.; Hu, S.; et al. MXene Ti3C2 Decorated G-C3N4/ZnO Photocatalysts with Improved Photocatalytic Performance for CO2 Reduction. Nano Mater. Sci. 2023, 5, 237–245. [Google Scholar] [CrossRef]
- Ma, J.; Miao, T.J.; Tang, J. Charge Carrier Dynamics and Reaction Intermediates in Heterogeneous Photocatalysis by Time-Resolved Spectroscopies. Chem. Soc. Rev. 2022, 51, 5777–5794. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, M.K.; Sheppard, L.R.; Bak, T.; Nowotny, J. Defect Chemistry of Titanium Dioxide. Application of Defect Engineering in Processing of TiO2-Based Photocatalysts. J. Phys. Chem. C 2008, 112, 5275–5300. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.; Yin, K.; Dong, S.; Ren, Z.; Yuan, F.; Yu, T.; Zou, Z.; Liu, J.M. Visible-Light Photocatalytic Properties of Weak Magnetic BiFeO3 Nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, C.; Luo, Z.; Zhu, M.; Li, B.; Zhou, L.; Zhang, G. Anchoring Co3O4 Nanoparticles on Conjugated Polyimide Ultrathin Nanosheets: Construction of a Z-Scheme Nano-Heterostructure for Enhanced Photocatalytic Performance. RSC Adv. 2023, 13, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhang, L. Efficient Visible Light Driven Photocatalytic Removal of RhB and NO with Low Temperature Synthesized in(OH)XSy Hollow Nanocubes: A Comparative Study. Environ. Sci. Technol. 2011, 45, 3027–3033. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-Scheme Photocatalysts: Principles, Synthesis, and Applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]


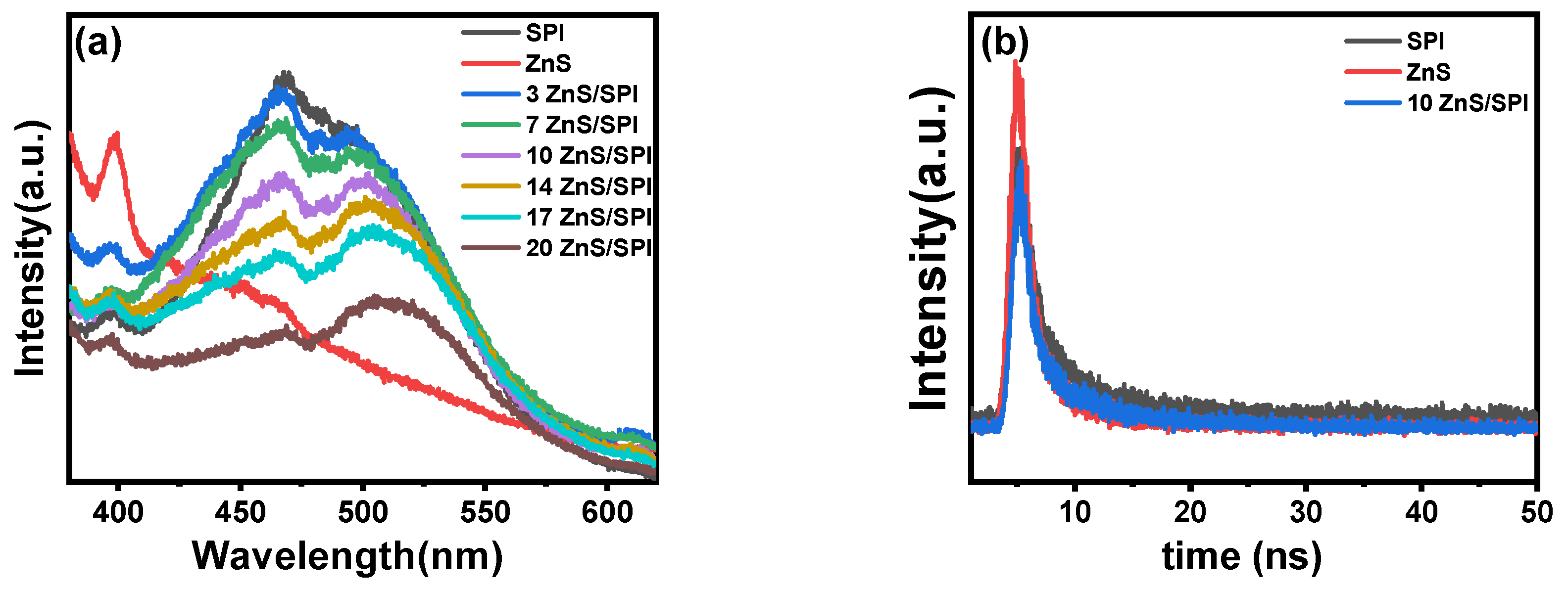
| Samples% | A1 (%) | τ1 (ns) | A2 (%) | τ2 (ns) | A3 (%) | τ3 (ns) | <τavg> (ns) |
|---|---|---|---|---|---|---|---|
| SPI | 204.13 | 1.66 | 53.95 | 7.99 | / | / | 5.21 |
| ZnS | 191.80 | 1.45 | 216.77 | 1.45 | 22.32 | 9.03 | 3.38 |
| 10 ZnS/SPI | 187.57 | 1.06 | 64.61 | 4.93 | 10.67 | 21.95 | 9.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Li, X.; Zhang, L.; Ma, C.; Chen, Y.; Wang, X.; Wei, H.; Wang, P. In Situ Crystalline Growth ZnS Nanoparticles on Conjugated Polymer for Enhancement of the Photocatalytic Performance. Polymers 2025, 17, 575. https://doi.org/10.3390/polym17050575
Liu B, Li X, Zhang L, Ma C, Chen Y, Wang X, Wei H, Wang P. In Situ Crystalline Growth ZnS Nanoparticles on Conjugated Polymer for Enhancement of the Photocatalytic Performance. Polymers. 2025; 17(5):575. https://doi.org/10.3390/polym17050575
Chicago/Turabian StyleLiu, Baotong, Xuelian Li, Long Zhang, Chenghai Ma, Ying Chen, Xinyu Wang, Hongli Wei, and Pengfei Wang. 2025. "In Situ Crystalline Growth ZnS Nanoparticles on Conjugated Polymer for Enhancement of the Photocatalytic Performance" Polymers 17, no. 5: 575. https://doi.org/10.3390/polym17050575
APA StyleLiu, B., Li, X., Zhang, L., Ma, C., Chen, Y., Wang, X., Wei, H., & Wang, P. (2025). In Situ Crystalline Growth ZnS Nanoparticles on Conjugated Polymer for Enhancement of the Photocatalytic Performance. Polymers, 17(5), 575. https://doi.org/10.3390/polym17050575







