Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide
Abstract
1. Introduction
2. Results
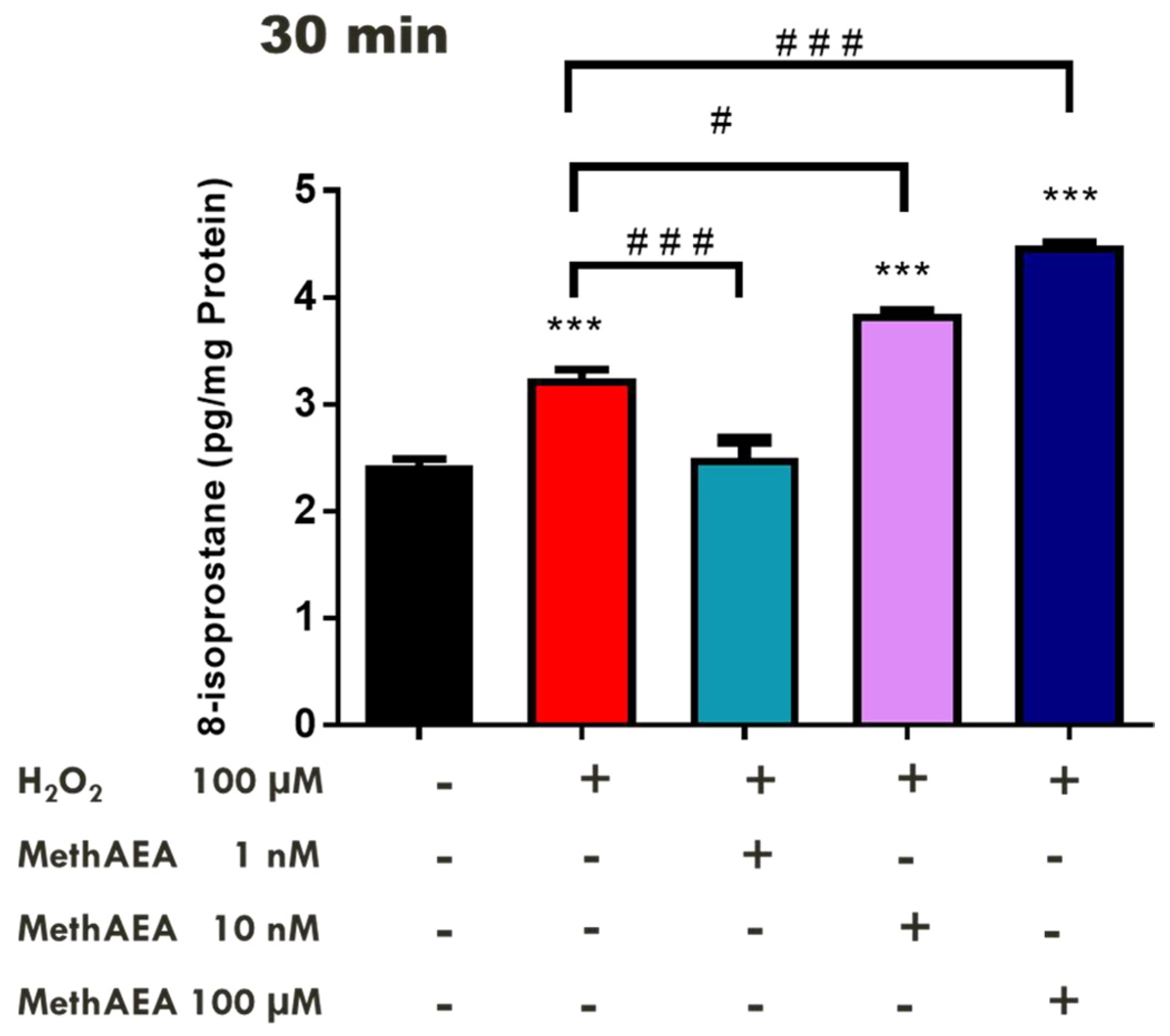
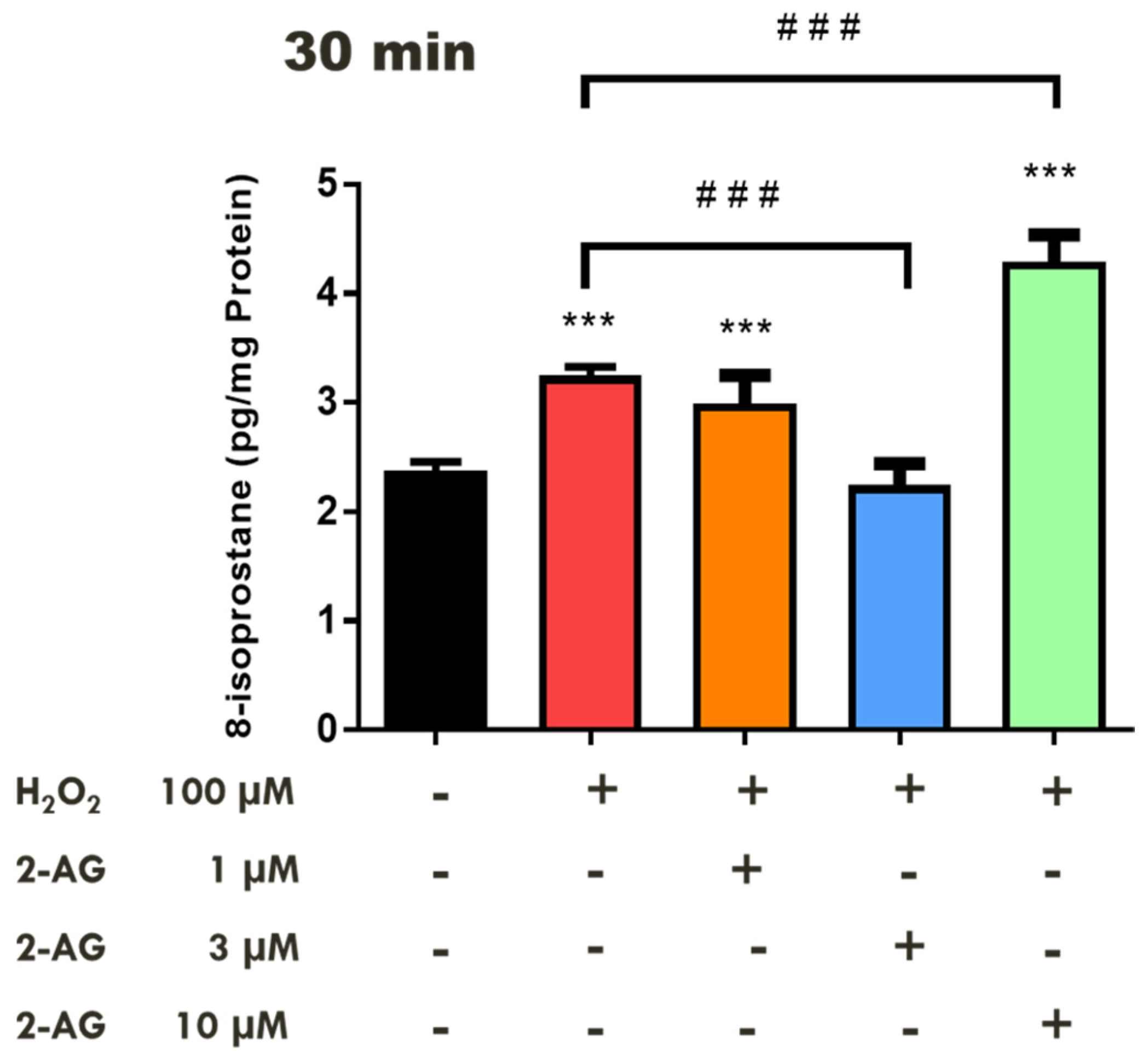
2.1. Effects of Cannabinoids on Lipid Peroxidation in the Bovine Retina
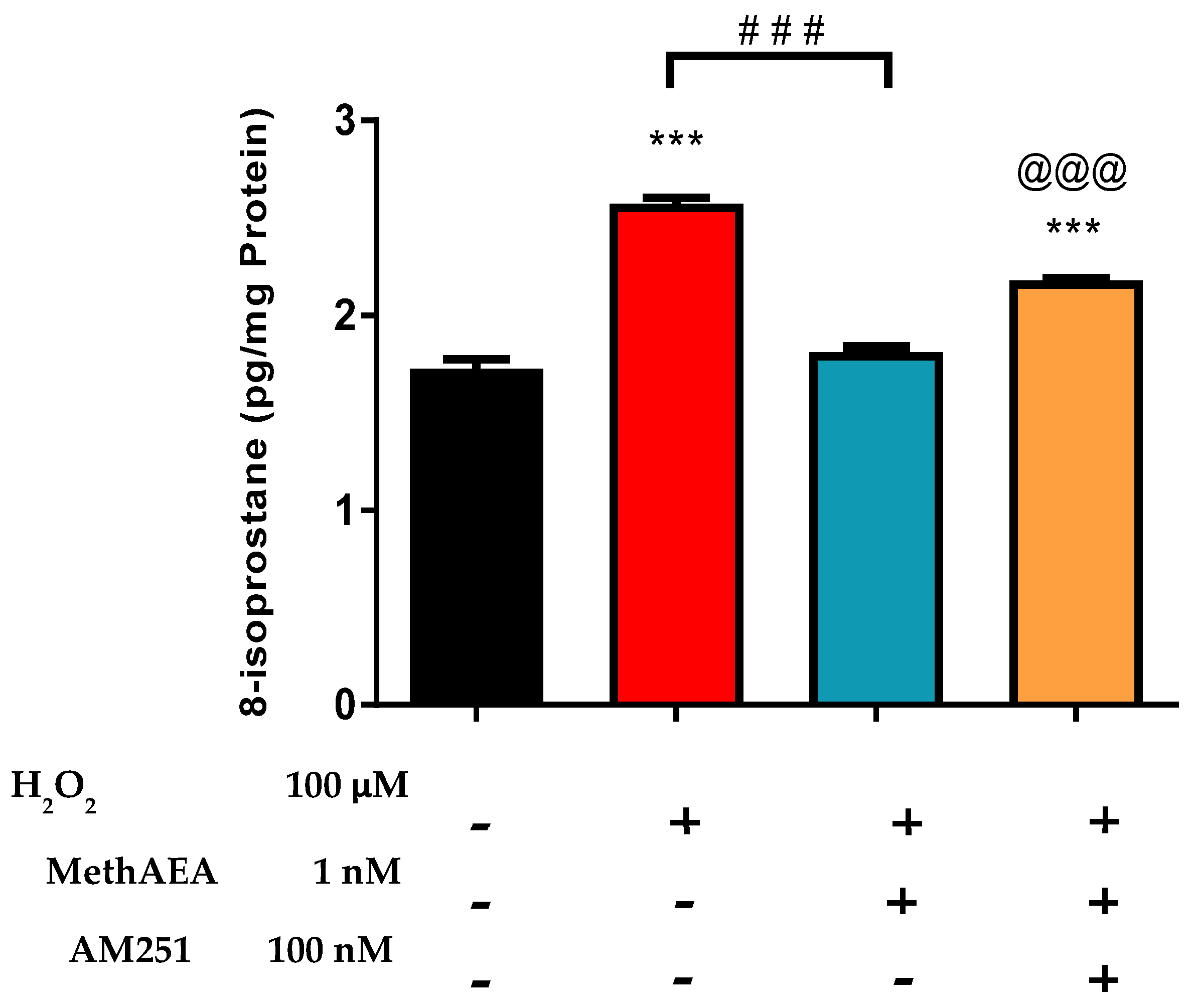
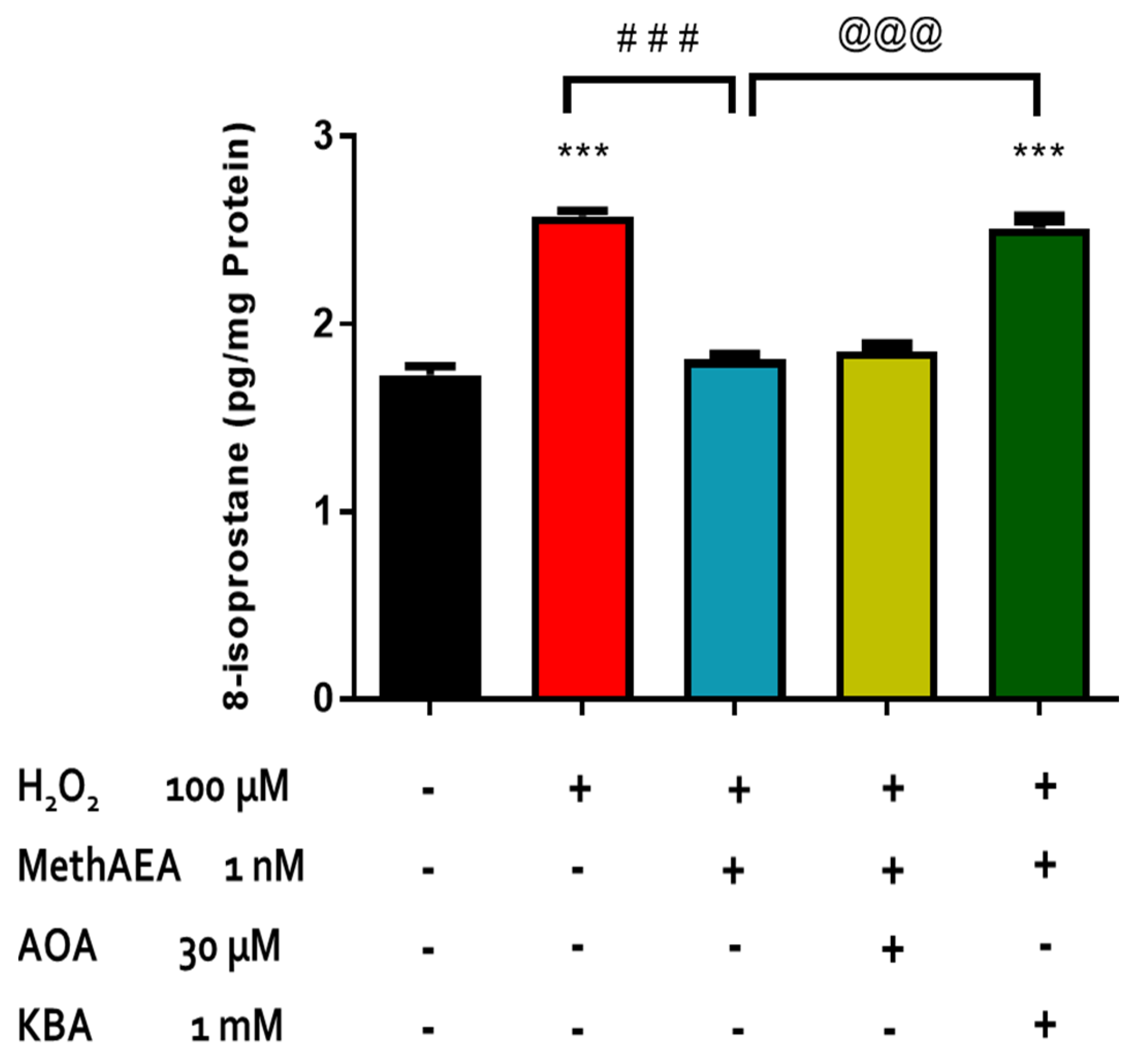
2.2. Involvement of CB1 Receptors and Intramurally Generated H2S in Cannabinoid-Mediated Neuroprotection in the Bovine Retina
3. Discussion
4. Methods
4.1. Chemicals
4.2. Tissue Preparations
4.3. 8-Isoprostane ELISA Assay
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R. The Endocannabinoid System: A Look Back and Ahead. Handb. Exp. Pharmacol. 2015, 231, vii–ix. [Google Scholar] [PubMed]
- Yazulla, S. Endocannabinoids in the retina: From marijuana to neuroprotection. Prog. Retin. Eye Res. 2008, 27, 501–526. [Google Scholar] [CrossRef] [PubMed]
- Katchan, V.; David, P.; Shoenfeld, Y. Cannabinoids and autoimmune diseases: A systematic review. Autoimmun. Rev. 2016, 15, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Kokona, D.; Georgiou, P.C.; Kounenidakis, M.; Kiagiadaki, F.; Thermos, K. Endogenous and Synthetic Cannabinoids as Therapeutics in Retinal Disease. Neural Plast. 2016, 2016, 8373020. [Google Scholar] [CrossRef]
- Nucci, C.; Gasperi, V.; Tartaglione, R.; Cerulli, A.; Terrinoni, A.; Bari, M.; De Simone, C.; Agro, A.F.; Morrone, L.A.; Corasaniti, M.T.; et al. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2997–3004. [Google Scholar] [CrossRef]
- Njie, Y.F.; He, F.; Qiao, Z.; Song, Z.H. Aqueous humor outflow effects of 2-arachidonylglycerol. Exp. Eye Res. 2008, 87, 106–114. [Google Scholar] [CrossRef]
- Njie, Y.F.; Qiao, Z.; Xiao, Z.; Wang, W.; Song, Z.H. N-arachidonylethanolamide-induced increase in aqueous humor outflow facility. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4528–4534. [Google Scholar] [CrossRef]
- Cairns, E.A.; Baldridge, W.H.; Kelly, M.E. The Endocannabinoid System as a Therapeutic Target in Glaucoma. Neural Plast. 2016, 2016, 9364091. [Google Scholar] [CrossRef]
- Chen, J.; Matias, I.; Dinh, T.; Lu, T.; Venezia, S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Finding of endocannabinoids in human eye tissues: Implications for glaucoma. Biochem. Biophys. Res. Commun. 2005, 330, 1062–1067. [Google Scholar] [CrossRef]
- Matias, I.; Wang, J.W.; Moriello, A.S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.S.; Moesgaard, B.; Hansen, H.H.; Petersen, G. N-Acylethanolamines and precursor phospholipids—Relation to cell injury. Chem. Phys. Lipids 2000, 108, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide as a physiological mediator: Its function and therapeutic applications. Nihon Yakurigaku Zasshi 2010, 136, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Yamada, M.; Kimura, H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J. Biol. Chem. 2011, 286, 39379–39386. [Google Scholar] [CrossRef]
- Nagai, Y.; Tsugane, M.; Oka, J.; Kimura, H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004, 18, 557–559. [Google Scholar] [CrossRef]
- Opere, C.A.; Zheng, W.D.; Zhao, M.; Lee, J.S.; Kulkarni, K.H.; Ohia, S.E. Inhibition of potassium- and ischemia-evoked [3H] D-aspartate release from isolated bovine retina by cannabinoids. Curr. Eye Res. 2006, 31, 645–653. [Google Scholar] [CrossRef]
- Opere, C.A.; Monjok, E.M.; Kulkarni, K.H.; Njie, Y.F.; Ohia, S.E. Regulation of [3H] D-aspartate release from mammalian isolated retinae by hydrogen sulfide. Neurochem. Res. 2009, 34, 1962–1968. [Google Scholar] [CrossRef]
- Telezhkin, V.; Brazier, S.P.; Cayzac, S.; Muller, C.T.; Riccardi, D.; Kemp, P.J. Hydrogen sulfide inhibits human BK(Ca) channels. Adv. Exp. Med. Biol. 2009, 648, 65–72. [Google Scholar]
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2011, 41, 113–121. [Google Scholar] [CrossRef]
- Osborne, N.N.; Ji, D.; Majid, A.S.A.; Fawcett, R.J.; Sparatore, A.; Del Soldato, P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Investig. Ophthalmol. Vis. Sci. 2010, 51, 284–294. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Zhao, F.; Zhao, P.Q.; Kang, X.L. Cannabinoid receptor 1 blockade protects human retinal pigment epithelial cells from oxidative injury. Mol. Vis. 2013, 19, 357–366. [Google Scholar] [PubMed]
- Krishnan, G.; Chatterjee, N. Endocannabinoids alleviate proinflammatory conditions by modulating innate immune response in muller glia during inflammation. Glia 2012, 60, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.F.; Wang, J.; Guan, J.; Zhou, L.; Sheng, Y.; Zhao, J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br. J. Pharmacol. 2013, 169, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A.; Stella, N.; Piomelli, D.; Mackie, K.; Karten, H.J.; Maguire, G. Cannabinoid CB1 receptors and ligands in vertebrate retina: Localization and function of an endogenous signaling system. Proc. Natl. Acad. Sci. USA 1999, 96, 14565–14570. [Google Scholar] [CrossRef] [PubMed]
- Smart, D.; Gunthorpe, M.J.; Jerman, J.C.; Nasir, S.; Gray, J.; Muir, A.I.; Chambers, J.K.; Randall, A.D.; Davis, J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br. J. Pharmacol. 2000, 129, 227–230. [Google Scholar] [CrossRef]
- Kapoor, A.; Thiemermann, C. Hydrogen sulfide, neurogenic inflammation, and cardioprotection: A tale of rotten eggs and vanilloid receptors. Crit. Care Med. 2010, 38, 728–730. [Google Scholar] [CrossRef]
- Abadji, V.; Lin, S.; Taha, G.; Griffin, G.; Stevenson, L.A.; Pertwee, R.G.; Makriyannis, A. (R)-methanandamide: A chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994, 37, 1889–1893. [Google Scholar] [CrossRef]
- Romano, M.R.; Lograno, M.D. Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: Evidences for CB1 receptors, nitric oxide and potassium channels. Br. J. Pharmacol. 2006, 147, 917–925. [Google Scholar] [CrossRef]
- Romano, M.R.; Lograno, M.D. Involvement of the peroxisome proliferator-activated receptor (PPAR) alpha in vascular response of endocannabinoids in the bovine ophthalmic artery. Eur. J. Pharmacol. 2012, 683, 197–203. [Google Scholar] [CrossRef]
- Njie-Mbye, Y.F.; Kulkarni-Chitnis, M.; Opere, C.A.; Barrett, A.; Ohia, S.E. Lipid peroxidation: Pathophysiological and pharmacological implications in the eye. Front. Physiol. 2013, 4, 366. [Google Scholar] [CrossRef]
- Kulkarni-Chitnis, M.; Njie-Mbye, Y.F.; Mitchell, L.; Robinson, J.; Whiteman, M.; Wood, M.E.; Opere, C.A.; Ohia, S.E. Inhibitory action of novel hydrogen sulfide donors on bovine isolated posterior ciliary arteries. Exp. Eye Res. 2015, 134, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Bush, L.; Robinson, J.; Opere, C.; Njie-Mbye, Y.F. A Neuroprotective Action of Cannabinoids in The Bovine Isolated Retina: Role of Hydrogen Sulfide. In Proceedings of the Annual Meeting of the Japanese Pharmacological Society WCP2018 (The 18th World Congress of Basic and Clinical Pharmacology), Kyoto, Japan, 1–6 July 2018; Japanese Pharmacological Society: Tokyo, Japan, 2018; p. PO2-1. [Google Scholar]
- Bush, L.; Robinson, J.; Okolie, A.; Muili, F.; Opere, C.A.; Whiteman, M.; Ohia, S.E.; Mbye, Y.F.N. Neuroprotective Actions of Hydrogen Sulfide-Releasing Compounds in Isolated Bovine Retinae. Pharmaceuticals 2024, 17, 1311. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N- methyl-D-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Binelli, A. Oxidative and genetic responses induced by Delta-9-tetrahydrocannabinol (Delta-9-THC) to Dreissena polymorpha. Sci. Total Environ. 2014, 468–469, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Rangel-López, E.; Colín-González, A.L.; Paz-Loyola, A.L.; Pinzón, E.; Torres, I.; Serratos, I.N.; Castellanos, P.; Wajner, M.; Souza, D.O.; Santamaría, A. Cannabinoid receptor agonists reduce the short-term mitochondrial dysfunction and oxidative stress linked to excitotoxicity in the rat brain. Neuroscience 2015, 285, 97–106. [Google Scholar] [CrossRef]
- Beltowski, J. Endogenous hydrogen sulfide in perivascular adipose tissue: Role in the regulation of vascular tone in physiology and pathology. Can. J. Physiol. Pharmacol. 2013, 91, 889–898. [Google Scholar] [CrossRef]
- Wallace, J.L.; Flannigan, K.L.; McKnight, W.; Wang, L.; Ferraz, J.G.; Tuitt, D. Pro-resolution, protective and anti-nociceptive effects of a cannabis extract in the rat gastrointestinal tract. J. Physiol. Pharmacol. 2013, 64, 167–175. [Google Scholar]
- Castany, S.; Carcole, M.; Leanez, S.; Pol, O. The role of carbon monoxide on the anti-nociceptive effects and expression of cannabinoid 2 receptors during painful diabetic neuropathy in mice. Psychopharmacology 2016, 233, 2209–2219. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. The endocannabinoid system: ‘NO’ longer anonymous in the control of nitrergic signalling? J. Mol. Cell Biol. 2017, 9, 91–103. [Google Scholar] [CrossRef]
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar]
- Sarzani, R. Endocannabinoids, blood pressure and the human heart. J. Neuroendocrinol. 2008, 20 (Suppl. S1), 58–62. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Laprevote, V. The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications. Neural Plast. 2016, 2016, 2916732. [Google Scholar] [CrossRef]
- Paloczi, J.; Varga, Z.V.; Hasko, G.; Pacher, P. Neuroprotection in Oxidative Stress-Related Neurodegenerative Diseases: Role of Endocannabinoid System Modulation. Antioxid. Redox Signal. 2017, 29, 75–108. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chavez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; Leanez, S.; Motterlini, R.; Pol, O. Treatment with carbon monoxide-releasing molecules and an HO-1 inducer enhances the effects and expression of micro-opioid receptors during neuropathic pain. Anesthesiology 2013, 118, 1180–1197. [Google Scholar] [CrossRef]
- Carcole, M.; Castany, S.; Leanez, S.; Pol, O. Treatment with a heme oxygenase 1 inducer enhances the antinociceptive effects of micro-opioid, delta- opioid, and cannabinoid 2 receptors during inflammatory pain. J. Pharmacol. Exp. Ther. 2014, 351, 224–232. [Google Scholar] [CrossRef]
- Schurman, L.D.; Lichtman, A.H. Endocannabinoids: A Promising Impact for Traumatic Brain Injury. Front. Pharmacol. 2017, 8, 69. [Google Scholar] [CrossRef]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef]
- Lepicier, P.; Bouchard, J.F.; Lagneux, C.; Lamontagne, D. Endocannabinoids protect the rat isolated heart against ischaemia. Br. J. Pharmacol. 2003, 139, 805–815. [Google Scholar] [CrossRef]
- Cernak, I.; Vink, R.; Natale, J.; Stoica, B.; Lea PMt Movsesyan, V.; Ahmed, F.; Knoblach, S.M.; Fricke, S.T.; Faden, A.I. The “dark side” of endocannabinoids: A neurotoxic role for anandamide. J. Cereb. Blood Flow. Metab. 2004, 24, 564–578. [Google Scholar] [CrossRef]
- Movsesyan, V.A.; Stoica, B.A.; Yakovlev, A.G.; Knoblach, S.M.; Lea PMt Cernak, I.; Vink, R.; Faden, A.I. Anandamide-induced cell death in primary neuronal cultures: Role of calpain and caspase pathways. Cell Death Differ. 2004, 11, 1121–1132. [Google Scholar] [CrossRef]
- Kello, M.; Mikes, J.; Jendzelovsky, R.; Koval, J.; Fedorocko, P. PUFAs enhance oxidative stress and apoptosis in tumour cells exposed to hypericin-mediated PDT. Photochem. Photobiol. Sci. 2010, 9, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.E.; Mitchell, T.W.; Else, P.L. Phospholipid peroxidation: Lack of effect of fatty acid pairing. Lipids 2012, 47, 451–460. [Google Scholar] [CrossRef]
- Navarrete, C.M.; Fiebich, B.L.; de Vinuesa, A.G.; Hess, S.; de Oliveira, A.C.; Candelario-Jalil, E.; Caballero, F.J.; Calzado, M.A.; Munoz, E. Opposite effects of anandamide and N-arachidonoyl dopamine in the regulation of prostaglandin E and 8-iso-PGF formation in primary glial cells. J. Neurochem. 2009, 109, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, J.E.; Oh, S.M.; Cha, W.J.; Hah, J.H.; Sung, M.W. Anticancer effects of anandamide on head and neck squamous cell carcinoma cells via the production of receptor-independent reactive oxygen species. Head Neck 2015, 37, 1187–1192. [Google Scholar] [CrossRef]
- Jarvinen, T.; Pate, D.W.; Laine, K. Cannabinoids in the treatment of glaucoma. Pharmacol. Ther. 2002, 95, 203–220. [Google Scholar] [CrossRef]
- Wang, M.T.; Danesh-Meyer, H.V. Cannabinoids and the eye. Surv. Ophthalmol. 2021, 66, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Payne, S. Eicosanoids in bovine retinal microcirculation. J. Ocul. Pharmacol. Ther. 1997, 13, 139–149. [Google Scholar] [CrossRef] [PubMed]
- LeDay, A.M.; Kulkarni, K.H.; Opere, C.A.; Ohia, S.E. Arachidonic acid metabolites and peroxide-induced inhibition of [3H]D-aspartate release from bovine isolated retinae. Curr. Eye Res. 2004, 28, 367–372. [Google Scholar] [CrossRef]
- Matsuda, K.; Ohnishi, K.; Misaka, E.; Yamazaki, M. Decrease of urinary prostaglandin E2 and prostaglandin F2 alpha excretion by nonsteroidal anti-inflammatory drugs in rats. Relationship to anti-inflammatory activity. Biochem. Pharmacol. 1983, 32, 1347–1352. [Google Scholar] [CrossRef]
- Zhan, G.-L.; Ohia, S.; Camras, C.; Ohia, E.; Wang, Y. Superior cervical ganglionectomy-induced lowering of intraocular pressure in rabbits: Role of prostaglandins and neuropeptide Y. Gen. Pharmacol. Vasc. Syst. 1999, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bush, L.; Okolie, A.; Robinson, J.; Muili, F.; Opere, C.A.; Ohia, S.E.; Njie Mbye, Y.F. Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide. Pharmaceuticals 2025, 18, 117. https://doi.org/10.3390/ph18010117
Bush L, Okolie A, Robinson J, Muili F, Opere CA, Ohia SE, Njie Mbye YF. Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide. Pharmaceuticals. 2025; 18(1):117. https://doi.org/10.3390/ph18010117
Chicago/Turabian StyleBush, Leah, Anthonia Okolie, Jenaye Robinson, Fatima Muili, Catherine A. Opere, Sunny E. Ohia, and Ya Fatou Njie Mbye. 2025. "Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide" Pharmaceuticals 18, no. 1: 117. https://doi.org/10.3390/ph18010117
APA StyleBush, L., Okolie, A., Robinson, J., Muili, F., Opere, C. A., Ohia, S. E., & Njie Mbye, Y. F. (2025). Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide. Pharmaceuticals, 18(1), 117. https://doi.org/10.3390/ph18010117





