Effectiveness of Pharmacokinetic-Guided Hydroxyurea Dose Individualization in Patients with Sickle Cell Anemia: A Mini-Review
Abstract
:1. Introduction
2. Results and Discussion
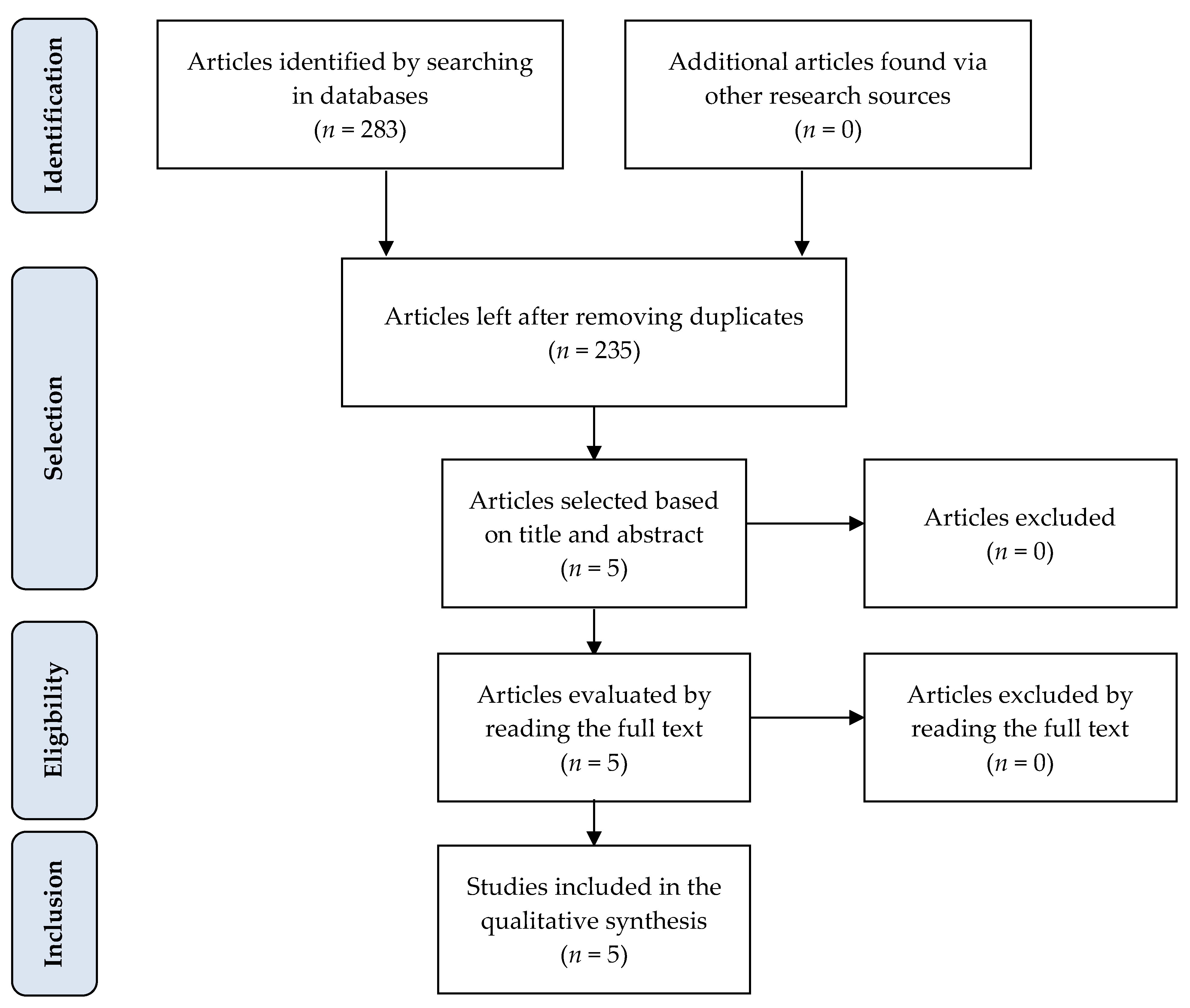
2.1. Selection and Qualitative Analysis of Articles
2.2. Main Findings from the Selected Articles
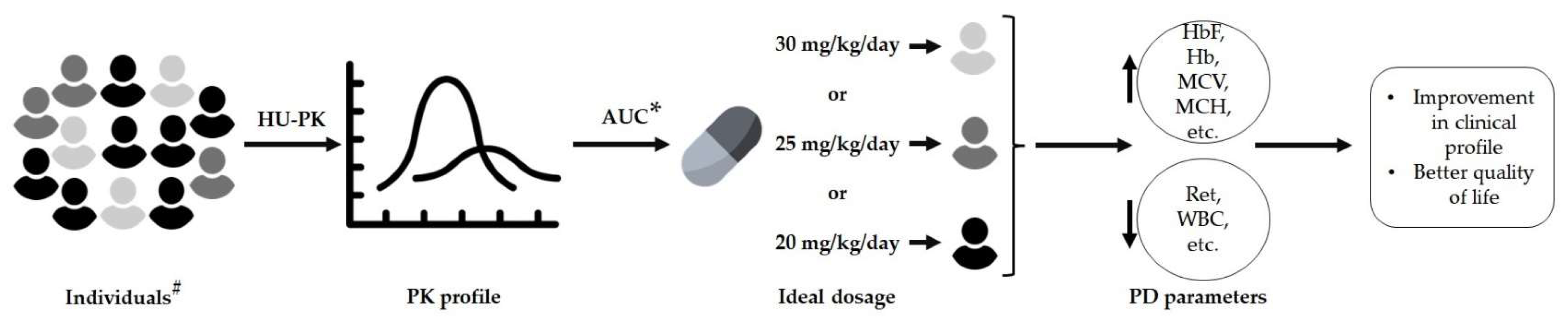
2.3. Dose Adjustment Using the Population Pharmacokinetic Model
2.4. Sample Collection for Pharmacokinetic Analysis
2.5. Pharmacokinetics and Pharmacodynamic Parameters
2.6. Limitations and Perspectives
3. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle Cell Disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.F.; Conran, N.; Fertrin, K.Y. Anemia Falciforme. In Tratado de Hematologia; Atheneu: São Paulo, Brazil, 2013; pp. 205–223. [Google Scholar]
- Habara, A.; Steinberg, M.H. Minireview: Genetic basis of heterogeneity and severity in sickle cell disease. Exp. Biol. Med. 2016, 241, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahouédéhou, S.C.M.A.; Adorno, E.V.; da Guarda, C.C.; Ndidi, U.S.; Carvalho, S.P.; Santiago, R.P.; Aleluia, M.M.; de Oliveira, R.M.; de Souza Gonçalves, M. Hydroxyurea in the Management of Sickle Cell Disease: Pharmacogenomics and Enzymatic Metabolism. Pharm. J. 2018, 18, 730–739. [Google Scholar] [CrossRef] [PubMed]
- McGann, P.T.; Ware, R.E. Hydroxyurea Therapy for Sickle Cell Anemia. Expert Opin. Drug Saf. 2015, 14, 1749–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, R.N.; Turner, C.; Dick, M.; Height, S.E.; Awogbade, M.; Inusa, B.; Okpala, I.; O’Driscoll, S.; Thein, S.L.; Rees, D.C. The Measurement of Urinary Hydroxyurea in Sickle Cell Anaemia. Br. J. Haematol. 2005, 130, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.K.; Patel, R.K.; Shah, V.; Nainiwal, L.; Trivedi, B. Hydroxyurea in Sickle Cell Disease: Drug Review. Indian J. Hematol. Blood Transfus. 2014, 30, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Yakubu, M.; Kim-Shapiro, D.B.; King, S.B. Rat Liver-Mediated Metabolism of Hydroxyurea to Nitric Oxide. Free Radic. Biol. Med. 2006, 40, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Sassi, H.; Bachir, D.; Habibi, A.; Astier, A.; Galactéros, F.; Hulin, A. No Effect of CYP450 and P-Glycoprotein on Hydroxyurea in Vitro Metabolism. Fundam. Clin. Pharmacol. 2010, 24, 83–90. [Google Scholar] [CrossRef]
- Yan, J.-H.; Ataga, K.; Kaul, S.; Olson, J.S.; Grasela, D.M.; Gothelf, S.; Kutlar, A.; Orringer, E. The Influence of Renal Function on Hydroxyurea Pharmacokinetics in Adults with Sickle Cell Disease. J. Clin. Pharmacol. 2005, 45, 434–445. [Google Scholar] [CrossRef]
- Hoppe, C.; Neumayr, L. Sickle Cell Disease. Hematol./Oncol. Clin. N. Am. 2019, 33, 355–371. [Google Scholar] [CrossRef]
- Effective Public Healthcare Panacea Project. Quality Assessment Tool for Quantitative Studies. Available online: https://www.ephpp.ca/quality-assessment-tool-for-quantitative-studies/ (accessed on 17 March 2021).
- Dong, M.; McGann, P.T.; Mizuno, T.; Ware, R.E.; Vinks, A.A. Development of a Pharmacokinetic-Guided Dose Individualization Strategy for Hydroxyurea Treatment in Children with Sickle Cell Anaemia: Population PK of Hydroxyurea in Paediatric Patients with Sickle Cell Anaemia. Br. J. Clin. Pharm. 2016, 81, 742–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGann, P.T.; Niss, O.; Dong, M.; Marahatta, A.; Howard, T.A.; Mizuno, T.; Lane, A.; Kalfa, T.A.; Malik, P.; Quinn, C.T.; et al. Robust Clinical and Laboratory Response to Hydroxyurea Using Pharmacokinetically Guided Dosing for Young Children with Sickle Cell Anemia. Am. J. Hematol. 2019, 94, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.T.; Niss, O.; Dong, M.; Pfeiffer, A.; Korpik, J.; Reynaud, M.; Bonar, H.; Kalfa, T.A.; Smart, L.R.; Malik, P. Early initiation of hydroxyurea (hydroxycarbamide) using individualised, pharmacokinetics-guided dosing can produce sustained and nearly pancellular expression of fetal haemoglobin in children with sickle cell anaemia. Br. J. Haematol. 2021, 194, 617–625. [Google Scholar] [CrossRef]
- Meier, E.R.; Creary, S.E.; Heeney, M.M.; Dong, M.; Appiah-Kubi, A.O.; Nelson, S.C.; Niss, O.; Piccone, C.; Quarmyne, M.-O.; Quinn, C.T.; et al. Hydroxyurea Optimization through Precision Study (HOPS): Study Protocol for a Randomized, Multicenter Trial in Children with Sickle Cell Anemia. Trials 2020, 21, 983. [Google Scholar] [CrossRef] [PubMed]
- Paule, I.; Sassi, H.; Habibi, A.; Pham, K.P.; Bachir, D.; Galactéros, F.; Girard, P.; Hulin, A.; Tod, M. Population Pharmacokinetics and Pharmacodynamics of Hydroxyurea in Sickle Cell Anemia Patients, a Basis for Optimizing the Dosing Regimen. Orphanet J. Rare Dis. 2011, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazon, C.; Sabo, A.-N.; Becker, G.; Lessinger, J.-M.; Kemmel, V.; Paillard, C. Optimizing Hydroxyurea Treatment for Sickle Cell Disease Patients: The Pharmacokinetic Approach. JCM 2019, 8, 1701. [Google Scholar] [CrossRef] [Green Version]
- Ware, R.E.; Despotovic, J.M.; Mortier, N.A.; Flanagan, J.M.; He, J.; Smeltzer, M.P.; Kimble, A.C.; Aygun, B.; Wu, S.; Howard, T.; et al. Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics of Hydroxyurea Treatment for Children with Sickle Cell Anemia. Blood 2011, 118, 4985–4991. [Google Scholar] [CrossRef] [Green Version]
- Batchelor, H.K.; Marriott, J.F. Paediatric Pharmacokinetics: Key Considerations: Paediatric Pharmacokinetics. Br. J. Clin. Pharm. 2015, 79, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Estepp, J.H.; Melloni, C.; Thornburg, C.D.; Wiczling, P.; Rogers, Z.; Rothman, J.A.; Green, N.S.; Liem, R.; Brandow, A.M.; Crary, S.E.; et al. Pharmacokinetics and Bioequivalence of a Liquid Formulation of Hydroxyurea in Children with Sickle Cell Anemia. J. Clin. Pharmacol. 2016, 56, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Wiczling, P.; Liem, R.I.; Panepinto, J.A.; Garg, U.; Abdel-Rahman, S.M.; Kearns, G.L.; Neville, K.A. Population Pharmacokinetics of Hydroxyurea for Children and Adolescents with Sickle Cell Disease. J. Clin. Pharmacol. 2014, 54, 1016–1022. [Google Scholar] [CrossRef]
- Tantawy, A.A.G.; Adly, A.A.M.; Ismail, E.A.R.; Abdelazeem, M. Clinical Predictive Value of Cystatin C in Pediatric Sickle Cell Disease: A Marker of Disease Severity and Subclinical Cardiovascular Dysfunction. Clin. Appl. Thromb./Hemost. 2017, 23, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Objective | Number of Participants | Principal Results |
|---|---|---|---|---|
| [13] | USA | Develop an HU pharmacokinetic model in a pediatric population in combination with Bayesian estimation to individualize HU dosages in pediatric patients with SCA. | 96 children |
|
| [14] | USA | Simplify and decrease HU dose-escalation so that children receive an optimal dose based on individualized pharmacokinetic parameters. | 51 children |
|
| [15] | USA | Investigate the utility of flow cytometric F-cell analysis and demonstrate sustained and near-pancellular or pancellular HbF distribution in SCA children undergoing an early and individualized pharmacokinetics-guided dosing of HU. | 48 children |
|
| [16] | USA | Validate the feasibility and benefits of the pharmacokinetic-guided dose approach in a multicentric study. | 104 children |
|
| [17] | France | Develop PK-PD population models for HU, seeking to assess the exposure-efficacy relationship and variability. Compare continuous and interrupted dosing regimens and develop recommendations for monitoring treatment response. | 97 adults |
|
| Study | Age (Years) | Gender | Health | Biomarkers | AUC0-∞ | CL | MTD | HbF | MCV |
|---|---|---|---|---|---|---|---|---|---|
| (Male/Female) | Conditions | (mg L−1 h−1) | (Lh–1 70 kg–1) | (mg/kg/Day) | (%) | (fL) | |||
| [13] | 1.9–16.5 | 42/21 | SCA | Serum creatinine, direct glomerular filtration rate, serum cystatin C, urine albumin, HbF | 91.1–115.7 | 19.56 | 14.2–35.5 | -- | -- |
| [14] | 0.5–21.0 | 29/21 * | SCA | HbF, Hb, MCV, ARC, WBC, ANC, platelets | 67–91 | 9.7–14.0 | 26.7 ± 4.8 | 33.3 ± 9.1 | 91.9 ± 15.3 |
| [15] | 2.3–21.9 | 27/21 | SCA | HbF, Hb, Hct, MCV, ARC, RDW, ANC, platelets, F-cell, F-reticulocytes, F/F-cell | 67–91 ** | 9.7–14.0 ** | 17.8–38.6 | ≥31.5 | ≈ 70–120 ¥ |
| [16] | xx | xx | SCA | Reticulocyte count, HbF, cystatin C | xx | xx | xx | xx | xx |
| [17] | 18–54 | 29/68 | SCA or S/β-thalassemia | Hb, HbF, MCV, MCH, PMN, platelets, bilirubin, LDH, ferritin, AST, ALT, creatinine, urea | -- | 10.4–12.9 | -- | 3.9–41.6 | 81–131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Neres, J.S.; Yahouédéhou, S.C.M.A.; Goncalves, M.S. Effectiveness of Pharmacokinetic-Guided Hydroxyurea Dose Individualization in Patients with Sickle Cell Anemia: A Mini-Review. Pharmaceuticals 2023, 16, 857. https://doi.org/10.3390/ph16060857
dos Santos Neres JS, Yahouédéhou SCMA, Goncalves MS. Effectiveness of Pharmacokinetic-Guided Hydroxyurea Dose Individualization in Patients with Sickle Cell Anemia: A Mini-Review. Pharmaceuticals. 2023; 16(6):857. https://doi.org/10.3390/ph16060857
Chicago/Turabian Styledos Santos Neres, Joelma Santana, Sètondji Cocou Modeste Alexandre Yahouédéhou, and Marilda Souza Goncalves. 2023. "Effectiveness of Pharmacokinetic-Guided Hydroxyurea Dose Individualization in Patients with Sickle Cell Anemia: A Mini-Review" Pharmaceuticals 16, no. 6: 857. https://doi.org/10.3390/ph16060857
APA Styledos Santos Neres, J. S., Yahouédéhou, S. C. M. A., & Goncalves, M. S. (2023). Effectiveness of Pharmacokinetic-Guided Hydroxyurea Dose Individualization in Patients with Sickle Cell Anemia: A Mini-Review. Pharmaceuticals, 16(6), 857. https://doi.org/10.3390/ph16060857






