Prediction of Cell Migration in MDA-MB 231 and MCF-7 Human Breast Cancer Cells Treated with Albizia Lebbeck Methanolic Extract Using Multilinear Regression and Artificial Intelligence-Based Models
Abstract
1. Introduction
2. Results and Discussion
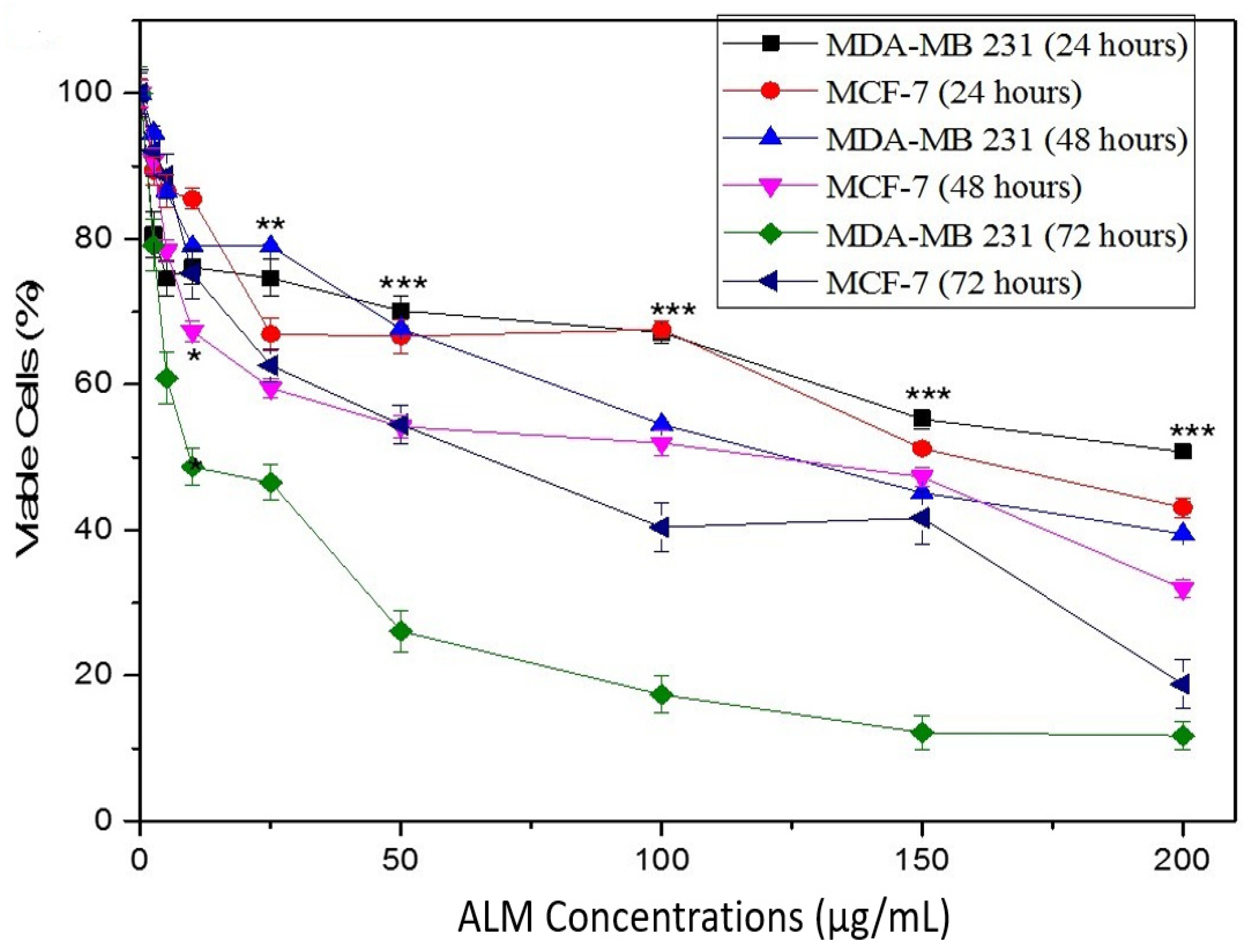
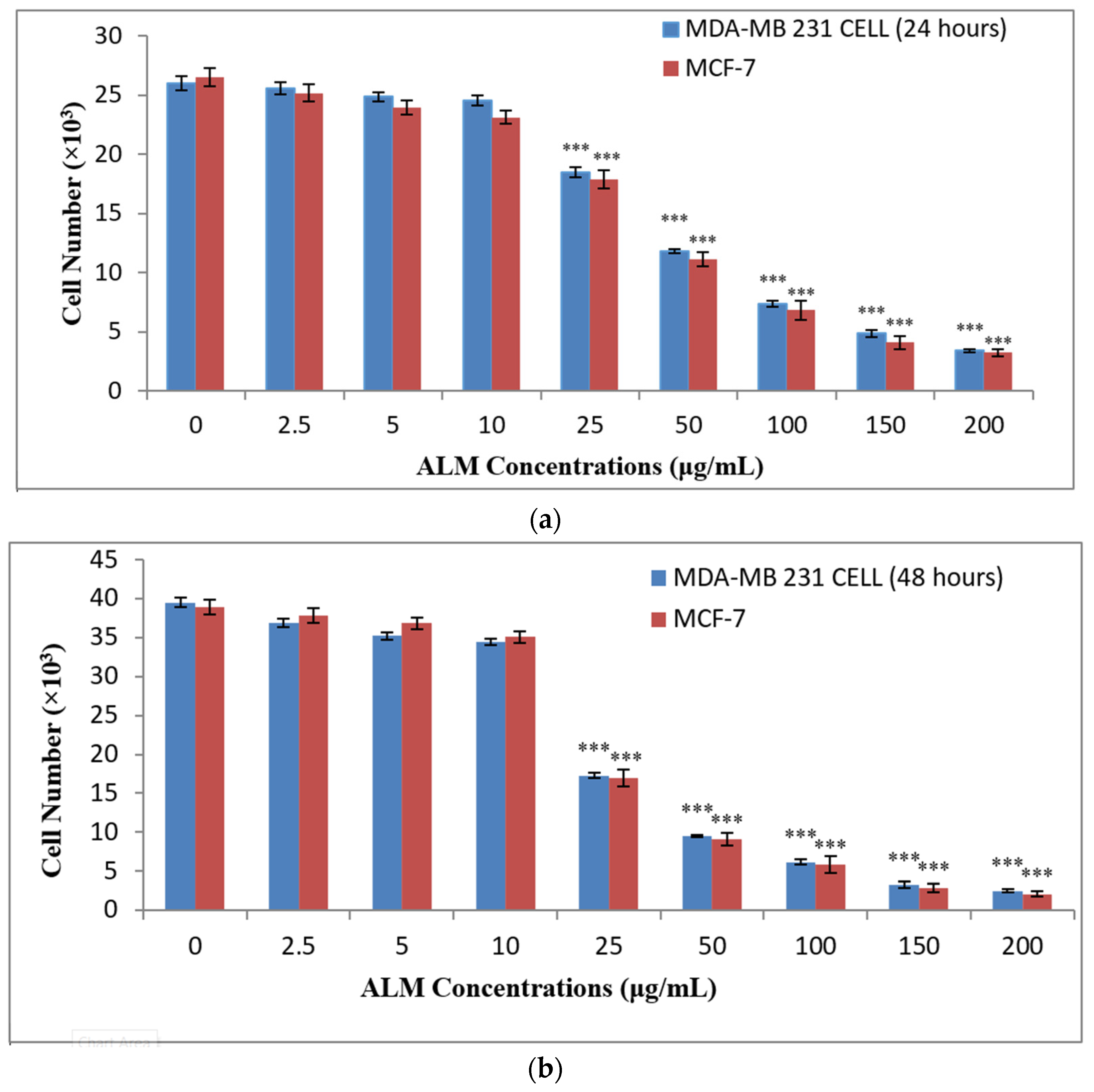
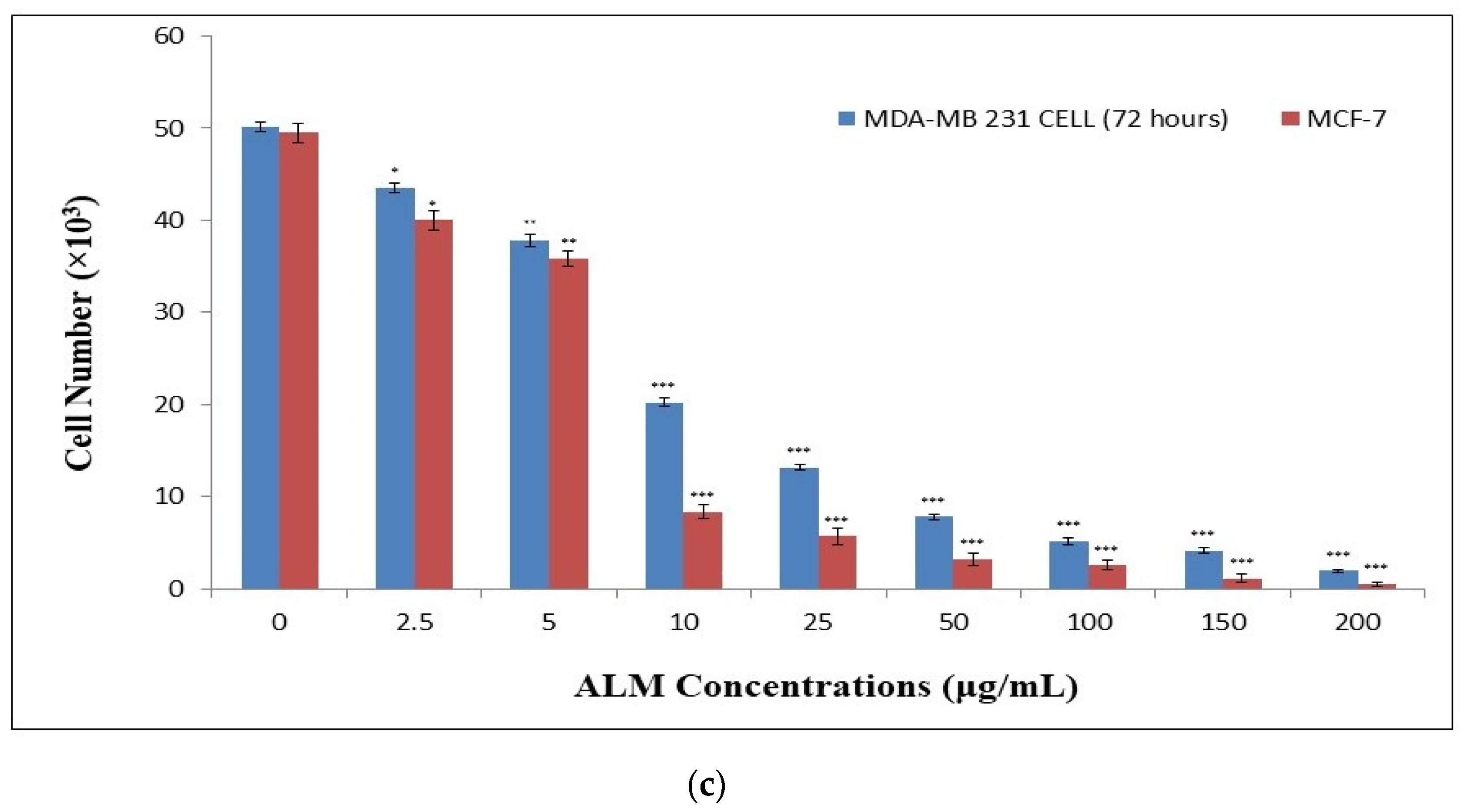
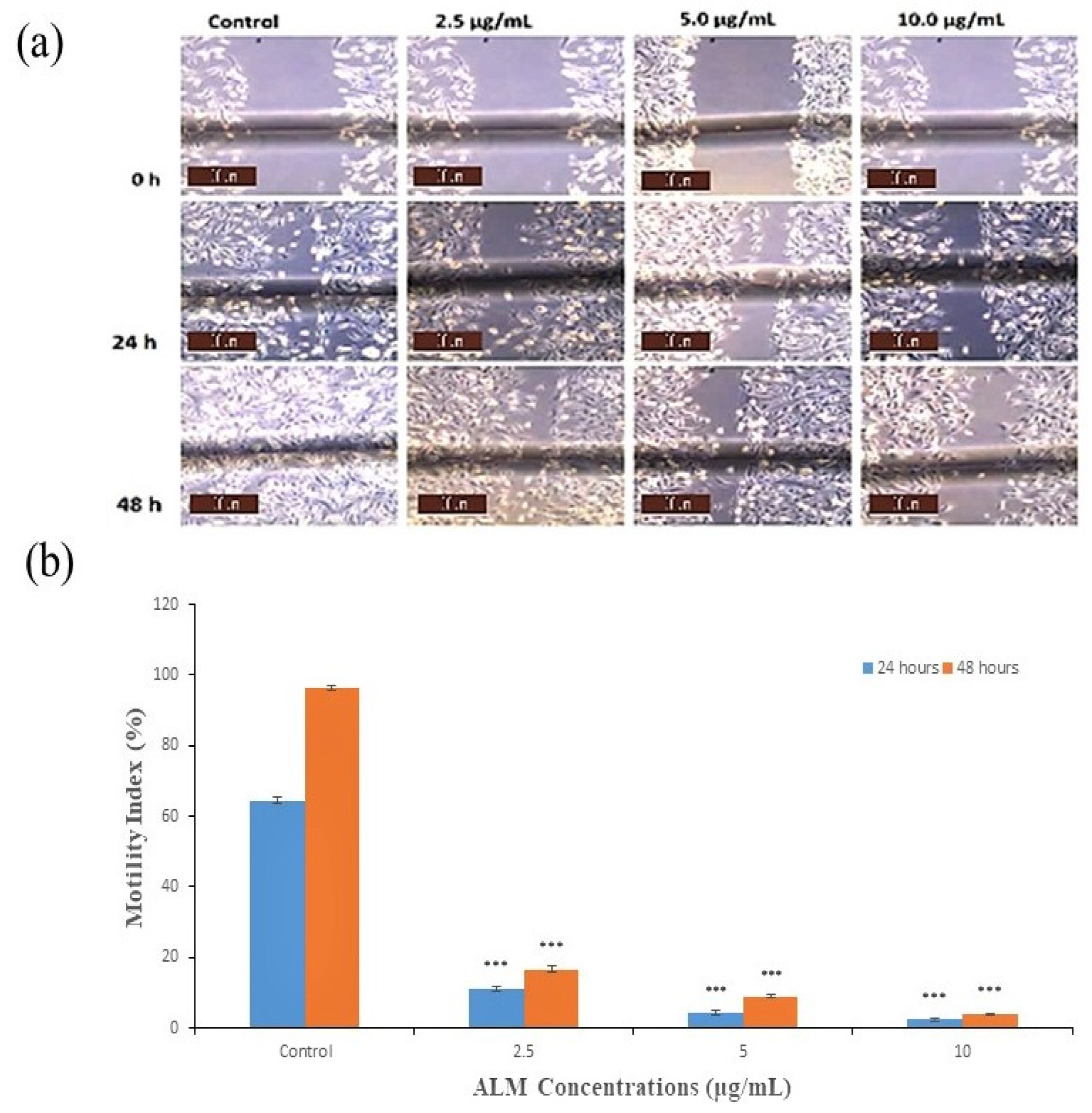
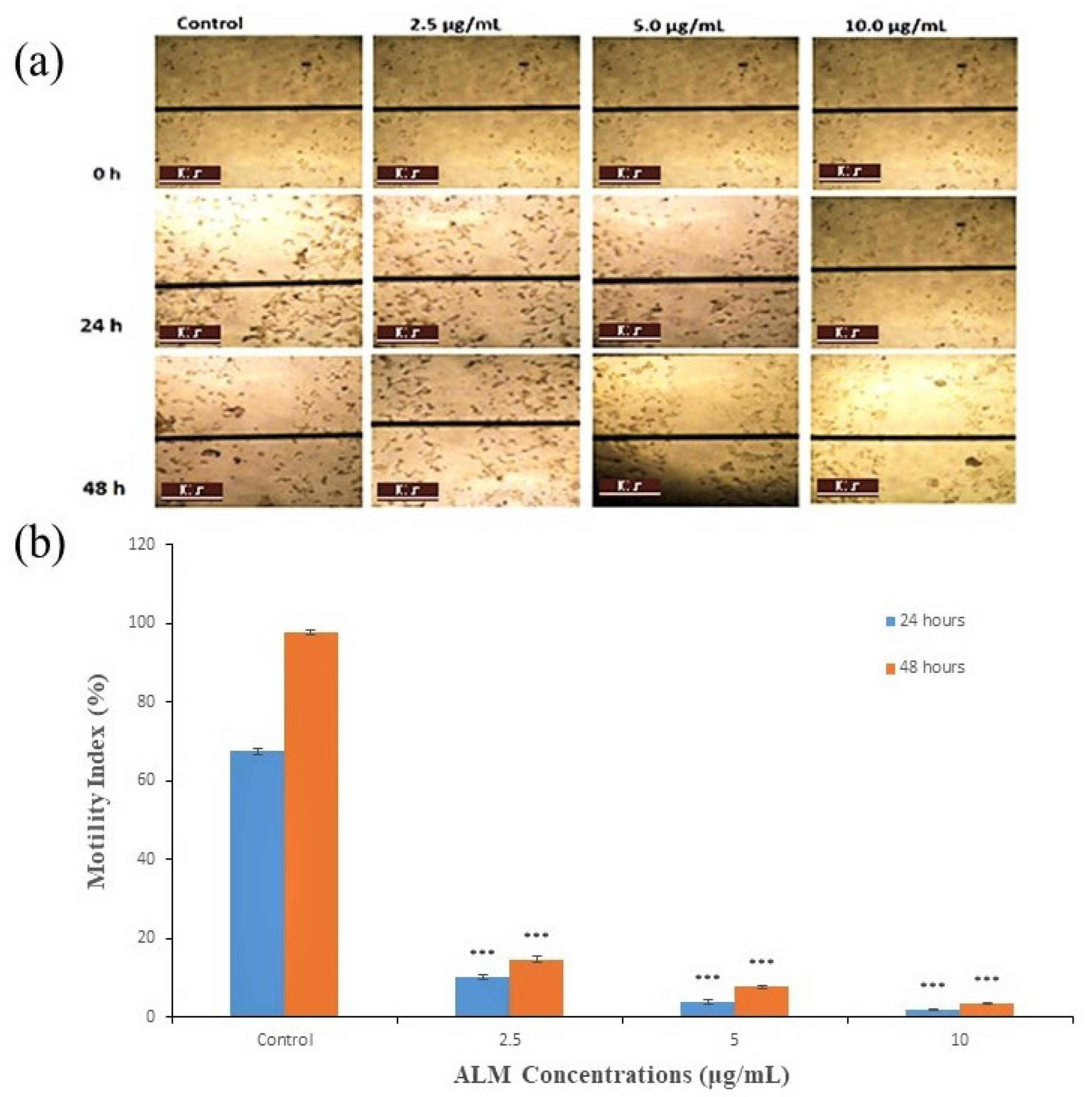
2.1. Experimental Results
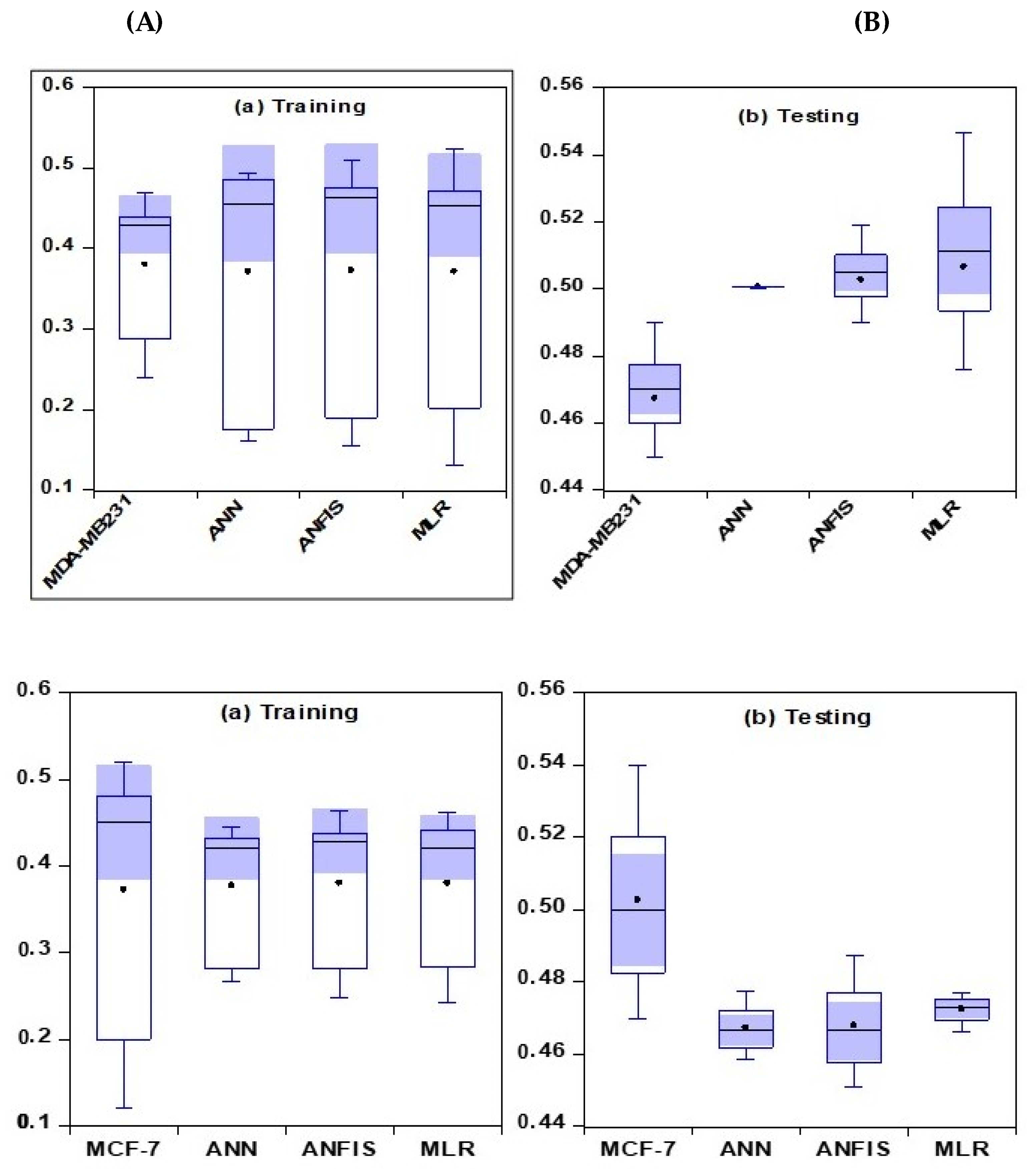
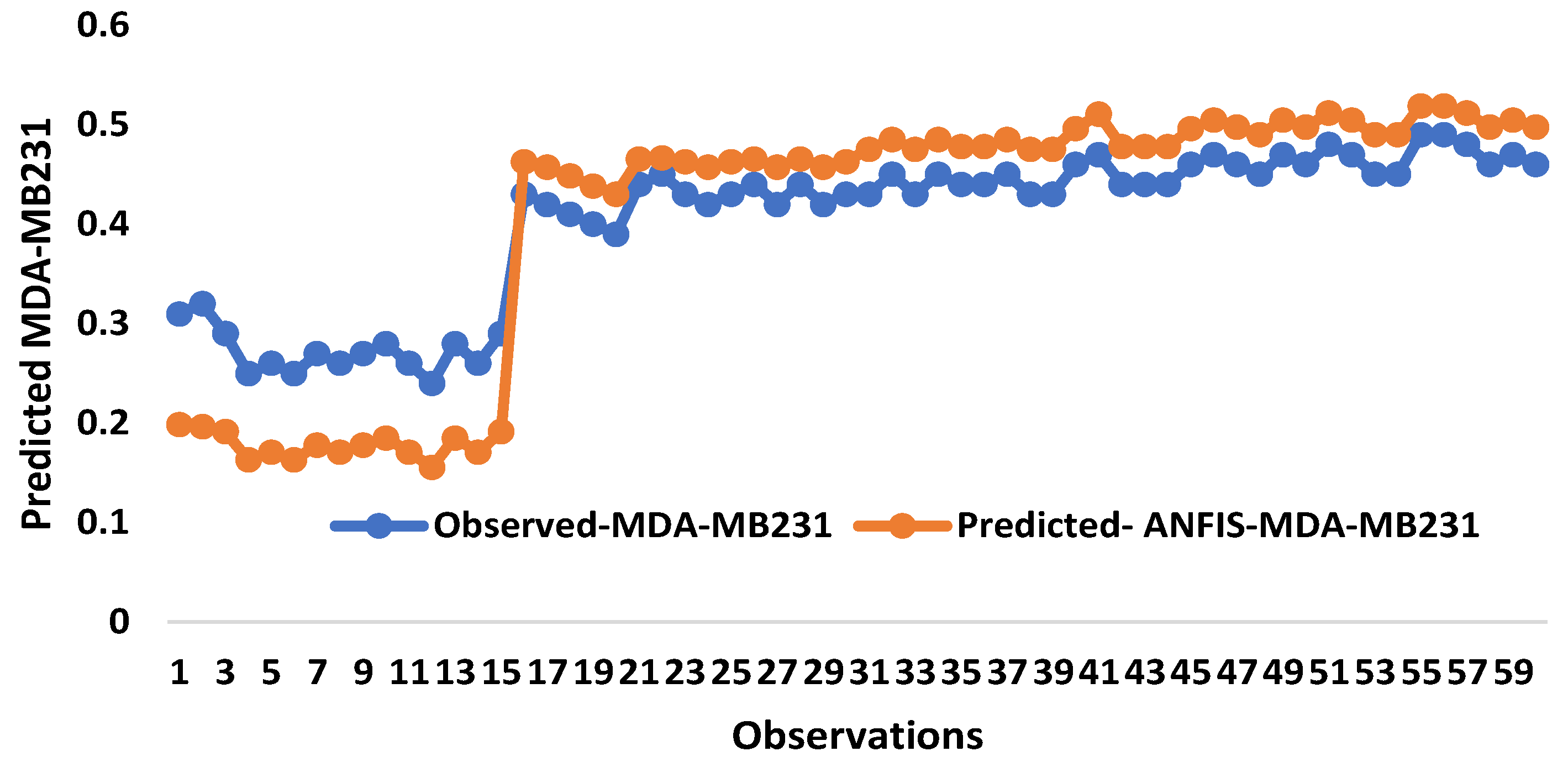
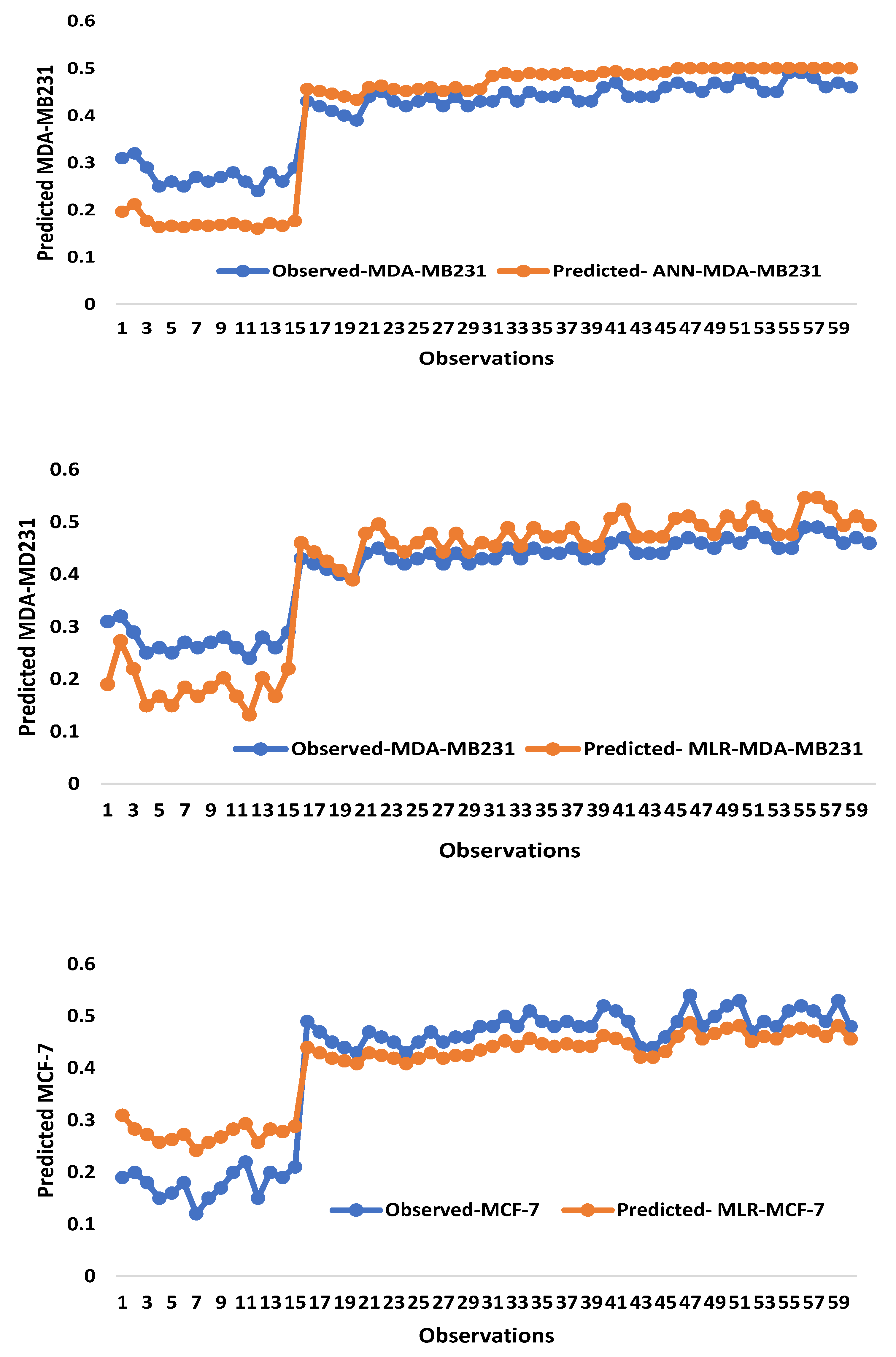
2.2. Artificial Intelligence-Based Models
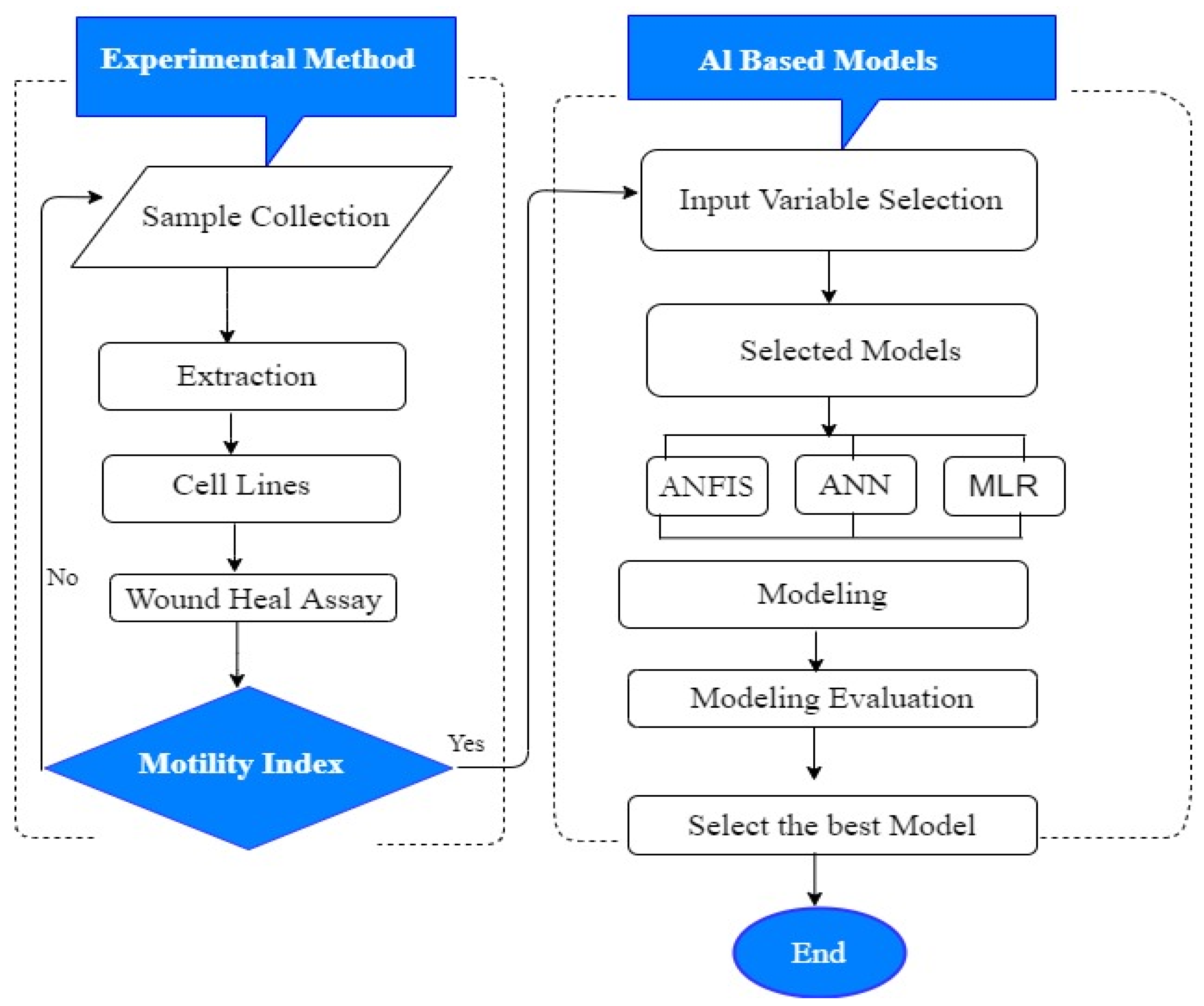
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. Determination of Phytochemical Composition of the Extract
3.3. Cell Lines and Culture Conditions
3.4. Toxicity Assay
3.5. Proliferation Assay
3.6. Wound-Heal Assay
3.7. Proposed Methodology
3.8. Back Propagation Neural Network (BPNN)
3.9. Adaptive Neuro-Fuzzy Inference System (ANFIS)
3.10. Multilinear Regression (MLR)
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.E.; Fedewa, S.A.; Sauer, A.G.; Kramer, J.L.; Smith, R.A.; Jemal, A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. Cancer J. Clin. 2016, 66, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumor-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Rep. Reg. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Eccles, S.A.; Box, C.; Court, W. Cell migration/invasion assays and their application in cancer drug discovery. Biotechnol. Ann. Rev. 2005, 11, 391–421. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. Tumor cell-organ microenvironment interations in the pathogenesis of cancer metastasis. Endocr. Rev. 2007, 28, 297–321. [Google Scholar] [CrossRef]
- Gao, C.-F.; Xie, Q.; Su, Y.-L.; Koeman, J.; Khoo, S.K.; Gustafson, M.; Knudsen, B.S.; Hay, R.; Shinomiya, N.; Woude, G.F.V. Proliferation and invasion: Plasticity in tumor cells. Proc. Natl. Acad. Sci. USA 2005, 102, 10528–10533. [Google Scholar] [CrossRef]
- Mazzocca, A.; Coppari, R.; De Franco, R.; Cho, J.-Y.; Libermann, T.A.; Pinzani, M.; Toker, A. A secreted form of ADAM9 promotes carcinoma invasion through tumor–stromal interactions. Cancer Res. 2005, 65, 4728–4738. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005, 15, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kavaz, D.; Abubakar, A.L.; Rizaner, N.; Umar, H. Biosynthesized ZnO nanoparticles using Albizia lebbeck extract induced biochemical and morphological alterations in Wistar rats. Molecules 2021, 26, 3864. [Google Scholar] [CrossRef] [PubMed]
- Al-Massarani, S.M.; El Gamal, A.A.; Abd El Halim, M.F.; Al-Said, M.S.; Abdel-Kader, M.S.; Basudan, O.A.; Alqasoumi, S.I. New acyclic secondary metabolites from the biologically active fraction of Albizia lebbeck flowers. Saudi Pharm. J. 2016, 25, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.K.; Gadge, P.P.; Shinde, A.A.; Padul, M.V.; Kachole, M.S. Characterization of the AlTI13 protein from Indian siris (Albizia lebbeck) that inhibits the growth of cotton bollworm (Helicoverpa armigera). J. Asia Pac. Entomol. 2014, 17, 319–325. [Google Scholar] [CrossRef]
- Missanjo, E.; Maya, C.; Kapira, D.; Banda, H.; Kamanga-Thole, G. Effect of Seed Size and Pretreatment Methods on Germination of Albizia lebbeck; ISRN Botany: New York, NY, USA, 2013; pp. 1–4. [Google Scholar]
- Kennedy, P.M.; Lowry, J.; Coates, D.B.; Oerlemans, J. Utilisation of tropical dry season grass by ruminants is increased by feeding fallen leaf of siris (Albizia lebbeck). Anim. Feed. Sci. 2002, 96, 175–192. [Google Scholar] [CrossRef]
- Muhammad, Z.; Muhammed, S.A. Compositional Studies and Antioxidant Potential of Albizia lebeck (L.) Benth. pods and seeds. Turkish J. Biol. 2013, 37, 25–32. [Google Scholar]
- Noté, O.P.; Jihu, D.; Antheaume, C.; Zeniou, M.; Pegnyemb, D.E.; Guillaume, D.; Chneiwess, H.; Kilhoffer, M.C.; Lobstein, A. Triterpenoid saponins from Albizia lebbeck (L.) Benth and their inhibitory effect on the survival of high grade human brain tumor cells. Carbohydr. Res. 2015, 404, 26–33. [Google Scholar] [CrossRef]
- Umar, H.; Kavaz, D.; Rizaner, N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 2019, 14, 87–100. [Google Scholar] [CrossRef]
- Hasan, S. Prediction of Breast Cancer Type Based on Artificial Intelligence Technique. Int. J. Adv. Sci. Res. Eng. Technol. 2019, 5, 43–50. [Google Scholar] [CrossRef]
- Pan, C.; Schoppe, O.; Parra-Damas, A.; Cai, R.; Todorov, M.I.; Gondi, G.; von Neubeck, B.; Böğürcü-Seidel, N.; Seidel, S.; Sleiman, K.; et al. Deep Learning Reveals Cancer Metastasis and Therapeutic Antibody Targeting in the Entire Body. Cell 2019, 179, 1661–1676.e19. [Google Scholar] [CrossRef]
- Liu, Y.; Kohlberger, T.; Norouzi, M.; Dahl, G.E.; Smith, J.L.; Mohtashamian, A.; Olson, N.; Peng, L.H.; Hipp, J.D.; Stumpe, M.C.; et al. Artificial intelligence–based breast cancer nodal metastasis detection insights into the black box for pathologists. Arch. Pathol. Lab. Med. 2019, 143, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Sadoughi, F.; Kazemy, Z.; Hamedan, F.; Owji, L.; Rahmanikatigari, M.; Azadboni, T.T. Artificial intelligence methods for the diagnosis of breast cancer by image processing: A review. Breast Cancer 2018, 10, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Khosla, A.; Gargeya, R.; Irshad, H.; Beck, A.H. Deep Learning for Identifying Metastatic Breast Cancer. arXiv 2016, arXiv:1606.05718. [Google Scholar]
- Roya, K.; Fatemeh, G. Screening of total phenol and flavonoid content, antioxidant and antibacterial activities of the methanolic extracts of three Silene species from Iran. Int. J. Agric. Crop Sci. 2013, 5, 305–312. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid, and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef]
- Kavaz, D.; Umar, H.; Shehu, S. Synthesis, characterization, antimicrobial and antimetastatic activity of silver nanoparticles synthesized from Ficus ingens leaf. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S1193–S1203. [Google Scholar] [CrossRef]
- El-Fayoumy, E.A.; Shanab, S.M.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement. Med. Ther. 2021, 21, 51. [Google Scholar] [CrossRef]
- Maryam, I.; Huzaifa, U.; Hindatu, H.; Zubaida, S. Nanoencapsulation of essential oils with enhanced antimicrobial activity: A new way of combating antimicrobial Resistance. J. Pharmacogn. Phytochem. 2015, 4, 165–170. [Google Scholar]
- Kampf, G.; Hollingsworth, A. Comprehensive bactericidal activity of an ethanol-based hand gel in 15 seconds. Ann. Clin. Microbiol. Antimicrob. 2008, 7, 2. [Google Scholar] [CrossRef]
- Musa, M.A.; Badisa, V.L.D.; Latinwo, L.M.; Cooperwood, J.; Sinclair, A.; Abdullah, A. Cytotoxic activity of new acetoxycoumarin derivatives in cancer cell lines. Anticancer Res. 2011, 31, 2017–2022. [Google Scholar] [PubMed]
- Ezez, D.; Mekonnen, N.; Tefera, M. Phytochemical analysis of Withania somnifera leaf extracts by GC-MS and evaluating antioxidants and antibacterial activities. Int. J. Food Prop. 2023, 26, 581–590. [Google Scholar] [CrossRef]
- Shin, S.Y.; Park, J.; Jung, Y.; Lee, Y.H.; Koh, D.; Yoon, Y.; Lim, Y. Anticancer activities of cyclohexenone derivatives. Appl. Biol. Chem. 2020, 63, 82. [Google Scholar] [CrossRef]
- Ochieng, P.J.; Hussain, A.; Dombi, J.; Krész, M. An efficient weighted network centrality approach for exploring mechanisms of action of the Ruellia herbal formula for treating rheumatoid arthritis. Appl. Netw. Sci. 2023, 8, 7. [Google Scholar] [CrossRef]
- Tao, T.; He, C.; Deng, J.; Huang, Y.; Su, Q.; Peng, M.; Yi, M.; Darko, K.O.; Zou, H.; Yang, X. A novel synthetic derivative of quercetin, 8-trifluoromethyl-3,5,7,3’,4’-O-pentamethyl-quercetin, inhibits bladder cancer growth by targeting the AMPK/mTOR signaling pathway. Oncotarget 2017, 8, 71657–71671. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Mikołajczak, P.; Kujawski, R.; Wielgus, K.; Klejewski, A.; Wolski, H.; Seremak-Mrozikiewicz, A. Pharmacological Effect of Quercetin in Hypertension and its Potential Application in Pregnancy-Induced Hypertension: Review of In Vitro, In Vivo, and Clinical Studies. Evid.-Based Complement. Altern. Med. 2018, 2018, 7421489. [Google Scholar] [CrossRef]
- Oğraş, T.T.; Tahtasakal, E.; Öztürk, S. In vitro production of tropane alkaloids from Brugmansia suaveolens. Int. J. Second. Metab. 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Vasanthi, P. Invitro cytotoxicity study of leaf extract of A. lebbeck exract on different canncer cell lines. Int. J. Biotechnol. Biochem. 2013, 9, 313–318. [Google Scholar]
- Isbilen, O.; Rizaner, N.; Volkan, E. Anti-proliferative and cytotoxic activities of Allium autumnale P. H. Davis (Amaryllidaceae) on human breast cancer lines MCF-7 and MDA-MB 231. BMC Complement. Altern. Med. 2018, 18, 30. [Google Scholar] [CrossRef]
- Lambertini, E.; Piva, R.; Khan, M.T.; Lampronti, L.; Bianchi, N.; Borgatti, M.; Gambari, R. Effects of extracts from Bangladeshi medicinal plants on in vitro proliferation of human breast cancer cell lines and expression of estrogen receptor alpha gene. Int. J. Oncol. 2004, 24, 419–423. [Google Scholar] [CrossRef]
- Choene, M.; Motadi, L. Validation of the Antiproliferative Effects of Euphorbia tirucalli Extracts in Breast Cancer Cell Lines. Mol. Biol. 2016, 50, 98–110. [Google Scholar] [CrossRef]
- Arya, M.; Bott, S.R.; Shergill, I.S.; Ahmed, H.U.; Williamson, M.; Patel, H.R. The metastatic cascade in prostate cancer. Surg. Oncol. 2006, 15, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.K.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef] [PubMed]
- Gumushan-Aktas, H.; Altun, S. Effects on Hedera helix L. extracts on rat prostate cancer cell proliferation and motility. Oncol. Lett. 2016, 12, 2985–2991. [Google Scholar] [CrossRef]
- Kavaz, D.; Umar, H.; Aliyu, M.A. Green Synthesized Metallic Oxide Nanomaterials for Diverse Application. J Nanoworld. 2022, 8, 14–22. [Google Scholar]
- Abba, S.I.; Pham, Q.B.; Usman, A.G.; Linh, N.T.T.; Aliyu, D.S.; Nguyen, Q.; Bach, Q.-V. Emerging evolutionary algorithm integrated with kernel principal component analysis for modelling the performance of a water treatment plant. J. Water Process. Eng. 2020, 33, 101081. [Google Scholar] [CrossRef]
- Hadi, S.J.; Abba, S.I.; Sammen, S.S.; Salih, S.Q.; Al-Ansari, N.; Yaseen, Z.M. Non-Linear Input Variable Selection Approach Integrated with Non-Tuned Data Intelligence Model for Streamflow Pattern Simulation. IEEE Access 2019, 7, 141533–141548. [Google Scholar] [CrossRef]
- Pham, Q.B.; Abba, S.I.; Usman, A.G.; Linh, N.T.T.; Gupta, V.; Malik, A.; Costache, R.; Vo, N.D.; Tri, D.Q. Potential of Hybrid Data Intelligence Algorithms for Multi-Station Modelling of Rainfall. Water Resour. Manag. 2019, 33, 5067–5087. [Google Scholar] [CrossRef]
- Yaseen, Z.M.; Jaafar, O.; Deo, R.C.; Kisi, O.; Adamowski, J.; Quilty, J.; El-shafie, A. Stream-flow forecasting using extreme learning machines: A case study in a semiarid region in Iraq. Water Resour. Manag. 2019, 33, 5067–5087. [Google Scholar] [CrossRef]
- Yaseen, Z.M.; Sulaiman, S.O.; Deo, R.C.; Chau, K.-W. An enhanced extreme learning machine model for river flow forecasting: State-of-the-art, practical applications in water resource engineering area and future research direction. J. Hydrol. 2018, 569, 387–408. [Google Scholar] [CrossRef]
- Ghali, U.M.; Usman, A.G.; Chellube, Z.M.; Degm, M.A.A.; Hoti, K.; Umar, H.; Abba, S.I. Advanced chromatographic technique for performance simulation of anti-Alzheimer agent: An ensemble machine learning approach. SN Appl. Sci. 2020, 2, 1871. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Kumar, J.; Dhar, P.; Tayade, A.B.; Gupta, D.; Chaurasia, O.P.; Upreti, D.K.; Toppo, K.; Arora, R.; Suseela, M.R.; Srivastava, R.B. Chemical Composition and Biological Activities of Trans-Himalayan Alga Spirogyra porticalis (Muell.) Cleve. PLoS ONE 2015, 10, e0118255. [Google Scholar] [CrossRef]
- Fraser, S.P.; Ding, Y.; Liu, A.; Foster, C.S.; Djamgoz, M.B. Tetrodoxin suppresses morphological enhancement of the metastatic Mat-LyLu rat prostate cancer cell line. Cell Tissue Res. 1999, 295, 505–512. [Google Scholar] [CrossRef]
- Jang, J.-S.R. ANFIS: Adaptive-Network-Based Fuzzy Inference System. IEEE Trans. Syst. Man Cybern. 1993, 23, 665–685. [Google Scholar] [CrossRef]
- Khademi, F.; Jamal, S.M.; Deshpande, N.; Londhe, S. Predicting strength of recycled aggregate concrete using Artificial Neural Network, Adaptive Neuro-Fuzzy Inference System and Multiple Linear Regression. Int. J. Sustain. Built Environ. 2016, 5, 355–369. [Google Scholar] [CrossRef]
- Elkiran, G.; Nourani, V.; Abba, S.; Abdullahi, J. Artificial intelligence-based approaches for multi-station modelling of dissolve oxygen in river. Glob. J. Environ. Sci. Manag. 2018, 4, 439–450. [Google Scholar] [CrossRef]



| S/n | RT | Peak Area | Area % | Compound Detected | Biological Activity |
|---|---|---|---|---|---|
| 1 | 13.58 | 96,734 | 0.61 | Cytidine, N-acetyl-(CAS) | Antimicrobial [28] |
| 2 | 30.91 | 236,928 | 1.49 | 1-Hexadecanol, 2-methyl-(CAS) | Anti-cancer [29,30] |
| 3 | 31.61 | 88,990 | 0.56 | 1-Hexadecanol, 2-methyl-(CAS) | Anti-cancer, antioxidant and antimicrobial [29,30] |
| 4 | 32.31 | 579,671 | 3.63 | 2-Propenoic acid, tetradecyl ester | Anti-tumour, anti-inflammatory, anti-mutagenic, [31] |
| 5 | 33.32 | 369,272 | 2.31 | Hexadecanoic acid, 2,3 dihydroxypropyl ester | Anti-tumour [32] |
| 6 | 36.54 | 445,820 | 2.79 | Hexadecanoic acid, 2,3-dihydroxypropylester | Antioxidant, antimicrobial [33] |
| 7 | 38.02 | 189,611 | 1.19 | QUERCETIN 7,3’,4’-TRIMETHOXY | Anti-tumour, [26] anti-hypertensive [34] |
| 8 | 38.46 | 802,577 | 5.03 | Dodecanoic acid, 2,3-bis(acetyloxy)propyl ester | Anti-inflammatory, antibacterial [35] |
| 9 | 39.29 | 466,439 | 2.92 | Hexadecanoic acid, 2,3-dihydroxypropyl ester | Anti-tumour [32] |
| 10 | 39.64 | 315,914 | 1.98 | Hexadecanoic acid, 2,3-dihydroxypropyl ester | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 11 | 40.40 | 220,048 | 1.38 | 2-Myristynoyl pantetheine | - |
| 12 | 41.62 | 321,977 | 2.02 | QUERCETIN 7,3’,4’-TRIMETHOXY | Anti-hypertensive [34] |
| 13 | 42.49 | 1,104,712 | 6.93 | Cyclopropanetetradecanoic acid, 2-octyl-, methyl ester | Antibacterial [35] |
| 14 | 43.05 | 561,757 | 3.52 | Hexadecanoic acid, 2,3-dihydroxypropyl ester | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 15 | 43.70 | 93,671 | 0.59 | QUERCETIN 7,3’,4’-TRIMETHOXY | Antioxidant, anti-tumour [36] anti-hypertensive [37] |
| 16 | 43.96 | 500,990 | 3.14 | TRANS-2-PHENYL-1,3-DIOXOLANE-4-METHYL OCTADEC-9,12,15-TRIENOATE | Anti-cancer [38] |
| 17 | 44.50 | 160,315 | 1.01 | Hexadecanoic acid, 2,3 dihydroxypropyl ester | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 18 | 44.82 | 102,210 | 0.64 | Hexadecanoic acid, 2,3-dihydroxypropyl ester | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 19 | 45.52 | 415,295 | 2.60 | TRANS-2-PHENYL-1,3-DIOXOLANE-4-METHYL OCTADEC-9,12,15-TRIENOATE | Anti-cancer [38] |
| 20 | 45.75 | 180,204 | 1.13 | Hexadecanoic acid, 2,3-dihydroxypropyl ester | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 21 | 46.34 | 1,532,251 | 9.61 | Dotriacontane | Anti-inflammatory, anti-thrombotic, antiviral [33] |
| 22 | 47.11 | 242,196 | 1.52 | “Hexadecanoic acid, 2,3-dihydroxypropyl ester” | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 23 | 47.40 | 217,328 | 1.36 | Hexadecanoic acid, 2,3-dihydroxypropyl ester (CAS) | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 24 | 47.99 | 252,815 | 1.58 | “Hexadecanoic acid, 2,3-dihydroxypropyl ester” | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 25 | 48.38 | 307,595 | 1.93 | QUERCETIN 7,3’,4’-TRIMETHOXY | Antioxidant, anti-tumour [36], anti-hypertensive [37] |
| 26 | 49.33 | 2,392,813 | 15.00 | Nonacosane (CAS) | Antimicrobial [35] |
| 27 | 49.93 | 109,234 | 0.68 | Octadecanoic acid, 2,3-dihydroxypropyl ester | Anti-proliferative, anti-cancer [32,34] |
| 28 | 50.48 | 281,862 | 1.77 | “Hexadecanoic acid, 2,3-dihydroxypropyl ester” | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 29 | 51.39 | 766,970 | 4.81 | “Hexadecanoic acid, 2,3-dihydroxypropyl ester” | Anti-tumour [32], antioxidant, antimicrobial [33] |
| 30 | 51.87 | 92,228 | 0.58 | “9,12,15-Octadecatrienoic acid, 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl)oxy]methyl]ethyl ester, (Z,Z,Z)” | Anti-proliferative, anti-cancer [32,34] |
| 31 | 52.09 | 2,502,977 | 15.69 | Heptacosane (CAS) | Antimicrobial [35] |
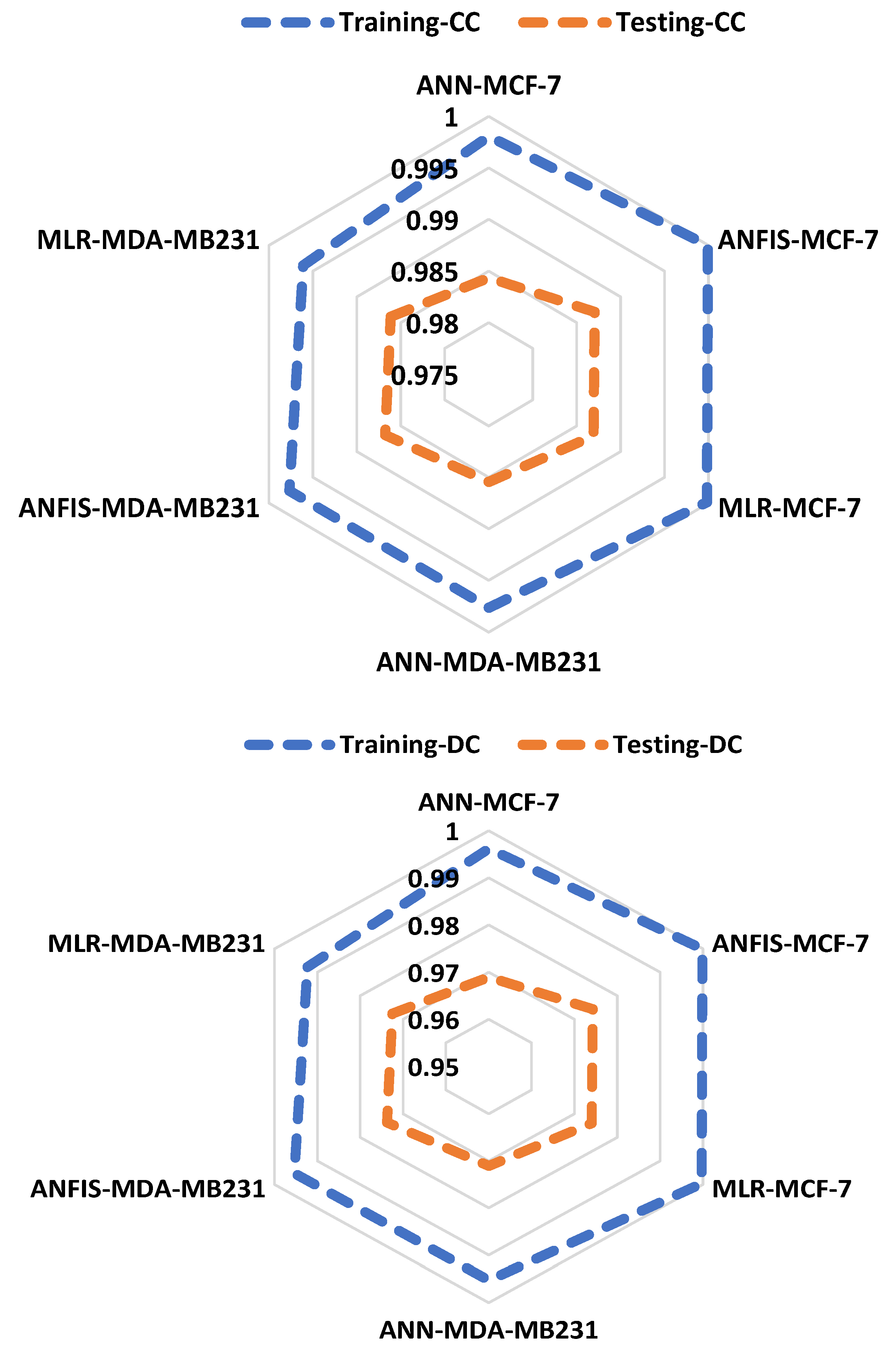
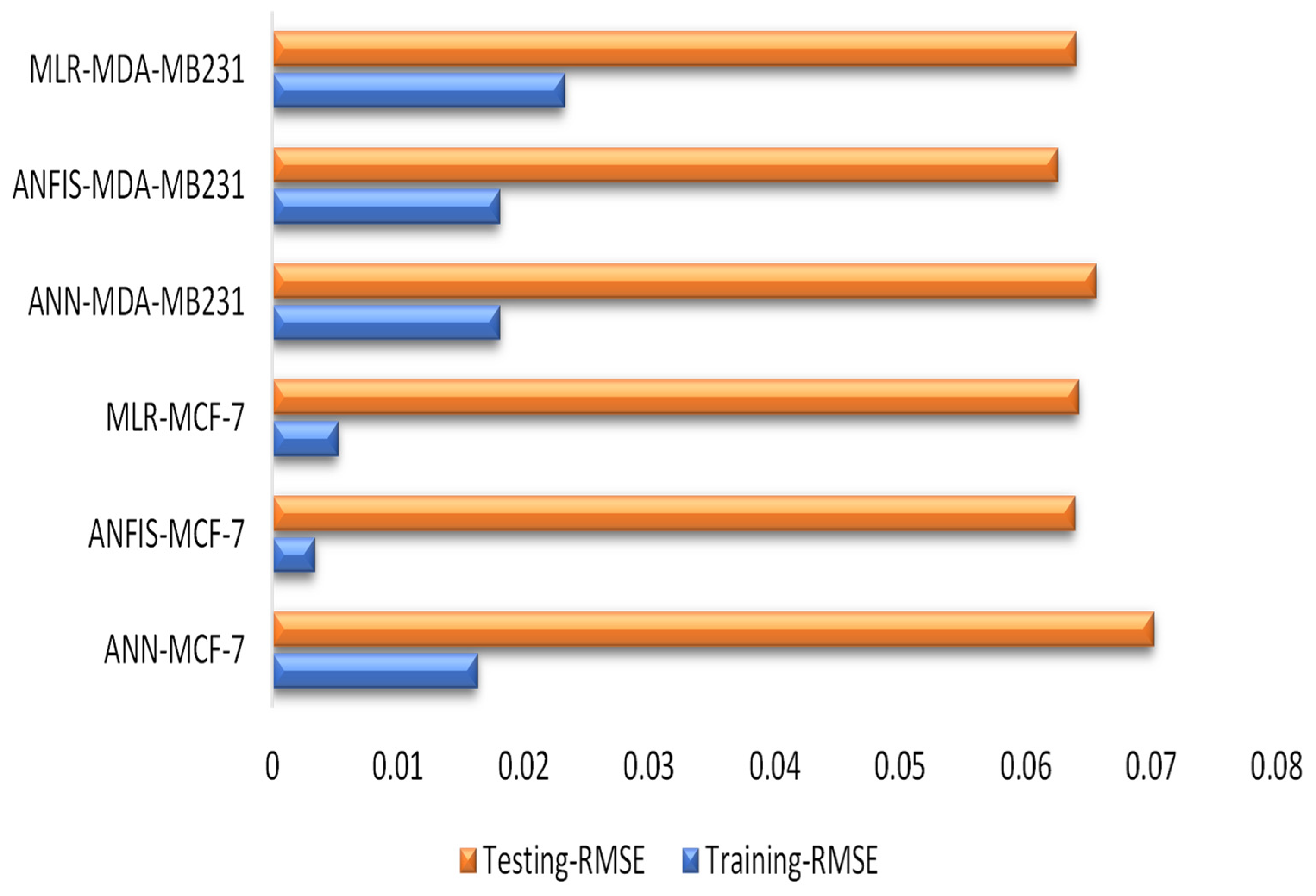
| Training | Testing | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DC | RMSE | MSE | CC | DC | RMSE | MSE | CC | ||
| ANN-MCF-7 | 0.9962 | 0.0163 | 0.0003 | 0.9981 | 0.9689 | 0.0702 | 0.0049 | 0.9843 | |
| ANFIS-MCF-7 | 0.9998 | 0.0033 | 0.0000 | 0.9999 | 0.9742 | 0.0639 | 0.0041 | 0.9870 | |
| MLR-MCF-7 | 0.9996 | 0.0052 | 0.0000 | 0.9998 | 0.9740 | 0.0642 | 0.0041 | 0.9869 | |
| ANN-MDA-MB231 | 0.9953 | 0.0181 | 0.0003 | 0.9976 | 0.9711 | 0.0656 | 0.0043 | 0.9854 | |
| ANFIS-MDA-MB231 | 0.9953 | 0.0181 | 0.0003 | 0.9976 | 0.9737 | 0.0625 | 0.0039 | 0.9868 | |
| MLR-MDA-MB231 | 0.9922 | 0.0232 | 0.0005 | 0.9961 | 0.9725 | 0.0640 | 0.0041 | 0.9861 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, H.; Rizaner, N.; Usman, A.G.; Aliyu, M.R.; Adun, H.; Ghali, U.M.; Uzun Ozsahin, D.; Abba, S.I. Prediction of Cell Migration in MDA-MB 231 and MCF-7 Human Breast Cancer Cells Treated with Albizia Lebbeck Methanolic Extract Using Multilinear Regression and Artificial Intelligence-Based Models. Pharmaceuticals 2023, 16, 858. https://doi.org/10.3390/ph16060858
Umar H, Rizaner N, Usman AG, Aliyu MR, Adun H, Ghali UM, Uzun Ozsahin D, Abba SI. Prediction of Cell Migration in MDA-MB 231 and MCF-7 Human Breast Cancer Cells Treated with Albizia Lebbeck Methanolic Extract Using Multilinear Regression and Artificial Intelligence-Based Models. Pharmaceuticals. 2023; 16(6):858. https://doi.org/10.3390/ph16060858
Chicago/Turabian StyleUmar, Huzaifa, Nahit Rizaner, Abdullahi Garba Usman, Maryam Rabiu Aliyu, Humphrey Adun, Umar Muhammad Ghali, Dilber Uzun Ozsahin, and Sani Isah Abba. 2023. "Prediction of Cell Migration in MDA-MB 231 and MCF-7 Human Breast Cancer Cells Treated with Albizia Lebbeck Methanolic Extract Using Multilinear Regression and Artificial Intelligence-Based Models" Pharmaceuticals 16, no. 6: 858. https://doi.org/10.3390/ph16060858
APA StyleUmar, H., Rizaner, N., Usman, A. G., Aliyu, M. R., Adun, H., Ghali, U. M., Uzun Ozsahin, D., & Abba, S. I. (2023). Prediction of Cell Migration in MDA-MB 231 and MCF-7 Human Breast Cancer Cells Treated with Albizia Lebbeck Methanolic Extract Using Multilinear Regression and Artificial Intelligence-Based Models. Pharmaceuticals, 16(6), 858. https://doi.org/10.3390/ph16060858












