The Effect of Dietary Advice Aimed at Increasing Protein Intake on Oral Health and Oral Microbiota in Older Adults: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
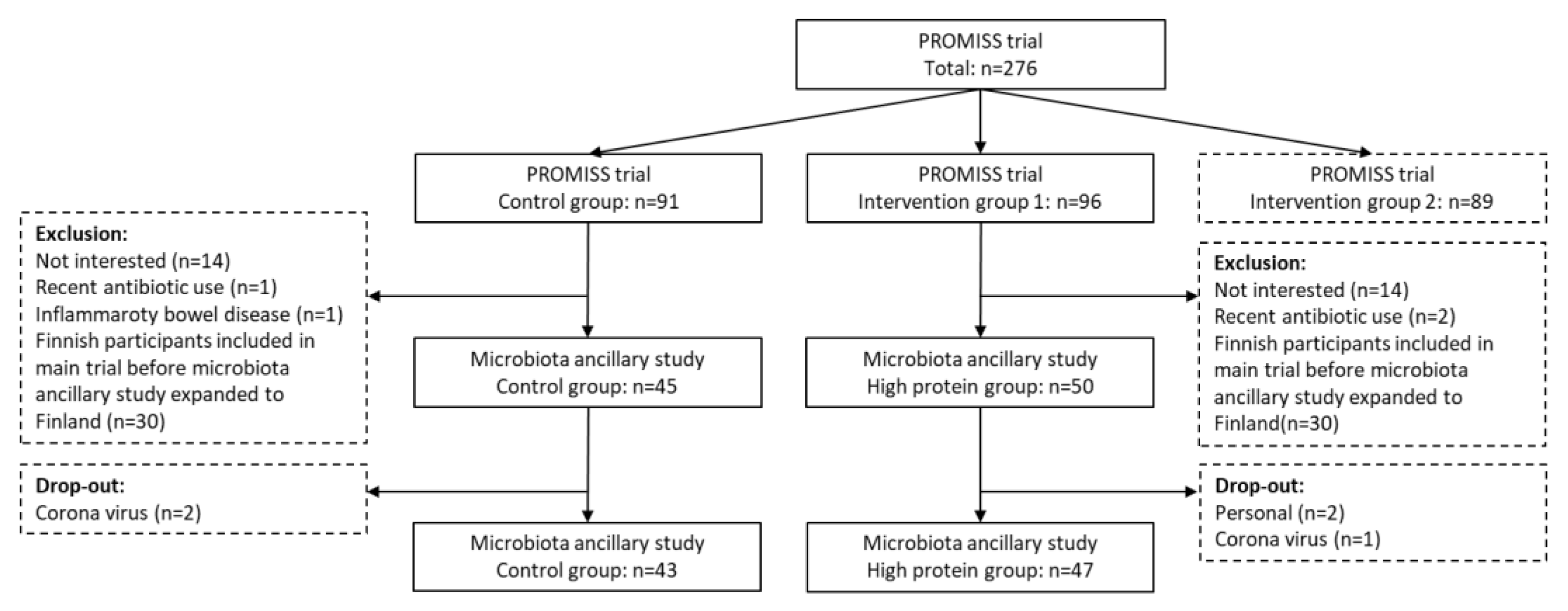
2.1. Participants
2.2. Intervention
2.3. Oral Health and Bio-Sampling
2.4. 16S rRNA Sequencing
2.5. Statistics
3. Results
3.1. Dietary Intake
3.2. Oral Health
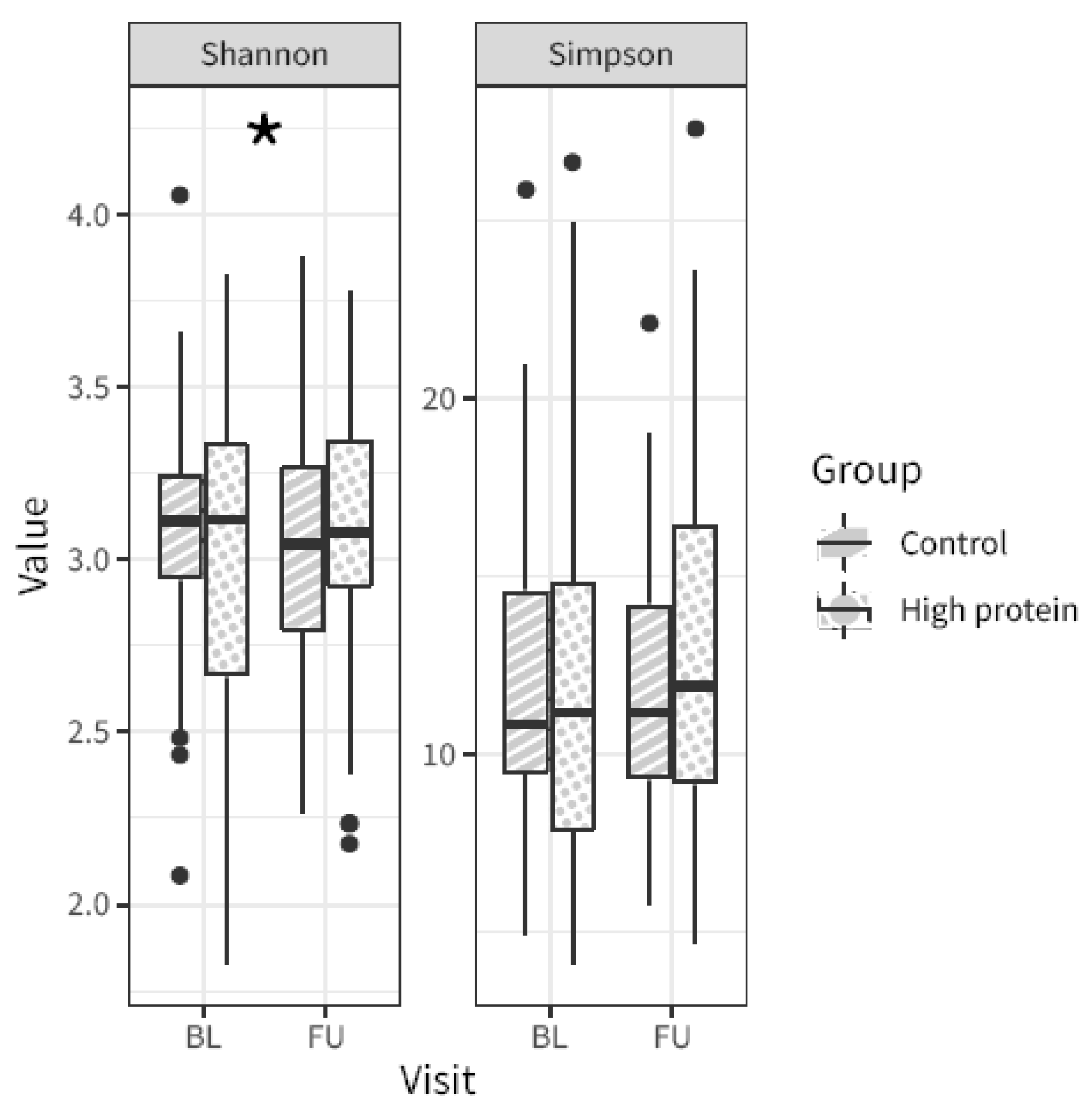
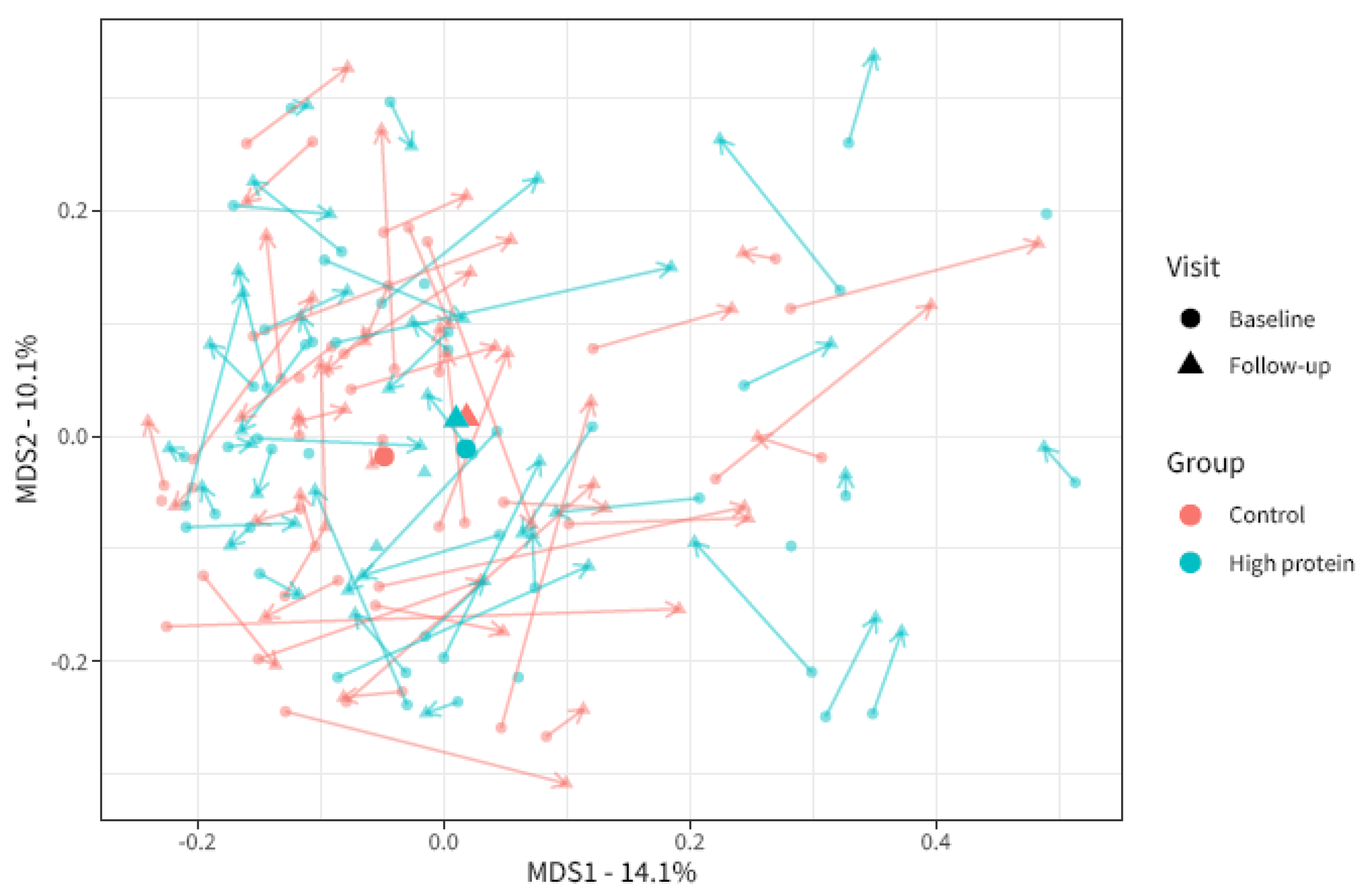
3.3. Oral Microbiota
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and oral health. Dis. Mon. 2019, 65, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Leij-Halfwerk, S.; Verwijs, M.H.; van Houdt, S.; Borkent, J.W.; Guaitoli, P.R.; Pelgrim, T.; Heymans, M.W.; Power, L.; Visser, M.; Corish, C.A.; et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: A systematic review and meta-analysis. Maturitas 2019, 126, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Gil-Montoya, J.A.; de Mello, A.L.; Barrios, R.; Gonzalez-Moles, M.A.; Bravo, M. Oral health in the elderly patient and its impact on general well-being: A nonsystematic review. Clin. Interv. Aging 2015, 10, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.A.; Warren, J.J.; Hand, J.S.; Xie, X.J.; Stumbo, P.J. Oral health, nutrient intake and dietary quality in the very old. J. Am. Dent. Assoc. 2002, 133, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Jin, B.H. Comparison of oral health status and daily nutrient intake between elders who live alone and elders who live with family: Based on the Korean National Health and Nutrition Examination Survey (KNHANES VI) (2013–2015). Gerodontology 2018, 35, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Taylor, G.W.; Manz, M.C.; Yoshihara, A.; Sato, M.; Muramatsu, K.; Watanabe, R.; Miyazaki, H. Oral health status: Relationship to nutrient and food intake among 80-year-old Japanese adults. Community Dent. Oral. Epidemiol. 2014, 42, 441–450. [Google Scholar] [CrossRef]
- Bomfim, R.A.; de Souza, L.B.; Corrente, J.E. Tooth loss and its relationship with protein intake by elderly Brazilians-A structural equation modelling approach. Gerodontology 2018, 35, 51–58. [Google Scholar] [CrossRef]
- Wysokinski, A.; Sobow, T.; Kloszewska, I.; Kostka, T. Mechanisms of the anorexia of aging—A review. Age 2015, 37, 9821. [Google Scholar] [CrossRef]
- Pflipsen, M.; Zenchenko, Y. Nutrition for oral health and oral manifestations of poor nutrition and unhealthy habits. Gen. Dent. 2017, 65, 36–43. [Google Scholar]
- Cattaneo, C.; Riso, P.; Laureati, M.; Gargari, G.; Pagliarini, E. Exploring Associations between Interindividual Differences in Taste Perception, Oral Microbiota Composition, and Reported Food Intake. Nutrients 2019, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public. Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on Dietary Reference Values for protein. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). EFSA J. 2012, 10, 2257. [Google Scholar]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Wijnhoven, H.A.H.; Jyvakorpi, S.K.; Suominen, M.H.; Niskanen, R.; Bosmans, J.E.; Brouwer, I.A.; Fluitman, K.S.; Klein, M.C.; Kuijper, L.D.; et al. Effectiveness and cost-effectiveness of personalised dietary advice aiming at increasing protein intake on physical functioning in community-dwelling older adults with lower habitual protein intake: Rationale and design of the PROMISS randomised controlled trial. BMJ Open 2020, 10, e040637. [Google Scholar] [PubMed]
- Fluitman, K.S.; Wijdeveld, M.; Davids, M.; van Ruiten, C.C.; Reinders, I.; Wijnhoven, H.A.H.; Keijser, B.J.; Visser, M.; Nieuwdorp, M.; IJzerman, R.G. Personalized Dietary Advice to Increase Protein Intake in Older Adults Does Not Affect the Gut Microbiota, Appetite or Central Processing of Food Stimuli in Community-Dwelling Older Adults: A Six-Month Randomized Controlled Trial. Nutrients 2023, 15, 332. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Kiesswetter, E.; Hengeveld, L.M.; Keijser, B.J.F.; Volkert, D.; Visser, M. Oral health determinants of incident malnutrition in community-dwelling older adults. J. Dent. 2019, 85, 73–80. [Google Scholar] [CrossRef]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef]
- Keijser, B.J.F.; Agamennone, V.; van den Broek, T.J.; Caspers, M.; van de Braak, A.; Bomers, R.; Havekes, M.; Schoen, E.; van Baak, M.; Mioch, D.; et al. Dose-dependent impact of oxytetracycline on the veal calf microbiome and resistome. BMC Genom. 2019, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Fluitman, K.S.; van den Broek, T.J.; Nieuwdorp, M.; Visser, M.; IJzerman, R.G.; Keijser, B.J.F. Associations of the oral microbiota and Candida with taste, smell, appetite and undernutrition in older adults. Sci. Rep. 2021, 11, 23254. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R package. 2018. Available online: https://cran.r-project.org/package=vegan (accessed on 7 September 2023).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.E.; Roussos, P. Dream: Powerful differential expression analysis for repeated measures designs. Bioinformatics 2021, 37, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.E.; Schadt, E.E. variancePartition: Interpreting drivers of variation in complex gene expression studies. BMC Bioinform. 2016, 17, 483. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Hall, M.W.; Singh, N.; Ng, K.F.; Lam, D.K.; Goldberg, M.B.; Tenenbaum, H.C.; Neufeld, J.D.; Beiko, R.G.; Senadheera, D.B. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. NPJ Biofilms Microbiomes 2017, 3, 2. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, H.; Mihindukulasuriya, K.A.; La Rosa, P.S.; Wylie, K.M.; Vishnivetskaya, T.; Podar, M.; Warner, B.; Tarr, P.I.; Nelson, D.E.; et al. Biogeography of the ecosystems of the healthy human body. Genome Biol. 2013, 14, R1. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.E.; Kim, H.N.; Jun, E.J.; Lee, J.H.; Kim, J.S.; Kim, J.B. Association of Self-Perceived Oral Health and Function with Clinically Determined Oral Health Status among Adults Aged 35(-)54 Years: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2018, 15, 1681. [Google Scholar] [CrossRef]
- Douglass, C.W.; Berlin, J.; Tennstedt, S. The validity of self-reported oral health status in the elderly. J. Public Health Dent. 1991, 51, 220–222. [Google Scholar] [CrossRef]
- Asakawa, M.; Takeshita, T.; Furuta, M.; Kageyama, S.; Takeuchi, K.; Hata, J.; Ninomiya, T.; Yamashita, Y. Tongue Microbiota and Oral Health Status in Community-Dwelling Elderly Adults. mSphere 2018, 3, 10–1128. [Google Scholar] [CrossRef]
| High Protein Group (n = 47) | Control Group (n = 43) | p-Value | |
|---|---|---|---|
| Characteristics | |||
| Age (years) | 74.6 ± 4.8 | 74.1 ± 4.7 | 0.572 |
| Sex (Male) | 28 (59.6) | 19 (44.2) | 0.205 |
| BMI (kg/m2) | 26.1 ± 2.9 | 26.8 ± 2.9 | 0.227 |
| MMSE | 29 (27–30) | 29 (27–30) | 0.573 |
| Education | 0.11 | ||
| Low | 3 (6.4) | 0 (0.0) | |
| Middle | 8 (17.0) | 13 (30.2) | |
| High | 36 (76.6) | 30 (69.8) | |
| Study site (Amsterdam) | 35 (74.5) | 33 (76.7) | 1 |
| Time to follow-up (days) | 182 (178–187) | 185 (178–191) | 0.187 |
| Oral Hygiene | |||
| Dentition | 0.842 | ||
| No teeth | 5 (10.9) | 6 (14.0) | |
| Some teeth | 7 (15.2) | 8 (18.6) | |
| Most to all teeth | 34 (73.9) | 29 (67.4) | |
| Teeth brushing | 0.698 | ||
| <2×/day | 17 (36.2) | 12 (27.9) | |
| 2×/day | 24 (51.1) | 25 (58.1) | |
| >2×/day | 6 (12.8) | 6 (14.0) | |
| Interdental cleaning | 0.802 | ||
| Never | 7 (14.9) | 6 (14.0) | |
| sporadic | 15 (31.9) | 11 (25.6) | |
| regularly | 25 (53.2) | 26 (60.5) | |
| Food intake | |||
| Energy intake (kcal/day) | 1701.9 ± 427.4 | 1611.0 ± 301.8 | 0.244 |
| Protein intake (g/kg aBW/day) | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.689 |
| Protein intake (g/day) | 62.9 ± 14.0 | 60.9 ± 10.4 | 0.457 |
| Carbohydrate intake (g/day) | 181.4 ± 56.4 | 170.9 ± 47.6 | 0.347 |
| Fat intake (g/day) | 67.3 ± 20.4 | 66.1 ± 18.3 | 0.771 |
| High Protein Group (n = 47) | Control Group (n = 43) | Differences at Follow-Up | |||
|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | OR (95%CI) | |
| Any oral discomfort (yes/no) | 26 (55.3) | 31 (66.0) | 35 (81.4) | 34 (79.1) | 0.9 (0.3–3.0) |
| Caries | 5 (10.6) | 5 (10.6) | 6 (14.0) | 6 (14.0) | 0.8 (0.2–2.9) |
| Bleeding gums | 4 (8.5) | 7 (14.9) | 7 (16.3) | 4 (9.3) | 3.7 (0.7–21.2) |
| Red/swollen gums | 4 (8.5) | 4 (8.5) | 6 (14.0) | 4 (9.3) | 1.0 (0.2–4.3) |
| Blisters/soars | 5 (10.6) | 6 (12.8) | 2 (4.7) | 6 (14.0) | 0.7 (0.2–2.5) |
| Toothache—hot/cold | 4 (8.5) | 2 (4.3) | 4 (9.3) | 4 (9.3) | 0.4 (0.1–2.6) |
| Toothache—chewing | 1 (2.1) | 2 (4.3) | 0 (0.0) | 3 (7.0) | 0.6 (0.1–3.8) |
| Lost/loose/broken teeth | 7 (14.9) | 7 (14.9) | 5 (11.6) | 4 (9.3) | 1.6 (0.4–6.3) |
| Halitosis | 4 (8.7) | 4 (8.5) | 5 (11.6) | 7 (16.3) | 0.5 (0.1–2.2) |
| Xerostomia | 14 (30.4) | 17 (36.2) | 21 (48.8) | 17 (39.5) | 1.6 (0.5–5.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fluitman, K.S.; van den Broek, T.; Reinders, I.; Wijnhoven, H.A.H.; Nieuwdorp, M.; Visser, M.; IJzerman, R.G.; Keijser, B.J.F. The Effect of Dietary Advice Aimed at Increasing Protein Intake on Oral Health and Oral Microbiota in Older Adults: A Randomized Controlled Trial. Nutrients 2023, 15, 4567. https://doi.org/10.3390/nu15214567
Fluitman KS, van den Broek T, Reinders I, Wijnhoven HAH, Nieuwdorp M, Visser M, IJzerman RG, Keijser BJF. The Effect of Dietary Advice Aimed at Increasing Protein Intake on Oral Health and Oral Microbiota in Older Adults: A Randomized Controlled Trial. Nutrients. 2023; 15(21):4567. https://doi.org/10.3390/nu15214567
Chicago/Turabian StyleFluitman, Kristina S., Tim van den Broek, Ilse Reinders, Hanneke A. H. Wijnhoven, Max Nieuwdorp, Marjolein Visser, Richard G. IJzerman, and Bart J. F. Keijser. 2023. "The Effect of Dietary Advice Aimed at Increasing Protein Intake on Oral Health and Oral Microbiota in Older Adults: A Randomized Controlled Trial" Nutrients 15, no. 21: 4567. https://doi.org/10.3390/nu15214567
APA StyleFluitman, K. S., van den Broek, T., Reinders, I., Wijnhoven, H. A. H., Nieuwdorp, M., Visser, M., IJzerman, R. G., & Keijser, B. J. F. (2023). The Effect of Dietary Advice Aimed at Increasing Protein Intake on Oral Health and Oral Microbiota in Older Adults: A Randomized Controlled Trial. Nutrients, 15(21), 4567. https://doi.org/10.3390/nu15214567




