Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome
Abstract
:1. Introduction
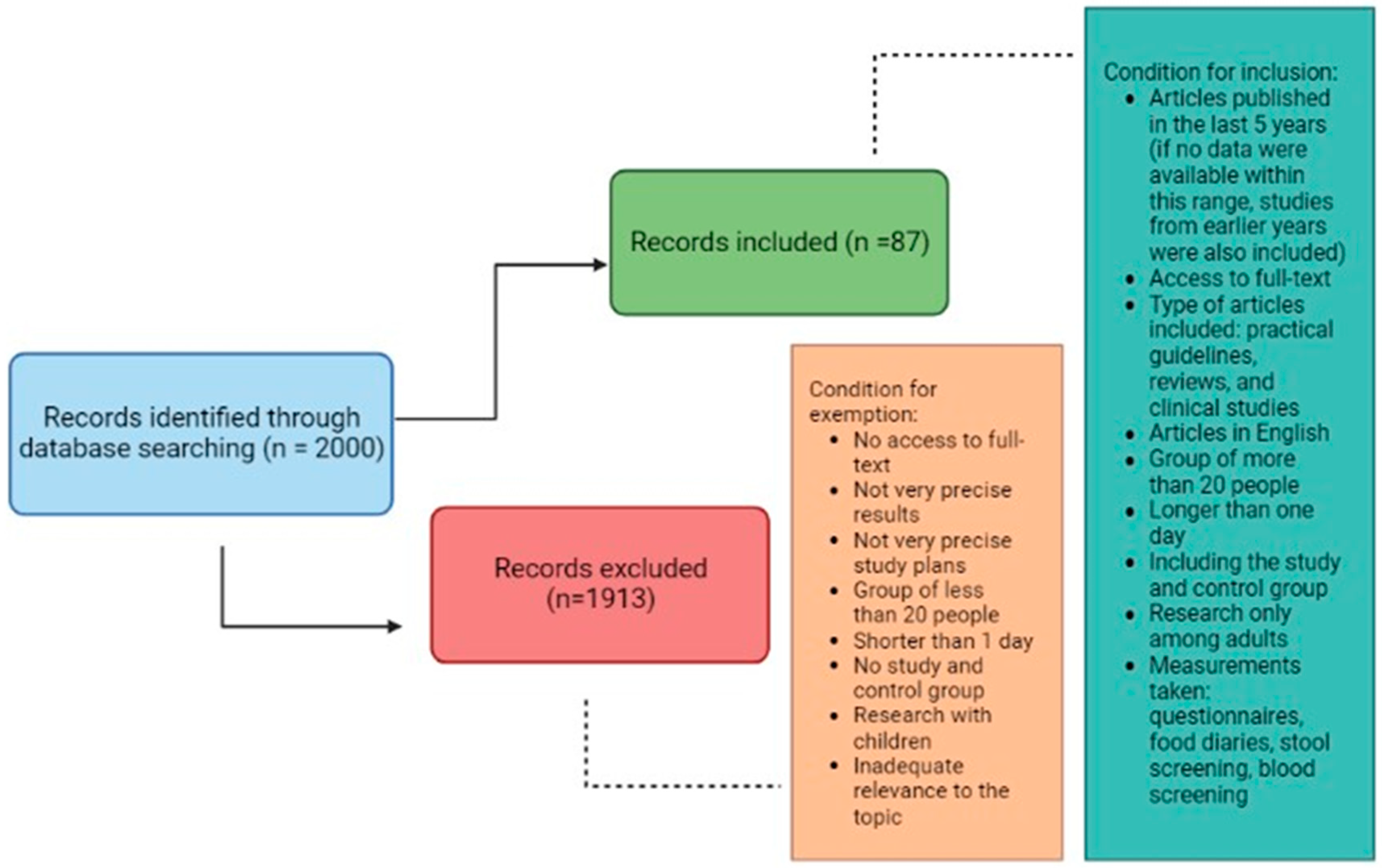
2. Materials and Methods
3. Food Choices
4. Food Groups
4.1. Vegetables and Fruit
4.2. Legume Seeds
4.3. Grain Products
4.4. Milk and Dairy Products
4.5. Meat, Fish, Eggs
5. Supplementation
5.1. Probiotics
5.2. Psyllium Husk
5.3. Vitamin D
6. Physical Activity
7. Conclusions
- The first intervention in terms of nutrition among individuals with IBS should be basic dietary recommendations recommended by NICE, based on principles of balanced nutrition. The second step, in the absence of therapeutic effects, is to consider the use of a low FODMAP diet. However, symptoms and the patient’s nutritional status should be monitored, as this diet does not always reduce symptom severity and improve the quality of life for patients. Prolonged use without specialist supervision may lead to deterioration in the patient’s nutritional status.
- Other unconventional diets, such as lactose-free and gluten-free diets, are not recommended for individuals with IBS, as there is no clear evidence regarding their effectiveness. Moreover, they may lead to a deterioration in nutritional status and overall health in individuals who follow them.
- Due to concerns about experiencing symptoms related to consuming specific foods, individuals with IBS often unjustifiably eliminate those foods from their diet, putting themselves at a high risk of nutritional deficiencies.
- All food groups should be included in the diet of individuals with IBS. However, it is important to individually adjust the quantity of products, cooking techniques, and presentation based on preferences and tolerances. The reintroduction of previously eliminated foods should start with small amounts and gradually increase to well-tolerated quantities.
- The results of studies conducted so far indicate promising outcomes of probiotic supplementation, psyllium, and vitamin D. However, further research is needed to definitively confirm their effectiveness.
- Regular physical activity is a crucial element in supporting IBS therapy. Physical exertion positively affects overall health, bodily functions, well-being, and mood. It may also provide benefits in terms of symptom severity and frequency. The most commonly recommended forms of physical activity among individuals with IBS include walking, cycling, swimming, yoga, or aerobics.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacy, B.E.; Chey, W.D.; Lembo, A.J. New and Emerging Treatment Options for Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2015, 11 (Suppl. 2), 1–19. [Google Scholar]
- Mulak, A.; Smereka, A.; Paradowski, L. Novelties and modifications in the Rome IV criteria. Gastroenterol. Klin. 2016, 8, 52–61. [Google Scholar]
- Borghini, R.; Donato, G.; Alvaro, D.; Picarelli, A. New insights in IBS-like disorders: Pandora’s box has been opened; a review. Gastroenterol. Hepatol. Bed Bench 2017, 10, 79–89. [Google Scholar]
- Altomare, A.; Di Rosa, C.; Imperia, E.; Emerenziani, S.; Cicala, M.; Guarino, M.P.L. Diarrhea Predominant-Irritable Bowel Syndrome (IBS-D): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2021, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Lim, D.Y.; Yeo, W.S. The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 2018, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.R.; Raker, J.M.; Whelan, K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 693–703. [Google Scholar] [CrossRef]
- Lewis, E.D.; Antony, J.M.; Crowley, D.C.; Piano, A.; Bhardwaj, R.; Tompkins, T.A.; Evans, M. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients. 2020, 12, 1159. [Google Scholar] [CrossRef]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [PubMed]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 683191. [Google Scholar] [CrossRef]
- Grodzinsky, E.; Hallert, C.; Faresjö, T.; Bergfors, E.; Faresjö, A.O. Could gastrointestinal disorders differ in two close but divergent social environments? Int. J. Health Geogr. 2012, 11, 5. [Google Scholar] [CrossRef]
- Radovanovic-Dinic, B.; Tesic-Rajkovic, S.; Grgov, S.; Petrovic, G.; Zivkovic, V. Irritable bowel syndrome—From etiopathogenesis to therapy. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2018, 162, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Melchior, C.; Algera, J.; Colomier, E.; Törnblom, H.; Simrén, M.; Störsrud, S. Food Avoidance and Restriction in Irritable Bowel Syndrome: Relevance for Symptoms, Quality of Life and Nutrient Intake. Clin. Gastroenterol. Hepatol. 2022, 20, 1290–1298.e4. [Google Scholar] [CrossRef] [PubMed]
- Galica, A.N.; Galica, R.; Dumitrașcu, D.L. Diet, fibers, and probiotics for irritable bowel syndrome. J. Med. Life. 2022, 15, 174–179. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Chey, W.D.; Harris, L.A.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M. ACG Task Force on Management of Irritable Bowel Syndrome. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113 (Suppl. 2), 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, M.; Sadeghi, O.; Hassanzadeh Keshteli, A.; Daghaghzadeh, H.; Esmaillzadeh, A.; Adibi, P. Physical activity in relation to irritable bowel syndrome among Iranian adults. PLoS ONE 2018, 13, e0205806. [Google Scholar] [CrossRef]
- Nunan, D.; Cai, T.; Gardener, A.D.; Ordóñez-Mena, J.M.; Roberts, N.W.; Thomas, E.T.; Mahtani, K.R. Physical activity for treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2022, 6, CD011497. [Google Scholar] [CrossRef]
- Algera, J.; Colomier, E.; Simrén, M. The Dietary Management of Patients with Irritable Bowel Syndrome: A Narrative Review of the Existing and Emerging Evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- NICE. Irritable Bowel Syndrome in Adults: Diagnosis and Management; NICE Clinical Guidelines, No. 61; National Institute for Health and Care Excellence (NICE): London, UK, 2017. Available online: https://www.nice.org.uk/guidance/cg61 (accessed on 18 December 2022).
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent advances in the treatment of irritable bowel syndrome. Pol. Arch. Intern. Med. 2021, 131, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Thomas, A.; Butler-Sanchez, M. Dietary Modification for the Restoration of Gut Microbiome and Management of Symptoms in Irritable Bowel Syndrome. Am. J. Lifestyle Med. 2021, 16, 608–621. [Google Scholar] [CrossRef]
- Colomier, E.; Van Oudenhove, L.; Tack, J.; Böhn, L.; Bennet, S.; Nybacka, S.; Störsrud, S.; Öhman, L.; Törnblom, H.; Simrén, M. Predictors of Symptom-Specific Treatment Response to Dietary Interventions in Irritable Bowel Syndrome. Nutrients 2022, 14, 397. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients with Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef]
- Böhmer, C.J.; Tuynman, H.A. The effect of a lactose-restricted diet in patients with a positive lactose tolerance test, earlier diagnosed as irritable bowel syndrome: A 5-year follow-up study. Eur. J. Gastroenterol. Hepatol. 2001, 13, 941–944. [Google Scholar] [CrossRef]
- Bijkerk, C.J.; de Wit, N.J.; Muris, J.W.; Whorwell, P.J.; Knottnerus, J.A.; Hoes, A.W. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009, 339, b3154. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Hookway, C.; Buckner, S.; Crosland, P.; Longson, D. Irritable bowel syndrome in adults in primary care: Summary of updated NICE guidance. BMJ 2015, 350, h701. [Google Scholar] [CrossRef] [PubMed]
- Aljada, B.; Zohni, A.; El-Matary, W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients 2021, 13, 3993. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e3. [Google Scholar] [CrossRef]
- Parker, T.J.; Woolner, J.T.; Prevost, A.T.; Tuffnell, Q.; Shorthouse, M.; Hunter, J.O. Irritable bowel syndrome: Is the search for lactose intolerance justified? Eur. J. Gastroenterol. Hepatol. 2001, 13, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality: Results From 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Törnblom, H.; Bengtsson, U.; Simrén, M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am. J. Gastroenterol. 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef]
- Singh, P.; Tuck, C.; Gibson, P.R.; Chey, W.D. The Role of Food in the Treatment of Bowel Disorders: Focus on Irritable Bowel Syndrome and Functional Constipation. Am. J. Gastroenterol. 2022, 117, 947–957. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Hausken, T. Diet in Irritable Bowel Syndrome (IBS): Interaction with Gut Microbiota and Gut Hormones. Nutrients 2019, 11, 1824. [Google Scholar] [CrossRef]
- Okawa, Y. A Discussion of Whether Various Lifestyle Changes can Alleviate the Symptoms of Irritable Bowel Syndrome. Healthcare 2022, 10, 2011. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.; Morden, A.; Bischof, D.; King, E.A.; Kosztowski, M.; Wick, E.C.; Stein, E.M. The role of fiber supplementation in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Ioniță-Mîndrican, C.B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- Muir, J. An Overview of Fiber and Fiber Supplements for Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2019, 15, 387–389. [Google Scholar]
- Cozma-Petruţ, A.; Loghin, F.; Miere, D.; Dumitraşcu, D.L. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J. Gastroenterol. 2017, 23, 3771–3783. [Google Scholar] [CrossRef] [PubMed]
- Ostgaard, H.; Hausken, T.; Gundersen, D.; El-Salhy, M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol. Med. Rep. 2012, 5, 1382–1390. [Google Scholar]
- Hughes, J.; Pearson, E.; Grafenauer, S. Legumes-A Comprehensive Exploration of Global Food-Based Dietary Guidelines and Consumption. Nutrients 2022, 14, 3080. [Google Scholar] [CrossRef]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of pulse consumption on metabolism and health: A systematic review of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 85–96. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; Curran, J.; Barr, S.I.; Slavin, J.; Puri, S.; Swaminathan, S.; Tapsell, L.; Patterson, C.A. Enhancing nutrition with pulses: Defining a recommended serving size for adults. Nutr. Rev. 2017, 75, 990–1006. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.M.; Guillamón, E.; Arribas, C. Autoclaved and Extruded Legumes as a Source of Bioactive Phytochemicals: A Review. Foods 2021, 10, 379. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Shevkani, K.; Singh, N.; Kaur, A. Bioactive constituents in pulses and their health benefits. J. Food Sci. Technol. 2017, 54, 858–870. [Google Scholar] [CrossRef]
- Reuzé, A.; Delvert, R.; Perrin, L.; Benamouzig, R.; Sabaté, J.M.; Bouchoucha, M.; Allès, B.; Touvier, M.; Hercberg, S.; Julia, C.; et al. Association between Self-Reported Gluten Avoidance and Irritable Bowel Syndrome: Findings of the NutriNet-Santé Study. Nutrients 2021, 13, 4147. [Google Scholar] [CrossRef]
- El-Salhy, M.; Gundersen, D. Diet in irritable bowel syndrome. Nutr. J. 2015, 14, 36. [Google Scholar] [CrossRef]
- McKenzie, Y.A.; Bowyer, R.K.; Leach, H.; Gulia, P.; Horobin, J.; O’Sullivan, N.A.; Pettitt, C.; Reeves, L.B.; Seamark, L.; Williams, M.; et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016, 29, 549–575. [Google Scholar] [CrossRef]
- Forsgård, R.A. Lactose digestion in humans: Intestinal lactase appears to be constitutive whereas the colonic microbiome is adaptable. Am. J. Clin. Nutr. 2019, 110, 273–279. [Google Scholar] [CrossRef]
- Szilagyi, A.; Ishayek, N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef]
- Pereira, P.M.; Vicente, A.F. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef]
- Maulu, S.; Nawanzi, K.; Abdel-Tawwab, M.; Khalil, H.S. Fish Nutritional Value as an Approach to Children’s Nutrition. Front. Nutr. 2021, 8, 780844. [Google Scholar] [CrossRef] [PubMed]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity and Colorectal Cancer. 2017. Available online: https://www.wcrf.org/sites/default/files/Colorectal-cancer-report.pdf (accessed on 29 December 2022).
- Diallo, A.; Deschasaux, M.; Latino-Martel, P.; Hercberg, S.; Galan, P.; Fassier, P.; Allès, B.; Guéraud, F.; Pierre, F.H.; Touvier, M. Red and processed meat intake and cancer risk: Results from the prospective NutriNet-Santé cohort study. Int. J. Cancer. 2018, 142, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.D.; Agudo, A.; Sánchez, M.J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Kassinen, A.; Krogius-Kurikka, L.; Mäkivuokko, H.; Rinttilä, T.; Paulin, L.; Corander, J.; Malinen, E.; Apajalahti, J.; Palva, A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007, 133, 24–33. [Google Scholar] [CrossRef]
- Moeen-Ul-Haq; Babar, A.N.; Hassan, M.K.; Ullah, F.; Ullah, A. Role of Lactobacillus plantarum 299v versus Placebo in symptomatic improvement of irritable bowel syndrome patients. J. Pak. Med. Assoc. 2022, 72, 404–408. [Google Scholar]
- Bednarska, O.; Biskou, O.; Israelsen, H.; Winberg, M.E.; Walter, S.; Keita, Å.V. A postbiotic fermented oat gruel may have a beneficial effect on the colonic mucosal barrier in patients with irritable bowel syndrome. Front. Nutr. 2022, 9, 1004084. [Google Scholar] [CrossRef]
- Gupta, A.K.; Maity, C. Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome: A prospective, interventional, randomized, double-blind, placebo-controlled clinical study [CONSORT Compliant]. Medicine 2021, 100, e23641. [Google Scholar] [CrossRef]
- Le Morvan de Sequeira, C.; Kaeber, M.; Cekin, S.E.; Enck, P.; Mack, I. The Effect of Probiotics on Quality of Life, Depression and Anxiety in Patients with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3497. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Li, J.; Long, Q.; Yue, C.C.; He, B.; Tang, X.G. The efficacy and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: A systematic review and meta-analysis based on seventeen randomized controlled trials. Int. J. Surg. 2020, 79, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, S.; Sajjad, S.; Sharma, S. Probiotics in Irritable Bowel Syndrome: A Review Article. Cureus 2023, 15, e36565. [Google Scholar] [CrossRef] [PubMed]
- Drakes, M.; Blanchard, T.; Czinn, S. Bacterial probiotic modulation of dendritic cells. Infect. Immun. 2004, 72, 3299–3309. [Google Scholar] [CrossRef]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef]
- Bretin, A.; Zou, J.; San Yeoh, B.; Ngo, V.L.; Winer, S.; Winer, D.A.; Reddivari, L.; Pellizzon, M.; Walters, W.A.; Patterson, A.D.; et al. Psyllium Fiber Protects Against Colitis Via Activation of Bile Acid Sensor Farnesoid X Receptor. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Garg, P. Psyllium Husk Should Be Taken at Higher Dose with Sufficient Water to Maximize Its Efficacy. J. Acad. Nutr. Diet. 2017, 117, 681. [Google Scholar] [CrossRef]
- Moayyedi, P.; Andrews, C.N.; MacQueen, G.; Korownyk, C.; Marsiglio, M.; Graff, L.; Kvern, B.; Lazarescu, A.; Liu, L.; Paterson, W.G.; et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J. Can. Assoc. Gastroenterol. 2019, 2, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Linsalata, M.; Riezzo, G.; Orlando, A.; D’Attoma, B.; Prospero, L.; Tutino, V.; Notarnicola, M.; Russo, F. The Relationship between Low Serum Vitamin D Levels and Altered Intestinal Barrier Function in Patients with IBS Diarrhoea Undergoing a Long-Term Low-FODMAP Diet: Novel Observations from a Clinical Trial. Nutrients 2021, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- De Vincentis, S.; Russo, A.; Milazzo, M.; Lonardo, A.; De Santis, M.C.; Rochira, V.; Simoni, M.; Madeo, B. How Much Vitamin D is Too Much? A Case Report and Review of the Literature. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1653–1659. [Google Scholar] [CrossRef]
- Nwosu, B.U.; Maranda, L.; Candela, N. Vitamin D status in pediatric irritable bowel syndrome. PLoS ONE 2017, 12, e0172183. [Google Scholar]
- Huang, H.; Lu, L.; Chen, Y.; Zeng, Y.; Xu, C. The efficacy of vitamin D supplementation for irritable bowel syndrome: A systematic review with meta-analysis. Nutr. J. 2022, 21, 24. [Google Scholar] [CrossRef]
- Yan, C.; Hu, C.; Chen, X.; Jia, X.; Zhu, Z.; Ye, D.; Wu, Y.; Guo, R.; Jiang, M. Vitamin D improves irritable bowel syndrome symptoms: A meta-analysis. Heliyon 2023, 9, e16437. [Google Scholar] [CrossRef]
- WHO Guideline. Physical Activity. Updated 5 October 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 18 March 2023).
- Daley, A.J.; Grimmett, C.; Roberts, L.; Wilson, S.; Fatek, M.; Roalfe, A.; Singh, S. The effects of exercise upon symptoms and quality of life in patients diagnosed with irritable bowel syndrome: A randomised controlled trial. Int. J. Sports Med. 2008, 29, 778–782. [Google Scholar] [CrossRef]
- Johannesson, E.; Simrén, M.; Strid, H.; Bajor, A.; Sadik, R. Physical activity improves symptoms in irritable bowel syndrome: A randomized controlled trial. Am. J. Gastroenterol. 2011, 106, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, E.; Ringström, G.; Abrahamsson, H.; Sadik, R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J. Gastroenterol. 2015, 21, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, L.; Naliboff, B.D.; Shapiro, D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: A pilot study. Psychol. Health Med. 2016, 21, 176–188. [Google Scholar] [CrossRef] [PubMed]

| Types FODMAP | The Main Products Rich in FODMAP |
|---|---|
| Fructans (fructooligosaccharides, inulin, oligofructose) | Wheat, vegetables (including artichokes, onion-la, garlic, cauliflower, asparagus, broccoli, mushrooms) |
| Galactooligosaccharides | Legumes, lentils, chickpeas (hummus) |
| Disaccharides (lactose) | Milk and milk products |
| Monosaccharides (fructose and high fructose/high glucose) | Honey, corn syrup, fruit juices in large quantities (pineapple, watermelon, pear, apple), fruit (including apples, mangoes, figs, watermelon, grapes) |
| Polyols (sorbitol, mannitol, xylitol, isomalt) | Dried prunes, apples, mushrooms, avocados, cauliflower, sweeteners for sugar-free products, including puddings, gelatine, chewing gum, mints, sweets |
| Country, Study, Year | Design, Population | Interventions | Main Findings |
|---|---|---|---|
| Australia, Biesiekierski et al., 2013 [24] | 1 stage: randomized, double-blind, placebo-controlled, cross-over trial, patients with IBS and non-celiac gluten sensitivity (n = 37) 2 stage: rechallenge, patients with IBS and non-celiac gluten sensitivity (n = 22) | 1 stage: High gluten (16 g/day) vs. Low gluten (2 g/day) vs. Whey (16 g/day), 1 week per intervention. 2 stage: Gluten (16 g/day) vs. Whey (16 g/day) vs. Placebo (no additional protein), 3 days. Run-in period of 2 weeks, gluten-free diet and low FODMAP diet | During reduction of FODMAP intake: improvement of gastrointestinal complaints in all subjects Diet containing gluten and whey protein: significant worsening of symptoms No evidence of a specific and dose-dependent response to gluten |
| Norway, Skodje et al., 2018 [23] | randomized, double-blind, placebo-controlled, cross-over trial, self-reported patients with non-celiac gluten sensitivity on gluten-free diet >6 months (n = 59) | Gluten-free diet (placebo-concealed muesli bars) vs. gluten-containing diet (5.7 g/day) vs. fructans containing diet (2.1 g/day), 7 days per intervention, 7 days washout | Significant differences in gastrointestinal symptoms between different dietary interventions Greatest symptoms among those consuming fructans No significant differences in symptoms between the placebo group and those consuming gluten |
| UK, Parker et al., 2001 [25] | No randomized controlled trial, observational interventional study 1 stage: patients with IBS were given lactose hydrogen breath tests (n = 122) 2 stage: patients with IBS and positive lactose hydrogen breath tests (n = 23) 3 stage: double-blind, placebo-controlled challenges, patients with IBS and positive lactose hydrogen breath tests and improving on the diet to confirm lactose intolerance (n = 9) 4 stage: patients who did not respond to the low lactose diet (n = 9) 5 stage: patients with IBS and negative lactose hydrogen breath tests (n = 35) Assessment of symptoms: before lactose hydrogen breath tests, 8 h after lactose hydrogen breath tests, every day during each dietary change | 2 stage: low lactose diet for 3 weeks 3 stage: diet containing 5 g vs. 10 g vs. 15 g of lactose vs. placebo 4 stage: followed either an exclusion or low fibre diet 5 stage: other dietary interventions | Before lactose hydrogen breath tests: no significant differences in symptoms After lactose hydrogen breath tests: symptoms in the positive group significantly worse Low lactose diet: improvement in 39% of people among those following the diet Exclusion diet: improvement in 50% of people among those following the diet Low fibre diet: improvement in 2/3 of those following the diet Lactose-free diet has no benefit among people with IBS regardless of test result |
| Netherlands, Bohmer et al., 2001 [26] | No randomized controlled trial, prospective observational study, patients with IBS and lactose malabsorption (n = 17) vs. patients with IBS and lactose tolerance (n = 53) | Low lactose diet and assessment of symptoms before, during, 6 weeks after and 5 years after starting the diet | Before lactose hydrogen breath tests: no significant differences in symptoms 6 weeks after starting diet: significant improvement in people with lactose malabsorption 5 years after starting the diet: significant improvement in people with lactose malabsorption Among people with IBS, it is very important to perform a lactose tolerance test and to include a lactose-free diet among those with a positive test result |
| Netherlands, Bijkerk et al., 2009 [27] | Randomized Controlled Trial, patients with IBS (n = 275) Observation of an increase in dietary fiber of the soluble (psyllium) or insoluble (bran) fraction in the diet | 12 weeks diet containing 10 g psyllium (n = 85) vs. 10 g bran (n = 97) vs. 10 g placebo (rice flour) (n = 93) | After 4 weeks and 2 months, a significant improvement in symptoms was noted among the psyllium group compared to the placebo group No significant effect of bran on symptoms compared to placebo After 12 weeks, a significant improvement in symptoms was noted among the psyllium group compared to placebo and the bran group |
| Sweden, Bohn et al., 2015 [28] | Multicenter Randomized Controlled Trial, patients with IBS (n = 75) Evaluation before and after intervention | 4 weeks traditional IBS diet (NICE guidelines) vs. Low FODMAP diet | During dietary intervention: relief of discomfort in both groups, with no significant difference between groups After 4 weeks of dietary intervention: 50% of those following the low FODMAP diet reported symptom relief vs. 46% of those following NICE recommendations reported symptom relief |
| Australia, Halmos et al., 2014 [29] | Single-centre, Randomized Controlled Trial, cross-over, patients with IBS (n = 30) vs. healthy control (n = 8) Evaluation before, during and after intervention | 21 days low FODMAP diet vs. typical Australian diet with a washout period of at least 21 days | During the diet: overall gastrointestinal symptoms were significantly reduced in the group on the low FODMAP diet compared to the control group. Flatulence, abdominal pain and gas also eased in the low FODMAP group. Reported stool consistency significantly better on the low FODMAP diet |
| Australia, Halmos et al., 2015 [30] | Single-blinded, randomised, cross-over trial, patients with IBS (n = 27) vs. healthy control (n = 6), Evaluation before, during and after intervention | 21 days low FODMAP diet vs. typical Australian diet with a washout period of at least 21 days | The low FODMAP diet group had higher stool pH, similar concentrations of short-chain fatty acids, and higher microbial diversity and reduced total bacteria compared to the control group. Low FODMAP diet significantly affects gut microbiota composition in the short term, long-term studies needed |
| Type of Diet | Dietary Assumptions | Effects on IBS Based on Research |
|---|---|---|
| Gluten-free diet | Elimination of gluten, i.e., products containing wheat, barley, rye, oats and related grains. It is recommended to eat, among other things, fruit, vegetables, fish, meat and gluten-free products [30]. | Research has shown that components of wheat may be responsible for causing some of the symptoms of IBS. However, there is no evidence that gluten is a factor. Therefore, a gluten-free diet should not be recommended as standard for people with IBS and more research is needed to assess the effect of gluten on IBS [17,23,31]. |
| Lactose-free diet | Limit consumption of lactose to 12 g/day. Eliminate the consumption of milk (cow, goat, sheep) and dairy products [33]. | Tests for lactose intolerance should be performed among patients with IBS. However, this diet should not be recommended to all people with IBS [17,24,32]. |
| High-fiber diet | Increasing the intake of foods rich in fibre of the water-soluble fraction and introducing additional amounts in the form of Psyllium seed husks [17]. | Strong research. Dietary fibre supplementation of the water-soluble fraction (e.g., Psyllium husks), may have a beneficial effect on the course of IBS [17,25]. |
| Low FODMAP diet | Elimination of products rich in fructans, galatooligosaccharides, disaccharides, monosaccharides and polyols [21]. | Low-quality research. Short-term use of the diet, may be beneficial in relieving symptoms. However, prolonged use reduces the diversity of the gut microbiota. Therefore, once IBS symptoms have abated, the diet should be expanded according to tolerance. More studies are needed to confirm efficacy [17,26,27,28]. |
| NICE guidelines | General recommendations such as eating regularly, avoiding skipping meals and large meals, drinking approximately 2 litres of fluids/day, limiting the consumption of alcoholic and carbonated beverages, reducing the intake of caffeine, fat, dietary fibre of the insoluble fraction, resistant starch and gas-enhancing products [17,19]. | Current recommendations given to every patient with IBS. The most effective and safe nutritional intervention [19,29]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L. Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome. Nutrients 2023, 15, 3662. https://doi.org/10.3390/nu15163662
Radziszewska M, Smarkusz-Zarzecka J, Ostrowska L. Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome. Nutrients. 2023; 15(16):3662. https://doi.org/10.3390/nu15163662
Chicago/Turabian StyleRadziszewska, Marcelina, Joanna Smarkusz-Zarzecka, and Lucyna Ostrowska. 2023. "Nutrition, Physical Activity and Supplementation in Irritable Bowel Syndrome" Nutrients 15, no. 16: 3662. https://doi.org/10.3390/nu15163662





