Zinc Status and Autism Spectrum Disorder in Children and Adolescents: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
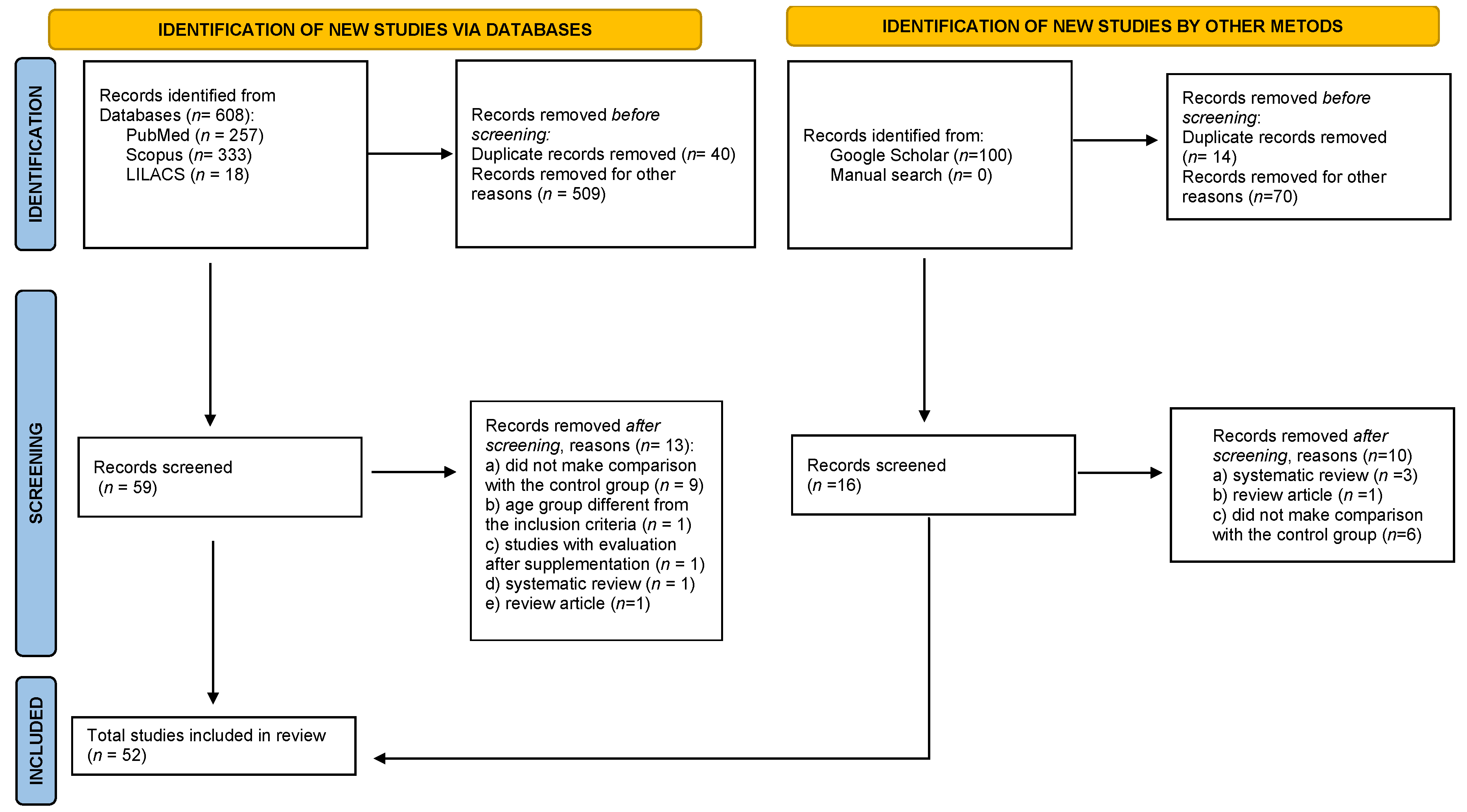
2.3. Selection of Studies
2.4. Data Extraction
2.5. Methodological Quality Assessment of Included Studies
3. Results
3.1. Study Characteristics
3.2. Methodological Quality of Studies
3.3. Main Results of Studies
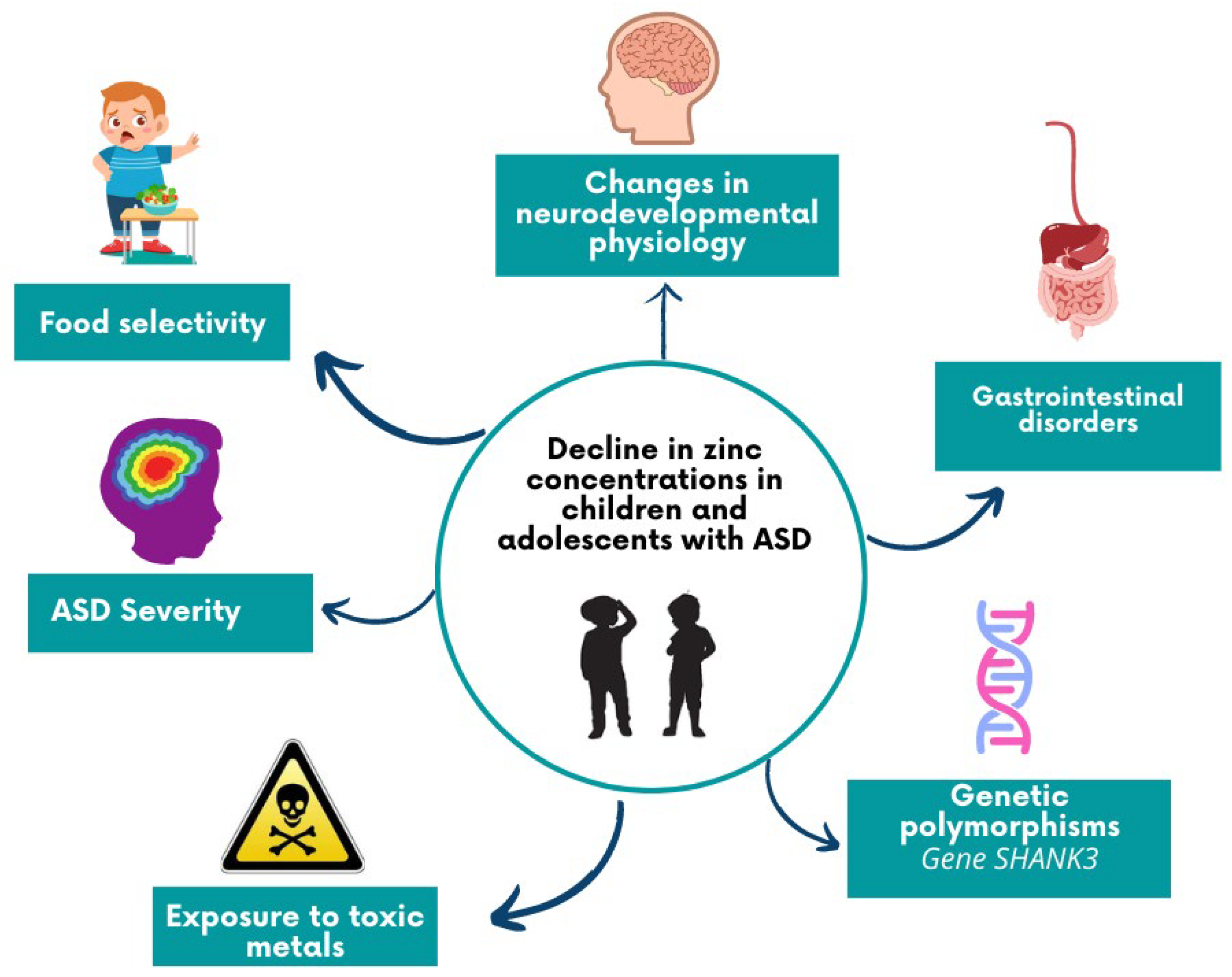
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA: Washington, DC, USA, 2013. [Google Scholar]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Tabatadze, T.; Zhorzholiani, L.; Kherkheulidze, M.; Kandelaki, E.I.T. Hair Heavy Metal and Essential Trace Element Concentration in Children with Autism Spectrum Disorder. Georgian Med. News 2015, 248, 77–82. [Google Scholar]
- Kambe, T.; Fukue, K.; Ishida, R.; Miyazaki, S. Overview of inherited zinc deficiency in infants and children. J. Nutr. Sci. Vitaminol. 2015, 61, S44–S46. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Karim, M.M. Metallothionein: A potential link in the regulation of zinc in nutritional immunity. Biol. Trace Elem. Res. 2018, 182, 1–13. [Google Scholar] [CrossRef]
- Vuralli, D.; Tumer, L.; Hasanoglu, A. Zinc deficiency in the pediatric age group is common but under evaluated. World J. Pediatr. 2017, 13, 360–366. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.; Singh, K.; Avasthi, K.; Kim, J.J. Neurobiology of zinc and its role in neurogenesis. Eur. J. Nutr. 2021, 60, 55–64. [Google Scholar] [CrossRef]
- Kawahara, M.; Tanaka, K.I.; Kato-Negishi, M. Nutrients Zinc, Carnosine, and Neurodegenerative Diseases. Available online: www.mdpi.com/journal/nutrients (accessed on 20 February 2021).
- Vela, G.; Stark, P.; Socha, M.; Sauer, A.K.; Hagmeyer, S.; Grabrucker, A.M. Zinc in gut-brain interaction in autism and neurological disorders. Neural Plast. 2015, 2015, 972791. [Google Scholar] [CrossRef]
- Al-Naama, N.; Mackeh, R.; Kino, T. C2H2-Type Zinc Finger Proteins in Brain Development, Neurodevelopmental, and Other Neuropsychiatric Disorders: Systematic Literature-Based Analysis. Front. Neurol. 2020, 11, 32. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Latorraca, C.d.O.C.; Rodrigues, M.; Pacheco, R.L.; Martimbianco, A.L.C.; Riera, R. Busca em bases de dados eletrônicas da área da saúde: Por onde começar. Diagn Trat. 2019, 24, 59–63. [Google Scholar]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2021. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 16 August 2021).
- Shearer, T.R.; Larson, K.; Neuschwander, J.; Gedney, B. Minerals in the hair and nutrient intake of autistic children. J. Autism Dev. Disord. 1982, 12, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.S.; Trentalange, M.J.; Zamichek, W.; Coleman, M. Trace elements in the hair of autistic and control children. J. Autism Dev. Disord. 1983, 13, 205–206. [Google Scholar] [CrossRef]
- Wecker, L.; Miller, S.B.; Cochran, S.R.; Dugger, D.L.; Johnson, W.D. Trace Element Concentrations in Hair from Autistic Children. J. Intellect. Disabil. Res. 1985, 29, 15–22. [Google Scholar] [CrossRef]
- Adams, J.B.; Holloway, C.E.; George, F.; Quig, D. Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Biol. Trace Elem. Res. 2006, 110, 193–209. [Google Scholar] [CrossRef]
- Al-ayadhi, L.Y. Heavy metals and trace elements in hair samples of autistic children in central Saudi Arabia. Neurosciences 2005, 10, 213–218. [Google Scholar]
- Al-Farsi, Y.M.; Waly, M.I.; Al-Sharbati, M.M.; Al-Shafaee, M.A.; Al-Farsi, O.A.; Al-Khaduri, M.M.; Gupta, I.; Ouhtit, A.; Al-Adawi, S.; Al-Said, M.F.; et al. Levels of heavy metals and essential minerals in hair samples of children with autism in Oman: A case-control study. Biol. Trace Elem. Res. 2013, 151, 181–186. [Google Scholar] [CrossRef]
- Yasuda, H.; Kobayashi, M.; Yasuda, Y.; Tsutsui, T. Estimation of autistic children by metallomics analysis. Sci. Rep. 2013, 3, 4–9. [Google Scholar] [CrossRef]
- Skalny, A.V.; Simashkova, N.V.; Klyushnik, T.P.; Grabeklis, A.R.; Radysh, I.V.; Skalnaya, M.G.; Tinkov, A.A. Analysis of Hair Trace Elements in Children with Autism Spectrum Disorders and Communication Disorders. Biol. Trace Elem. Res. 2017, 177, 215–223. [Google Scholar] [CrossRef]
- Zhai, Q.; Cen, S.; Jiang, J.; Zhao, J.; Zhang, H.; Chen, W. Disturbance of trace element and gut microbiota profiles as indicators of autism spectrum disorder: A pilot study of Chinese children. Environ. Res. 2019, 171, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Notova, S.V.; Chernova, L.N.; Skalny, A.A.; Burtseva, T.I.; Tinkov, A.A. Hair trace element concentrations in autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD). J. Trace Elem. Med. Biol. 2020, 61, 126539. [Google Scholar] [CrossRef] [PubMed]
- Fiłon, J.; Ustymowicz-Farbiszewska, J.; Karczewski, J.; Żendzian-Piotrowska, M. Analysis of trace element content in hair of autistic children. J. Elem. 2017, 22, 1285–1293. [Google Scholar] [CrossRef]
- Skalny, A.; Simashkova, N.; Skalnaya, A.A.; Klyushnik, T.P.; Bjørklund, G.; Skalnaya, M.G.; Tinkov, A.A. Assessment of gender and age effects on serum and hair trace element levels in children with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 1675–1684. [Google Scholar] [CrossRef]
- Lakshmi Priya, M.D.; Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 2011, 142, 148–158. [Google Scholar] [CrossRef]
- E Amen, N.; Eqani, S.A.M.A.S.; Khuram, F.; Alamdar, A.; Tahir, A.; Shah, S.T.A.; Nasir, A.; Javed, S.; Bibi, N.; Hussain, A.; et al. Environmental exposure pathway analysis of trace elements and autism risk in Pakistani children population. Sci. Total Environ. 2020, 712, 136471. [Google Scholar] [CrossRef]
- Blaurock-Busch, E.; Amin, O.R.; Dessoki, H.H.; Rabah, T. Toxic Metals and Essential Elements in Hair and Severity of Symptoms among Children with Autism. Maedica 2012, 7, 38–48. [Google Scholar]
- Yorbik, Ö.; Akay, C.; Sayal, A.; Cansever, A.; Söhmen, T.; Çavdar, A.O. Zinc Status in Autistic Children. J. Trace Elem. Exp. Med. 2004, 17, 101–107. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Skalnaya, M.G.; Simashkova, N.V.; Klyushnik, T.P.; Skalnaya, A.A.; Bjørklund, G.; Notova, S.V.; Kiyaeva, E.V.; Skalny, V.A. Association between catatonia and levels of hair and serum trace elements and minerals in autism spectrum disorder. Biomed. Pharmacother. 2019, 109, 174–180. [Google Scholar] [CrossRef]
- Adams, J.B.; Romdalvik, J.; Ramanujam, V.M.S.; Legator, M.S. Mercury, lead, and zinc in baby teeth of children with autism versus controls. J. Toxicol. Environ. Health Part A Curr. Issues 2007, 70, 1046–1051. [Google Scholar] [CrossRef]
- Wu, J.; Liu, D.J.; Shou, X.J.; Zhang, J.S.; Meng, F.C.; Liu, Y.Q.; Han, S.-P.; Zhang, R.; Jia, J.-Z.; Wang, J.Y.; et al. Chinese children with autism: A multiple chemical elements profile in erythrocytes. Autism Res. 2018, 11, 834–845. [Google Scholar] [CrossRef]
- Russo, A.J.; de Vito, R. Analysis of copper and zinc plasma concentration and the efficacy of zinc therapy in individuals with Asperger’s Syndrome, pervasive developmental disorder not otherwise specified (PDD-NOS) and autism. Biomark Insights 2011, 6, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.Y.; Jian, B.; Wu, C.; Jiang, C.Z.; Kang, Y.; Zhou, J.X.; Yang, F.; Liang, Y. A comparison of blood metal levels in autism spectrum disorder and unaffected children in Shenzhen of China and factors involved in bioaccumulation of metals. Environ. Sci. Pollut. Res. 2018, 25, 17950–17956. [Google Scholar] [CrossRef]
- Chehbani, F.; Gallello, G.; Brahim, T.; Ouanes, S.; Douki, W.; Gaddour, N.; Sanz, M.L.C. The status of chemical elements in the blood plasma of children with autism spectrum disorder in Tunisia: A case-control study. Environ. Sci. Pollut. Res. 2020, 27, 35738–35749. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Dungarwal, P.; Bagde, K.; Thakur, P.; Gajjar, P.; Kamath, A. Comparative evaluation of salivary zinc concentration in autistic and healthy children in mixed dentition age group-pilot study. Indian J. Dent. Res. 2019, 30, 43–46. [Google Scholar] [PubMed]
- Crăciun, E.C.; Bjørklund, G.; Tinkov, A.A.; Urbina, M.A.; Skalny, A.V.; Rad, F.; Dronca, E. Evaluation of whole blood zinc and copper levels in children with autism spectrum disorder. Metab. Brain Dis. 2016, 31, 887–890. [Google Scholar] [CrossRef]
- Wu, L.L.; Mao, S.S.; Lin, X.; Yang, R.W.; Zhu, Z. Evaluation of Whole Blood Trace Element Levels in Chinese Children with Autism Spectrum Disorder. Biol. Trace Elem. Res. 2019, 191, 269–275. [Google Scholar] [CrossRef]
- Sehgal, R.; Gulati, S.; Gupta, Y.K.; Sapra, S.; Mehta, M.; Pandey, R.M.; Kumar, G.; Srivastava, A.; Kabra, M. Blood heavy metal levels in children with autism spectrum disorder: A cross-sectional study from northern India. J. Nepal Paediatr. Soc. 2019, 39, 6–14. [Google Scholar] [CrossRef]
- Li, S.O.; Wang, J.L.; Bjørklund, G.; Zhao, W.N.Y.C. Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 2014, 25, 1216–1220. [Google Scholar] [CrossRef]
- Skalny, A.; Simashkova, N.; Klyushnik, T.P.; Grabeklis, A.R.; Radysh, I.; Skalnaya, M.G.; Nikonorov, A.A.; Tinkov, A.A. Assessment of serum trace elements and electrolytes in children with childhood and atypical autism. J. Trace Elem. Med. Biol. 2017, 43, 9–14. [Google Scholar] [CrossRef]
- Guo, M.; Li, L.; Zhang, Q.; Chen, L.; Dai, Y.; Liu, L.; Feng, J.; Cai, X.; Cheng, Q.; Chen, J.; et al. Vitamin and mineral status of children with autism spectrum disorder in Hainan Province of China: Associations with symptoms. Nutr. Neurosci. 2020, 23, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, D.U.; O’Donnell, S.M.; Lalor, A.; Grant, T.G.H. Zinc and vitamin A deficiency in a cohort of children with autism spectrum disorder. Child. Care Health Dev. 2019, 45, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.M.; Kredy, H.M.; Gazar, N.J. Clinical study of Vitamin D3 deficiency and some trace elements in autism. J. Glob. Pharma Technol. 2019, 11, 705–708. [Google Scholar]
- Al-Bazzaz, A.; A-Dahir, K.; Almashhadani, A.; Al-Ani, I. Estimation of fasting serum levels of glucose, zinc, copper, zinc/copper ratio and their relation to the measured lipid profile in autistic patients and non-autistic controls in Jordan. Biomed. Pharmacol. J. 2020, 13, 481–488. [Google Scholar] [CrossRef]
- Hawari, I.; Eskandar, M.B.; Alzeer, S. The Role of Lead, Manganese, and Zinc in Autism Spectrum Disorders (ASDs) and Attention-Deficient Hyperactivity Disorder (ADHD): A Case-Control Study on Syrian Children Affected by the Syrian Crisis. Biol. Trace Elem. Res. 2020, 197, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, F.; Adams, J.; Coleman, D.; Quig, D.; Hahn, J. Urinary essential elements of young children with autism spectrum disorder and their mothers. Res. Autism Spectr. Disord. 2020, 72, 101518. [Google Scholar] [CrossRef] [PubMed]
- Jory, J.; McGinnis, W.R. Red-cell trace minerals in children with autism. Am. J. Biochem. Biotechnol. 2008, 4, 101–104. [Google Scholar] [CrossRef]
- Pakyurek, M.; Azarang, A.; Iosif, A.M.; Nordahl, T.E. Assessment of Biometal Profile in Children with Autism Spectrum Disorder, with Attention Deficit Hyperactivity Disorder, or with Other Psychiatric Diagnoses: A Comparative Outpatient Study. Acta Psychopathol. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Yasuda, H.; Yonashiro, T.; Yoshida, K.; Ishii, T.; Tsutsui, T. Mineral Imbalance in Children with Autistic Disorders. Biomed. Res. Trace Elem. 2005, 16, 285–292. [Google Scholar]
- Elsheshtawy, E.; Tobar, S.; Sherra, K.; Atallah, S.; Elkasaby, R. Study of some biomarkers in hair of children with autism. Middle East Curr. Psychiatry 2011, 18, 6–10. [Google Scholar] [CrossRef]
- Semprún-Hernández, N.; Bohórquez-Visier, A.P.; Henríquez, A.B.; Bohórquez, R.C.; Medina, F.H.; Maury-Sintjago, E.; Ocando, N.M. Copper, zinc, calcium and magnesium profiles in subjects with autistic disorder according to their functioning level. Trace Elem. Electrolytes 2012, 29, 1–5. [Google Scholar] [CrossRef]
- Vergani, L.; Cristina, L.; Paola, R.; Luisa, A.M.; Shyti, G.; Edvige, V.; Minniti, G.; Grasselli, E.; Canesi, L.; Voci, A. Metals, metallothioneins and oxidative stress in blood of autistic children. Res. Autism Spectr. Disord. 2011, 5, 286–293. [Google Scholar] [CrossRef]
- Rezaei, M.; Rezaei, A.; Esmaeili, A.; Nakhaee, S.; Azadi, N.A.; Mansouri, B.; A Case-Control Study on the Relationship between Urine Trace Element Levels and Autism Spectrum Disorder among Iranian Children. Environ. Sci. Pollut Res. Int.; 2022; Aug 29 29(38). Available online: https://pubmed.ncbi.nlm.nih.gov/35352223/ (accessed on 31 August 2022).
- Zhao, G.; Liu, S.J.; Gan, X.-C.; Li, J.R.; Wu, X.X.; Liu, S.Y.; Jin, Y.-S.; Zhang, K.-R.; Wu, H.-M. Analysis of Whole Blood and Urine Trace Elements in Children with Autism Spectrum Disorders and Autistic Behaviors. Biol. Trace Elem. Res. 2011, 201, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.Q.; Behl, S.; Day, P.L.; Delgado, A.M.; Larson, N.B.; Stromback, L.R.; Huebner, A.R.; DeGrado, T.R.; Davis, J.M.; Jannetto, P.J.; et al. Evaluation of Zn, Cu, and Se Levels in the North American Autism Spectrum Disorder Population. 2021. Available online: www.frontiersin.org (accessed on 23 August 2022).
- Rashaid, A.H.B.; Nusair, S.D.; Alqhazo, M.T.; Adams, J.B.; Abu-Dalo, M.A.; Bashtawi, M.A. Heavy metals and trace elements in scalp hair samples of children with severe autism spectrum disorder: A case-control study on Jordanian children. J. Trace Elem. Med. Biol. 2021, 67, 126790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Yang, T.; Chen, J.; Chen, L.; Dai, Y.; Jia, F.Y.; Wu, L.J.; Hao, Y.; Li, L.; Zhang, J.; et al. Association between Serum Trace Elements and Core Symptoms in Children with Autism Spectrum Disorder: A National Multicenter Survey. Zhongguo Dang Dai Er Ke Za Zhi 2021, 23, 445–450. [Google Scholar]
- Skogheim, T.S.; Weyde, K.V.F.; Engel, S.M.; Aase, H.; Surén, P.; Øie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Caspersen, I.H.; Hornig, M.; et al. Metal and Essential Element Concentrations during Pregnancy and Associations with Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder in Children. 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/33765546/ (accessed on 31 August 2022).
- Zhang, J.; Lin, J.; Zhao, X.; Yao, F.; Feng, C.; He, Z.; Cao, X.; Gao, Y.; Khan, N.U.; Chen, M.; et al. Trace Element Changes in the Plasma of Autism Spectrum Disorder Children and the Positive Correlation Between Chromium and Vanadium. 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/35006555/ (accessed on 31 August 2022).
- Ma, J.; Wu, J.; Li, H.; Wang, J.; Han, J.; Zhang, R. Association Between Essential Metal Elements and the Risk of Autism in Chinese Han Population. Biol. Trace Elem. Res. 2022, 200, 505–515. [Google Scholar] [CrossRef]
- Shahrol Abd Wahil, M.; Hasni, J.M.; Md Isa, Z. Assessment of Urinary Lead (Pb) and Essential Trace Elements in Autism Spectrum Disorder: A Case-Control Study among Preschool Children in Malaysia. Biol. Trace Element. Res. 2021, 200, 97–121. [Google Scholar] [CrossRef]
- Gaafar, M.; Hussein, H.; Nasr, S.; Amer, M. Plasma Concentrations of the Trace Elements Copper, Zinc, Lead and Selenium in Children with Autistic Spectrum Disorder at Zagazig University Hospitals. Egypt. J. Hosp. Med. 2021, 84, 2124–2129. [Google Scholar] [CrossRef]
- Auda, F.M.; Ali, A.M.; Dhyaa, S.; Auda, F.M. Levels of Heavy Metal and Trace Element among Children with Autism Spectrum Disorders. In Journal of Physics: Conference Series; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 1879. [Google Scholar]
- De Benoist, B.; Darnton-Hill, I.; Davidsson, L.; Fontaine, O.; Hotz, C. Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG interagency meeting on zinc status indicators. Food Nutr. Bull. 2007, 28 (Suppl. 3), 480–486. [Google Scholar] [CrossRef]
- Babaknejad, N.; Sayehmiri, F.; Sayehmiri, K.; Mohamadkhani, A.; Bahrami, S. The relationship between zinc levels and autism: A systematic review and meta-analysis. Iran J. Child Neurol. 2016, 10, 1–9. [Google Scholar]
- Saghazadeh, A.; Rezaei, N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: Higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 340–368. [Google Scholar] [CrossRef] [PubMed]
- King, J.C.; Brown, K.H.; Gibson, R.S.; Krebs, N.F.; Lowe, N.M.; Siekmann, J.H.; Raiten, D.J. Biomarkers of nutrition for development (BOND)-Zinc Review. J. Nutrition. Am. Soc. Nutr. 2016, 146, 858S–885S. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Berni Canani, R.; Fairweather-Tait, S.; Heinonen, M.; Korhonen, H.; la Vieille, S.; Marchelli, R.; Martin, A.; Naska, A.; Neuhäuser-Berthold, M.; et al. Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844–3846. [Google Scholar]
- Hotz, C. Dietary indicators for assessing the adequacy of population zinc intakes. Food Nutr. Bull. 2007, 28, S430–S453. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M. Assessing zinc in humans. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Grabrucker, S.; Jannetti, L.; Eckert, M.; Gaub, S.; Chhabra, R.; Pfaender, S.; Mangus, C.; Reddy, P.P.; Rankovic, V.; Schmeisser, M.J.; et al. Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain 2014, 137, 137–152. [Google Scholar] [CrossRef]
- Yasuda, H.; Tsutsui, T.; Suzuki, K. Metallomics analysis for assessment of toxic metal burdens in infants/children and their mothers: Early assessment and intervention are essential. Biomolecules 2021, 11, 6. [Google Scholar] [CrossRef]
- Portbury, S.D. Zinc Signal in Brain Diseases. Int. J. Mol. Sci. 2017, 18, 2506. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Giacconi, R.; Cipriano, C.; Muzzioli, M.; Fattoretti, P.; Bertoni-Freddari, C.; Isani, G.; Zambenedetti, P.Z.P. Zinc-bound metallothioneins as potential biological markers of ageing. Brain Res. Bull. 2001, 55, 147–153. [Google Scholar] [CrossRef]
- Bourassa, D.; Elitt, C.M.; McCallum, A.M.; Sumalekshmy, S.; McRae, R.L.; Morgan, M.T.; Siege, N.; Perry, J.W.; Rosenberg, P.A.; Fahrni, C.J. Chromis-1, a Ratiometric Fluorescent Probe Optimized for Two-Photon Microscopy Reveals Dynamic Changes in Labile Zn(II) in Differentiating Oligodendrocytes. ACS Sens. 2018, 3, 458–467. [Google Scholar] [CrossRef]
- Bou Khalil, R. The potential role of insulin-like growth factor-1 and zinc in brain growth of autism spectrum disorder children. Autism 2019, 23, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef] [PubMed]
- Hagmeyer, S.; Sauer, A.K.; Grabrucker, A.M. Prospects of zinc supplementation in autism spectrum disorders and Shankopathies such as Phelan McDermid syndrome. Front. Synaptic. Neurosci. 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Diolordi, L.; del Balzo, V.; Bernabei, P.; Vitiello, V.; Lorenzo, O.; Donini, M. Eating habits and dietary patterns in children with autism. Eat. Weight. Disord. 2014, 19, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Zamora, A.F.; Ramírez-Valenzuela, D.G.; Ramos-Jiménez, A. Food Selectivity and Its Implications Associated with Gastrointestinal Disorders in Children with Autism Spectrum Disorders. Nutrients 2022, 14, 2660. [Google Scholar] [CrossRef]
- Saravia, L.; Gonz Alez-Zapata, L.I.; Rendo-Urteaga, T.; Ramos, J.; Collese, T.S.; Bove, I.; Delgado, C.; Tello, F.; Iglesia, I.; Gonçalves Sousa, E.D.; et al. Development of a Food Frequency Questionnaire for Assessing Dietary Intake in Children and Adolescents in South America. Obesity 2018, 26, 31–40. [Google Scholar] [CrossRef]
- Dubourdieu, P.M.; Guerendiain, M. Dietary Intake, Nutritional Status and Sensory Profile in Children with Autism Spectrum Disorder and Typical Development. Nutrients 2022, 14, 2155. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55–S65. [Google Scholar] [CrossRef]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal issues and autism spectrum disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 501–513. [Google Scholar] [CrossRef]
- Kondaiah, P.; Singh Yaduvanshi, P.; Sharp, P.A.; Pullakhandam, R. Iron and Zinc Homeostasis and Interactions: Does Enteric Zinc Excretion Cross-Talk with Intestinal Iron Absorption? 2019. Available online: www.mdpi.com/journal/nutrients (accessed on 14 September 2022).
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc Homeostasis in Humans. 2000. Available online: https://pubmed.ncbi.nlm.nih.gov/10801944/ (accessed on 18 March 2021).
| Author/ Year | Country | Study Design | Quality | Number of Participants | Average Age | Biological Matrix | Zinc Concentration (ppm) | p-Value | Analytical Method | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD | Control | ASD | Control | ASD | Control | |||||||
| Shearer et al., 1982. [16] | USA | Case–Control | 3 | 12 | 12 | 8.0 (0.8) | 8.4 (0.6) | Hair | 175 (73) x | 158 (57) | >0.05 | ASS |
| Gentile et al., 1983. [17] | USA | Case–Control | 1 | 47 | 37 | NI | Hair | NI | NI | NI | ||
| Wecker et al., 1985. [18] | USA | Case–Control | 3 | 21 | 12 | 2–11 | Hair | 128 (116) x | 166 (112) | NI | ASS | |
| Adams et al., 2005. [19] | USA | Case–Control | 3 | 51 | 40 | 3–15 | Hair | 156 AP 163 | 147 | NI | ICP-OES | |
| Laila Y. Al-Ayadhi, 2005. [20] | Saudi Arabia | Case–Control | 3 | 65 | 80 | 8.8 (0.5) | Hair | 150 (20) x | 140 (8) | NI | AAS | |
| Al-Farsi et al., 2013. [21] | Oman | Case–Control | 5 | 27 | 27 | 3–14 | Hair | 5.4 (0.82) y | 2.9 (2.2) | 0.0001 | NI | |
| Tabatadze et al., 2015. [3] | Georgia | Case–Control | 3 | 30 | 30 | 4–5 | Hair | NI | NI | NI | ||
| Skalny et al., 2016. [22] | Russia | Case–Control | 5 | 74 | 74 | 5.12 (2.36) | 5.11 (2.34) | Hair | 124.6 (77.0–174.2)y | 113.3 (69.4–166.3) | 0.365 | ICP-MS |
| Skalny et al., 2016. [23] | Russia | Case–Control | 5 | 33 | 33 | 3–8 | Hair | 130.3 (77.0–175.6) y | 101.6 (59.5–149.8) | NI | ICP-OES | |
| Zhai et al., 2019. [24] | China | Case–Control | 3 | 68 | 58 | 4.96 (1.01) | 4.9 (0.97) | Hair | 78.00 x | 44.7 | <0.001 | ICP-MS |
| Skalny et al., 2020. [25] | Russia | Case–Control | 6 | 109 | 104 | 5.18 (1.00) | 5.1 (1.05) | Hair | 122.3 (86.6–152.9)y AA 110.0 (83.1–139.1) | 141.0 (103.9–162.2) | <0.05 | ICP-MS |
| Fiłon et al., 2017. [26] | Poland | Case–Control | 2 | 30 | 30 | 5.2 (1.5) | 5.09 (1.5) | Hair | 99.93 (67.50)x | 149.66 (42.56) | 0.000 | NI |
| Skalny et al. 2017. [27] | Russia | Case–Control | 4 | 70 | 70 | 6.4 (2.9) | 6.3 (2.9) | Hair/Plasma | 122 (77–169)y/0.97 (0.89–1.06) y | 132 (86–172)/0.98 (0.89–1.05) | 0.678 0.617 | ICP-DRC-MS |
| Priya MDL, Geetha A., 2011. [28] | India | Case–Control | 4 | 45 | 50 | 4–12 | Nail/Hair | c 150.83 (18.09) x b 192.02 (23.04) x a 187.44 (22.47)x/ c 130.46 (15.65) x b 172.81 (20.73) x a 171.92 (20.63) x | 193.98 (23.27)/171.68 (20.60) | 0.01 | AAS | |
| Amen et al., 2020. [29] | Pakistan | Case–Control | 5 | 90 | 76 | 9.8 (3.3) | Hair/Urine | NI | NI | ICP-MS | ||
| Blaurock-Busch et al., 2011. [30] | Saudi Arabia | Case–Control | 5 | 25 | 25 | 6.2 (2.3) | 5.2 (1.9) | Hair/Urine | 101.042 (52.0)x/2213 (1062.20) | 149.86 (58.51)/ 890 (450) | 0.003 0.32 | ICP-MS |
| Yorbik et al., 2004. [31] | Turkey | Case–Control | 3 | 45 | 41 | 6.5 (2.2) | 6.7 (2.5) | Plasma/Erythrocytes/Hair | 0.0198 (0.0025) x/0.0023 (0.0024)/131,7 (60) | 0.0257 (0.0024)/0.003 (0.0029)/184.0 (19) | NI | AAS |
| Tinkov et al., 2019. [32] | Russia | Case–Control | 2 | 60 | 30 | 4.7 (1.8) | 4.8 (2.2) | Hair/ Serum | 121.59 (77.17–156.1) y/127.45 (92.53–164.5) | 152.77 (99.02–179.6) | NI | ICP-MS |
| Adams et al., 2007. [33] | USA | Case–Control | 3 | 15 | 11 | 7.0 (1.7) | 6.1 (2.2) | Teeth | 100 (20) x | 98 (16) | NI | CV-ASS |
| Wu et al., 2018. [34] | China | Case–Control | 5 | 50 | 50 | 2–8 | Erythrocytes | Limits of quantification (LOQ) 0.00402 | NI | NI | AAS | |
| AJ Russo and Robert de Vito, 2011. [35] | USA | Cohort | 4 | 79 | 18 | 11.7 (5.62) | Plasma | 78.36 (20.32)x | 84.42 (24.18) | 0.3541 | ICP-MS | |
| Qin et al., 2018. [36] | China | Case–Control | 5 | 34 | 38 | 4.1 (0.8) | 4.2 (1.7) | Plasma | 4.30 (1.84) x | 5.05 (1.52) | <0.05 | ICP-OES |
| Chehbani et al., 2020. [37] | Tunisia | Case–Control | 2 | 89 | 70 | 7.5 (3.0) | 7.8 (3.4) | Plasma | 0.610 (0.166) x | 0.586 (0.179) | 0.37 | ICP-OES |
| Deshpande et al., 2019. [38] | India | Case–Control | 4 | 10 | 10 | 6–14 | Spittle | 0.0133 x | 0.0274 | NI | ICP-OES | |
| Crăciun et al., 2016. [39] | Romania | Case–Control | 3 | 28 | 28 | 5.8 (3.1) | 5.9 (2.9) | Blood | 5.54 (0.78) x | 6.14 (0.76) | 0.005 | ICP-SFMS |
| Wu et al., 2018. [40] | China | Case–Control | 5 | 113 | 141 | 4.9 (2.2) | 4.9 (2.1) | Blood | 0.122 (0.020) x | 0.129 (0.018) | 0.05 | ICP-MS |
| Sehgal et al., 2019. [41] | India | Case–Control | 6 | 60 | 60 | 3–12 years | Blood | 1.04 (0.91–1.18) x | 0.90(0.81–1.01) | 0.02 | ICP-AES | |
| Li et al., 2014. [42] | China | Case–Control | 5 | 60 | 60 | 3.78 (1.22) | Serum | 0.78 (0.07) x | 0.87 (0.08) | <0.001 | NI | |
| Skalny et al., 2016. [43] | Russia | Case–Control | 3 | 24 | 24 | 6.6 (1.4) | 6.5 (0.9) | Serum | 1.02 (0.19) x | 0.96 (0.12) | 0.114 | ICP-MS |
| Min Guo et al., 2018. [44] | China | Case–Control | 5 | 274 | 97 | 4.0 (1.1) | 4.2 (1.2) | Serum | NI | <000.1 | HPLC | |
| Sweetman et al., 2018. [45] | Ireland | Case–Control | 5 | 74 | 72 | 2–18 | Serum | 0.017 (0.002) x | 0.017 (0.003) | 0.86 | ICP-MS | |
| Sultan et al., 2019. [46] | Iraq | Case–Control | 2 | 90 | 30 | 2–10 | Serum | a 0.022 (0.002) x b 0.015 (0.001) c 0.014 (0.001) | 0.0037 (0.0034) | NI | AAS | |
| Al-Bazzaz et al., 2020. [47] | Jordan | Case–Control | 3 | 35 | 35 | 4–12 | Serum | 0.8434 (0.1828) x | 0.9589 (0.1091) | significant at 5% | NI | |
| Hawari et al., 2020. [48] | Syria | Case–Control | 4 | 31 | 30 | 3–12 | Serum | 0.8448 (0.1599) x | 0.7997 (0.1372) | NI | NI | |
| Qureshi et al., 2020. [49] | USA | Case–Control | 3 | 21 | 26 | 2–5 | Urine | 5.70 (2.34) | 7.44 (3.22) | NI | ICP-OES | |
| Joan Jory and Woody R. McGinnis, 2008.[50] | Canada | Case–Control | 3 | 20 | 15 | 3.90 (1.68) | 3.87 (1.06) | Erythrocytes | 0.2048 (0.0366) x | 0.2267 (0.0261) | 0.08 | ICP-MS |
| Pakyurek et al., 2018. [51] | USA | Case–Control | 4 | 15 | 12 | 5–18 | Blood | 0.708 (0.1239) x | 0.8633 (0.2946) | 0.1189 | NI | |
| Yasuda et al., 2005. [52] | Japan | Case–Control | 3 | 360 | 241 | 0–15 | Hair | 0.005 (0.0001) x | 0.00502 (0.000117) | 0.0904 | ICP-MS | |
| Elsheshtawy et al., 2010. [53] | Egypt | Case–Control | 7 | 32 | 32 | 4.1 (0.8) | 4 (0.8) | Hair | 304.99 (25.8) x | 419.5 (45.96) | 0.000 | AAS |
| Semprun-Hernández et al., 2012. [54] | Venezuela | Case–Control | 6 | 30 | 20 | 3–17 | Serum | 1.805 (0.577) x a 1.904 (0.572) b 1.683 (0.554) c 1.889 (0.667) | 2.194 (0.721) | >0.05 | AAS | |
| Vergani et al., 2011. [55] | Italy | Case–Control | 3 | 38 | 32 | 2–6 | Plasma | 1.021 (0.100) x | 0.808 (0.131) | 0.01 | ICP-AES | |
| Rezaei et al.,2022. [56] | Iran | Case–Control | 44 | 35 | 10–11 | Urine | 0.733 | 0.764 | 0.349 | ICP-MS | ||
| Zhao et al., 2022. [57] | China | Case–Control | 30 | 30 | 4.2 (1.53) | 8 (1.3) | Blood/ Urine | 3.8815 (3.319–4.369)/4.354 (1.535–8.069) | 4.155 (3.675–4.816)/ 4.833 (1.496–8.487) | 0.188 0.918 | ICP-MS | |
| Metha et al., 2021. [58] | USA | Case–Control | 52 | 22 | 2–4 | Plasma | 0.00075 (0.00016) | 0.00088 (0.00035) | 0.2309 | ICP-MS | ||
| Rashaid et a., 2021. [59] | Jordan | Case–Control | 50 | 50 | 4–12 | Hair | 185.96 ± 95.35 | 244.29 ± 183.40 | 0.038 | ICP-AES | ||
| Zhang et al., 2021. [60] | China | Case–Control | 1342 | 1293 | 2–7 | Plasma | 0.75 (0.64; 0.84) a,b 0.74 (0.66; 0.84) c 0.70 (0.62; 0.82) | 0.77 (0.66; 0.87) | 0,003 | ICP-MS | ||
| Skogheim et al., 2021. [61] | Norway | Case–Control | 397 | 1034 | NI | Blood | 49.66 (48.5–50.85) | 52.02 (51.39–52.66) | NI | ICP-SFMS | ||
| Zhang J. et al., 2022. [62] | China | Case–Control | 30 | 30 | 4.03 (1.1) | 4.21 (0.9) | Plasma | 3.988 (2.995–5.328) | 4.279 (3.681–5.306) | 0.261 | ICP-MS | |
| Jiahui et al., 2021. [63] | China | Case–Control | 92 | 91 | 2–8 | Serum | 0.8251 (0.7673–0.9079) | 0.8978 (0.8065–0.9489) | 0.002 | ICP-MS/ICP-AES | ||
| Whail et al., 2022. [64] | Malaysia | Case–Control | 81 | 74 | 3–6 | Urine | 0.3981 (0.2452) | 0.8888 (0.9015) | <0.001 | ICP-MS | ||
| Gaafar et al., 2021. [65] | Egypt | Case–Control | 42 | 21 | 3–11 | Plasma | 0.707 (0.099) | 1.14 (0.089) | <0.001 | NI | ||
| Auda et al., 2021. [66] | Iraq | Case–Control | 60 | 30 | 3–6 | Blood | 24.072 (7.359) | 33.952 (15.534) | 0.0021 | EDS | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Nascimento, P.K.d.S.B.; Oliveira Silva, D.F.; de Morais, T.L.S.A.; de Rezende, A.A. Zinc Status and Autism Spectrum Disorder in Children and Adolescents: A Systematic Review. Nutrients 2023, 15, 3663. https://doi.org/10.3390/nu15163663
do Nascimento PKdSB, Oliveira Silva DF, de Morais TLSA, de Rezende AA. Zinc Status and Autism Spectrum Disorder in Children and Adolescents: A Systematic Review. Nutrients. 2023; 15(16):3663. https://doi.org/10.3390/nu15163663
Chicago/Turabian Styledo Nascimento, Priscila Kelly da Silva Bezerra, David Franciole Oliveira Silva, Tássia Louise Sousa Augusto de Morais, and Adriana Augusto de Rezende. 2023. "Zinc Status and Autism Spectrum Disorder in Children and Adolescents: A Systematic Review" Nutrients 15, no. 16: 3663. https://doi.org/10.3390/nu15163663
APA Styledo Nascimento, P. K. d. S. B., Oliveira Silva, D. F., de Morais, T. L. S. A., & de Rezende, A. A. (2023). Zinc Status and Autism Spectrum Disorder in Children and Adolescents: A Systematic Review. Nutrients, 15(16), 3663. https://doi.org/10.3390/nu15163663





