Abstract
Background: Gestational diabetes mellitus (GDM) is prevalent with lasting health implications for the mother and offspring. Medical therapy is the foundation of GDM management, for achieving optimal glycemic control often requires treatment with insulin or metformin. Gut dysbiosis is a feature of GDM pregnancies, therefore, dietary manipulation of the gut microbiota may offer a new avenue for management. Probiotics are a relatively new intervention, which can reduce the mother’s blood sugar levels and, furthermore, adjust glucose and lipid metabolism in both mother and offspring. Objective: The aim of this systematic review and meta-analysis is to explore the effect of probiotics/synbiotics on glucose and lipid metabolism in women with GDM. Methods: A systematic search of the literature was conducted using the electronic databases Cochrane Library, Web of Science, PubMed, and EBOSCO, published between 1 January 2012 and 1 November 2022. A total of 11 randomized controlled clinical trials (RCTs) were analyzed. The indicators included fasting plasma glucose (FPG), fasting serum insulin (FSI), the homoeostatic model assessment for insulin resistance (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), total cholesterol (TC), HDL cholesterol, LDL cholesterol and triglycerides (TG), the mean weight at end of trial, and gestational weight gain (GWG). Results: Compared with the placebo, probiotics/synbiotics were associated with a statistically significant improvement in FPG (MD = −2.33, 95% CI = −4.27, −0.40, p = 0.02), FSI (MD = −2.47 95% CI = −3.82, −1.12, p = 0.0003), HOMA-IR (MD = −0.40, 95% CI = −0.74, −0.06, p = 0.02), and TC (MD = −6.59, 95% CI = −12.23,−−0.95, p = 0.02), while other factors had no significant difference. The subgroup analysis revealed that the kind of supplement led to heterogeneity for FPG and FSI, while heterogeneity was not found for others. Conclusion: Probiotics/synbiotics could control glucose and lipid metabolism in pregnant women with GDM. There was a significant improvement in FPG, FSI, HOMA-IR, and TC. The use of specific probiotic supplementation may be a promising prevention and therapeutic strategy for GDM. However, due to the heterogeneity among existing studies, further studies are warranted to address the limitations of existing evidence and better inform the management of GDM.
1. Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance that is first detected during pregnancy. GDM affects about 12.8% of pregnancies globally [1]. Women with GDM are associated with a high risk of pregnancy outcomes such as eclampsia, shoulder dystocia, cesarean section, while the risk of type 2 diabetes (T2DM), pancreatic cancer, and cardiovascular disease after pregnancy is increased [2,3]. The fetus needs high energy to ensure metabolism and growth in the uterus, of which 80% is provided by glucose [4]. During pregnancies, the mother’s blood glucose could be transported to the fetus through blood vessels in the placenta [5]. The level of fetal glucose could induce secretion of insulin by the fetal pancreas as well as insulin-like growth factors, which play an important role in organ growth and development and fat and glycogen reserves for preventing neonatal glycemic imbalances after birth [4]. In GDM mothers, the persistent high level of blood sugar could result in sustained increase in fetal insulin, which could change hypothalamic response to glucose and lead to long-term mediobasal hypothalamus gliosis as well as insulin resistance [6,7]. This suggests that, later in the newborn’s life, the incidence of congenital malformations, neonatal hypoglycemia, respiratory disorders, cardiometabolic disease, obesity, T2DM, and autism could increase and even neonatal death could occur [8,9,10]. GDM seriously endangers the quality of life for both mothers and children.
It has been proved that an imbalance in gut microbiota is associated with the occurrence of GDM [11]. GDM women had a reduction in alpha diversity when compared to that of normal women at both mid- and late gestation. Meanwhile, in GDM, the Firmicutes/Bacteroidetes (F/B) ratio increases in late pregnancy [12]. These changes in gut microbial composition correlate with fat mass accumulation, increasing blood glucose levels, and insulin resistance [13]. GDM women with gut microbial composition changes are also associated with long-term health burden on their offspring, such as the reduction in alpha diversity and Lactobacillus, and increased Escherichia and Parabacteroides, which could cause an imbalance in the gut flora [14].
In order to prevent the negative influence and decrease the risk factors of GDM, some researchers overviewed the effects of various interventions [15,16,17] (diet, exercise, diet and exercise combined, dietary supplements, pharmaceutical management such as metformin, and the management of other health issues). However, the evidence was of low to moderate quality. Recent research has tried to explore novel, effective, and safe treatment strategies at the population level. Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” by the World Health Organization (WHO) [18]. Synbiotics are regarded as indigestible food, which could stimulate and activate bacteria in the digestive tract [19]. It has shown that probiotic/synbiotic supplements are beneficial for pregnancy, which could alleviate insulin resistance and improve lipid metabolism [20]. However, the effect of probiotic/synbiotic supplementation on GDM is controversial, as some randomized controlled trials (RCTs) reported significant improvement in blood glucose control while there was no difference after intervention in other studies [21,22]. Therefore, this meta-analysis was to investigate the interaction between probiotic/synbiotic treatment in relation to glucose and lipid metabolism in GDM.
2. Method
2.1. Study Protocol
The protocol of this meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO), ID: CRD42023387754.
2.2. Search Strategy
This review followed the 2020 update of the PRISMA statement [23]. A systematic literature search was conducted using the electronic databases Web of Science, PubMed, EBOSCO, and Cochrane Library published between 1 January 2012 and 1 November 2022. The following search terms were used: (pregnan* OR gestation* OR matern* OR obstetric* OR gestational diabetes mellitus OR gestational diabetes OR GDM) AND (probiotic* OR synbiotic* OR lactobacill* OR streptococc* OR bifidobacter* OR saccharomy* OR yeast OR bacteria* OR acidophilus OR ferment* OR microorganism*) AND (glucose* OR insulin).
2.3. Inclusion and Exclusion Criteria
The inclusion criteria were (1) participants were pregnant women with GDM and had no other metabolic diseases, (2) probiotics or synbiotics were used as treatment, (3) participants avoided probiotic- or synbiotic-containing foods and other supplements during intervention, (4) no regular exercise intervention before and during pregnancy, (5) the indicators in studies, related to glucose and lipid metabolism, included, but were not limited to, fasting plasma glucose, fasting serum insulin, total cholesterol, triglycerides, (6) randomized controlled trials and published in English, (7) data reported by means and standard deviation and with the baseline. The exclusion criteria were (1) systematic reviews, case reports, commentaries, meta-analyses and abstracts, (2) animal experiments, (3) probiotics/synbiotics containing unknown bacteria, (4) the intervention group combined other treatment strategies except taking probiotics/synbiotics, (5) data extraction was insufficient.
2.4. Data Extraction and Quality Assessment
For each eligible study, the following information was extracted: first author name, published year, country of study, sample size, GDM diagnostic method, the age of participants, intervention duration and frequency, the variety of probiotic/synbiotic bacteria, the dose of supplements, weight at end of trial, gestational weight gain (GWG), fasting plasma glucose (FPG), fasting serum insulin (FSI), the homoeostatic model assessment for insulin resistance (HOMA-IR), quantitative insulin sensitivity check index (QUICKI), total cholesterol (TC), HDL cholesterol, LDL cholesterol, and triglycerides (TG). Two investigators assessed the quality of the included studies by Cochrane Collaboration Risk of Bias. Each study was evaluated from the following perspective: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other bias. The judgement of this perspective was marked as “low risk”, “high risk”, and “unclear risk” of bias.
2.5. Statistical Analysis
RevMan v.5.4.1 was used to conduct the meta-analysis. The mean difference and standard deviation were used to report the effect size of continuous data, and a 95% confidence interval (CI) was given, when p < 0.05 was considered as a statistically significant difference. The heterogeneity between studies was assessed by I2 and Cochran’s Q test, and p value ≤ 0.1 or an I2 ≥ 50% was regarded as showing heterogeneity. When heterogeneity existed, a random-effects model was applied, otherwise a fixed-effects model was chosen. Subgroup analysis was performed to identify the potential causes of heterogeneity.
3. Results
3.1. Study Selection
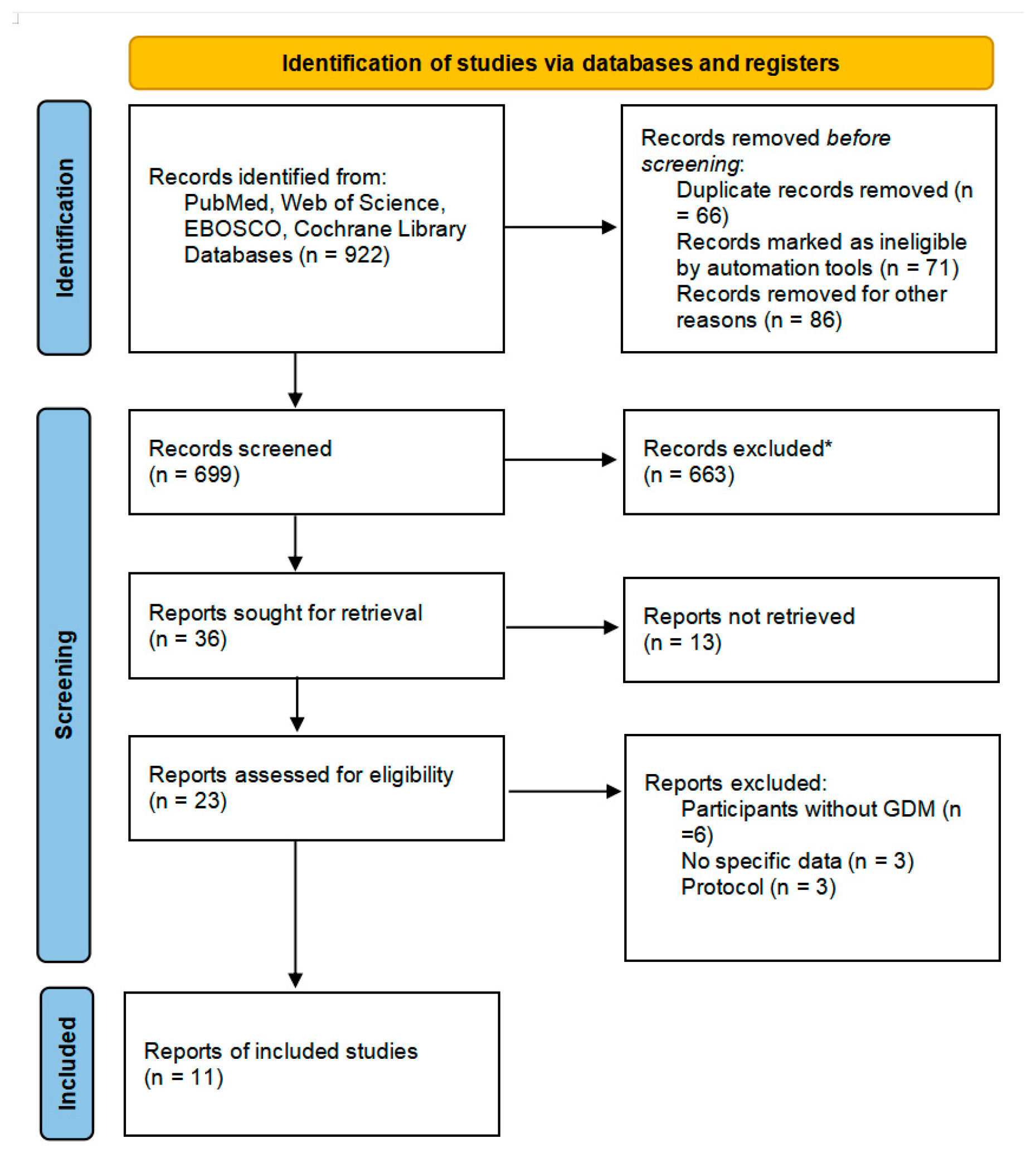
A total of 922 articles were retrieved by searching the four databases, among which 66 were excluded as duplications and 157 were irrelevant. After screening, 23 were retrieved and assessed for eligibility as part of the selection process. After selection, six studies were excluded as participants did not have GDM, three studies did not have specific data, and three protocols were excluded. Finally, 11 randomized controlled trials met the inclusion criteria and were included in this meta-analysis, as shown in Figure 1.
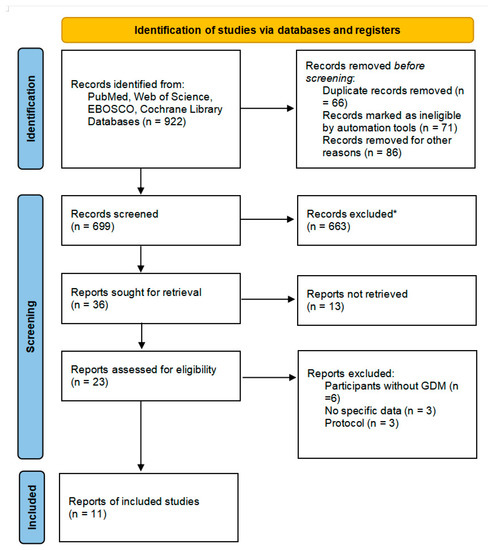
Figure 1.
Flow diagram describing inclusion and exclusion criteria. * Automation tools were used; 201 were excluded by automation tools applying filters (animals, review, meta-analysis, and year of publication); and 462 records were excluded by a human.
3.2. Description of Included Studies
The articles on RCTs were published from 2012 to 2022. Characteristics of included studies are shown in Table 1. Eight trials were conducted by Iranian researchers, while three were from Thailand, Turkey, and Ireland, respectively. These trials involved 390 participants in probiotics/synbiotics groups and 389 in placebo groups. The mean age of those participants ranged from 26.4 years to 33.5 years. Nine articles diagnosed patients with a “2 h 75 g oral glucose tolerance test”, one article did not mention the diagnostic criteria. Meanwhile, seven of them were based on the American Diabetes Association guidelines [24] and one was based on International Association of Diabetes and Pregnancy Study Groups [25]. The specific criteria are consistent among the different guidelines. One study used a “3 h 100 g oral glucose tolerance test” based on O’Sullivan’s diagnostic criteria [26], and the remaining article did not mention the details of either the diagnostic method or diagnostic criteria. Recent research demonstrated that no convincing evidence showed an advantage, except medical workload and costs [4]. Therefore, this review includes the entire studies. No patients received medicine therapy. The duration of intervention ranged from 4 weeks to 8 weeks. Eight RCTs chose probiotics, including Lactobacillus acidophilus, L. fermentum, L.casei, L. reuteri, L. salivarius, L. delbrueckii bulgaricus, Bifidobacterium bifidum, and Streptococcus thermophilus, as the intervention method. The remaining three RCTs used synbiotics, including L. acidophilus, L. casei, L. fermentum, L. gasseria, L. plantarum, Bififidobacterium bififidum, B.longum, B. infantis. Most of them took one capsule a day, only one study adopted two capsules a day. The daily consumption of probiotics/synbiotics varied from 1 × 109 CFU/capsule to 112.5 × 109 CFU/capsule. In our meta-analysis, a variety of after-intervention outcomes were reported including FPG, FSI, HOMA-IR, QUICKI, TC, HDL cholesterol, LDL cholesterol, TG, weight at end of trial, and GWG. The main finding of this meta- analysis is shown in Table 2.

Table 1.
Characteristics of included studies.

Table 2.
The main findings of this meta-analysis [36].
3.3. Risk of Bias of Included Studies and Quality of Evidence
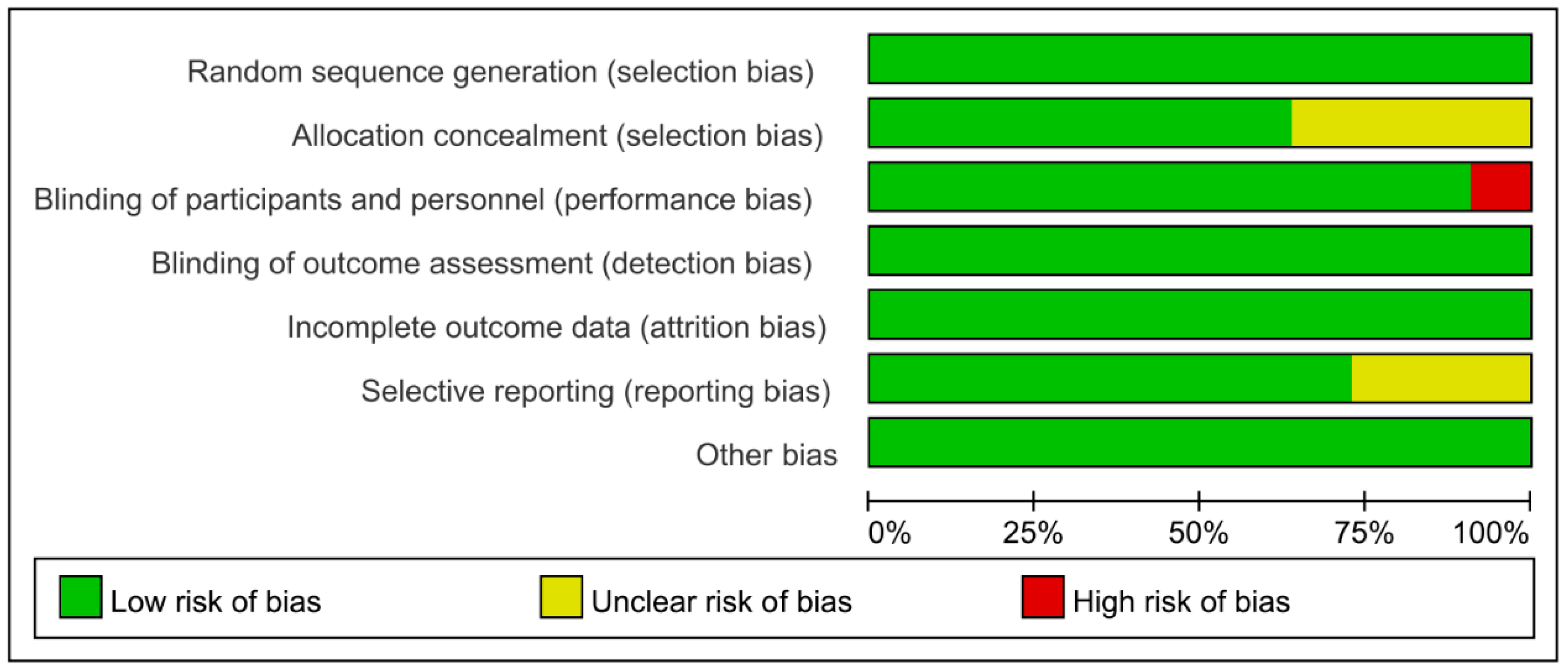
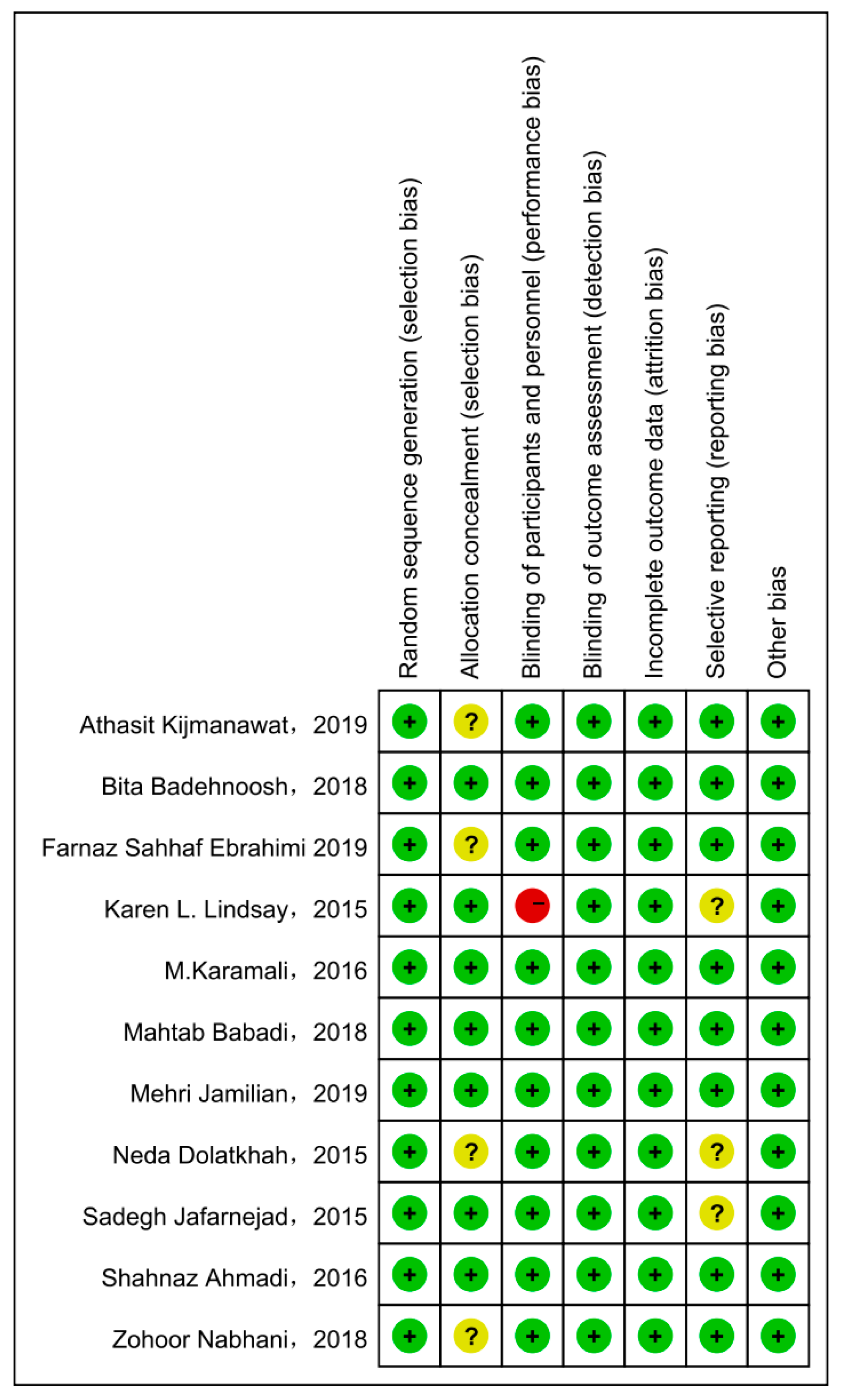
Cochrane Collaboration Risk of Bias was used with the included studies, and objectively evaluated the quality of evidence (i.e., “low risk”, “high risk”, or “unclear risk”). The rating of bias domains for included studies is shown in Figure 2. All studies had low risk in random sequence generation; among them, nine studies used a computer program, while two were conducted by trained staff. Blinding of outcome assessment and incomplete outcome data also showed 100% low risk. Around 50% had low risk in allocation concealment. Only one study had high risk in blinding of participants and personnel. The concrete bias of each study is shown in Figure 3.
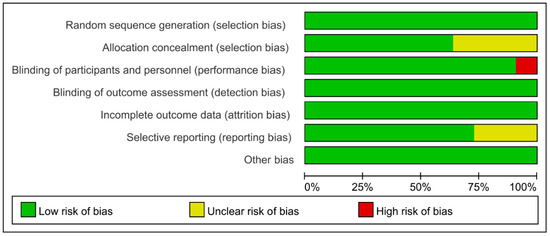
Figure 2.
Risk of bias for included studies.
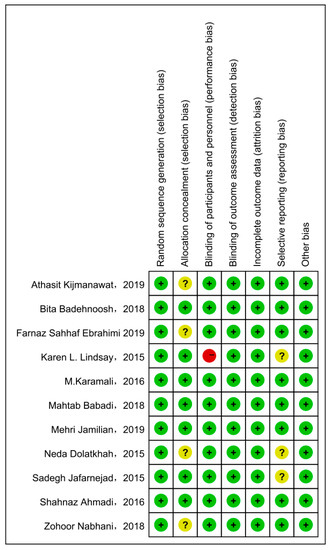
Figure 3.
The concrete bias of each study [21,22,27,28,29,30,31,32,33,34,35].
3.4. Glucose Control
3.4.1. Fasting Plasma Glucose
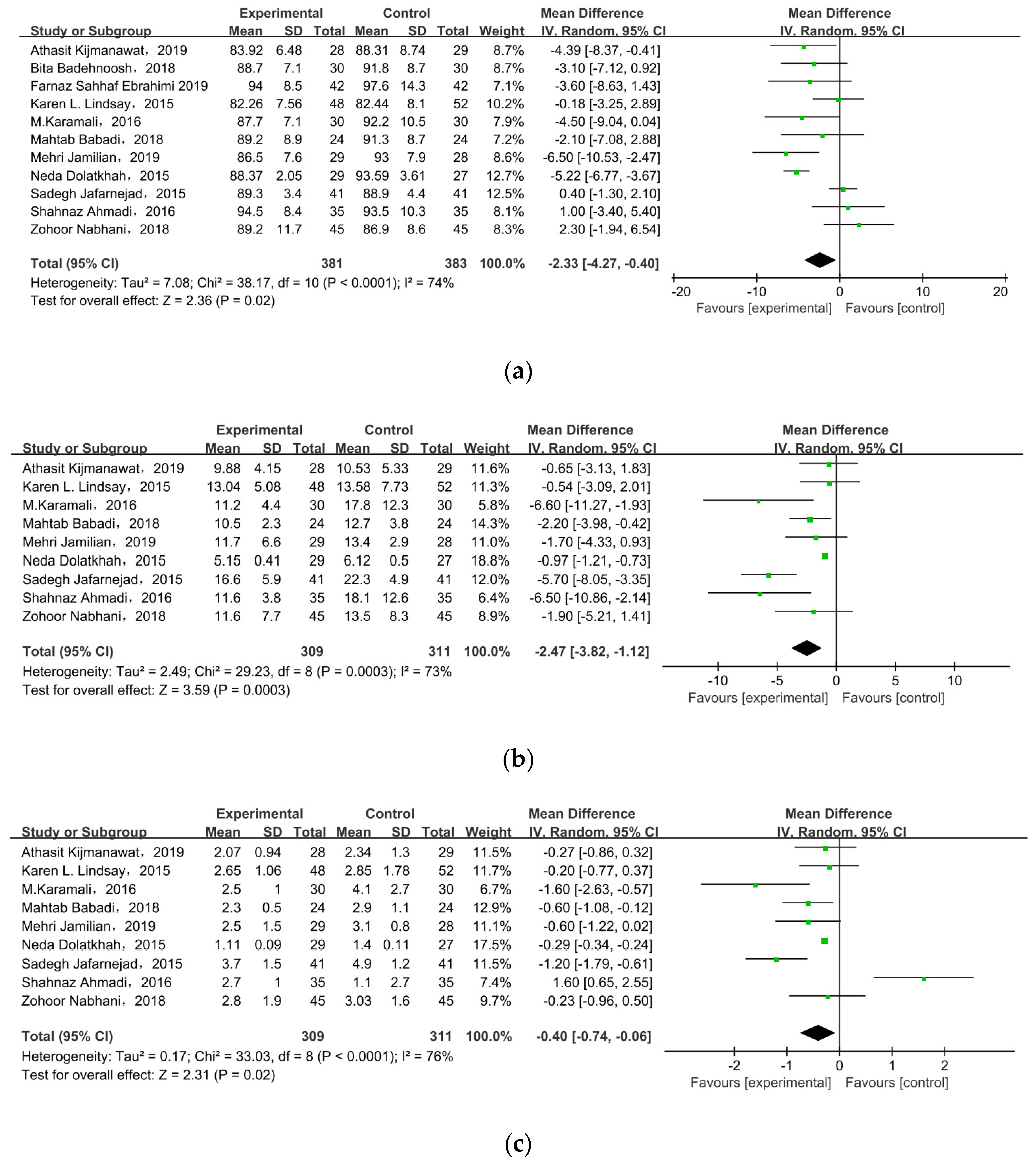
All of the included studies investigated the effect of probiotic/synbiotic supplements on FPG in GDM, as shown in Figure 4a. In the meta-analysis, the mean difference in FPG level was MD = −2.33, 95% CI = −4.27, −0.40, p = 0.02, meaning supplements statistically significantly improved FPG level among pregnant women with GDM.
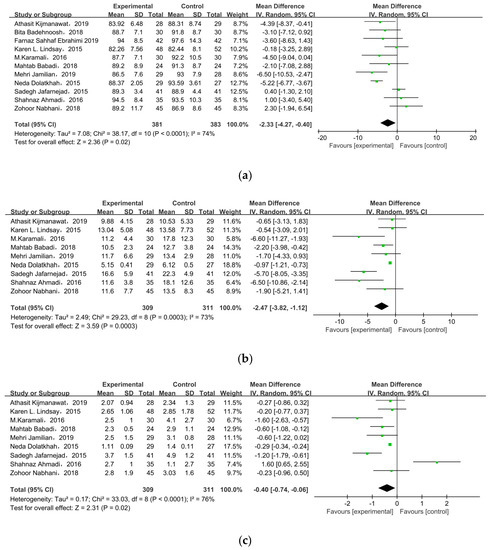
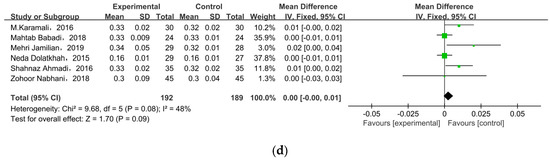
Figure 4.
Effect of probiotic supplementation on glucose control in pregnant women with GDM: (a) FPG (mg/dL), (b) FSI (mU/L), (c) HOMA-IR, and (d) QUICKI. FPG: fasting plasma glucose; FSI: fasting serum insulin; HOMA-IR: the homoeostatic model assessment for insulin resistance; QUICKI: quantitative insulin sensitivity check index [21,22,27,28,29,30,31,32,33,34,35].
3.4.2. Insulin
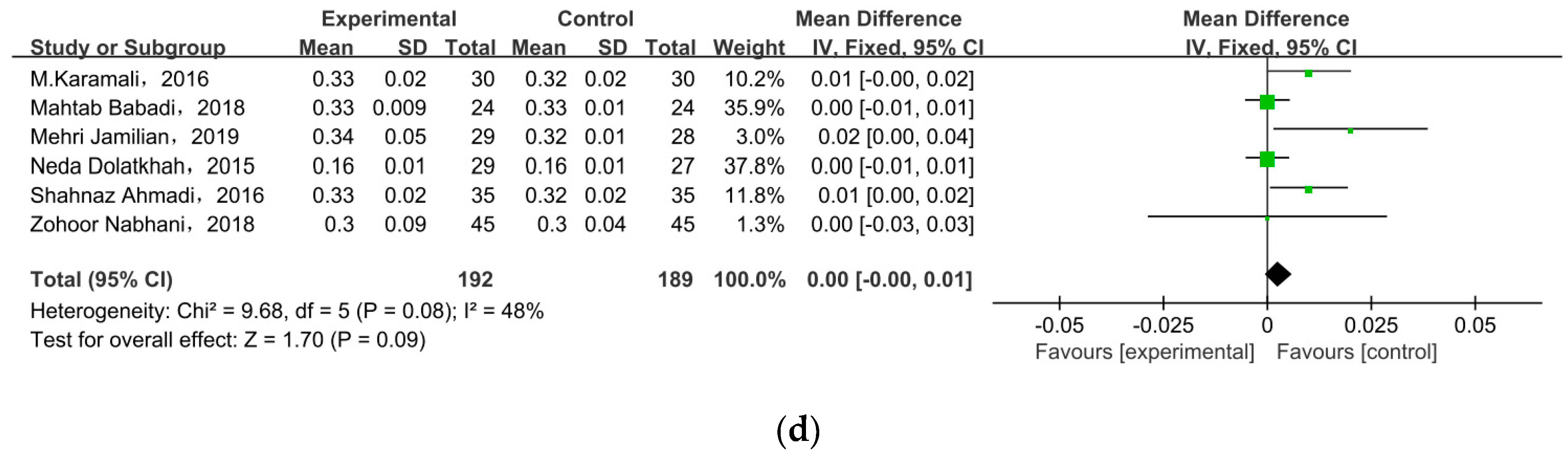
Nine of the eleven studies reported FSI as an outcome. The overall pooled estimate of the mean difference in FSI was MD = −2.47, 95% CI = −3.82, −1.12, p = 0.0003 (Figure 4b), indicating the intervention could improve FSI in women with GDM. HOMA-IR was observed in nine studies. After meta-analysis, we found a significant effect favoring probiotics/synbiotics, as shown in Figure 4c (MD = −0.40, 95% CI = −0.74, −0.06, p = 0.02). Six studies measured QUICKI, but there was no significant difference between the two groups (MD = 0.00, 95% CI = 0.00, 0.01, p = 0.09) (Figure 4d).
3.5. Lipid Profiles
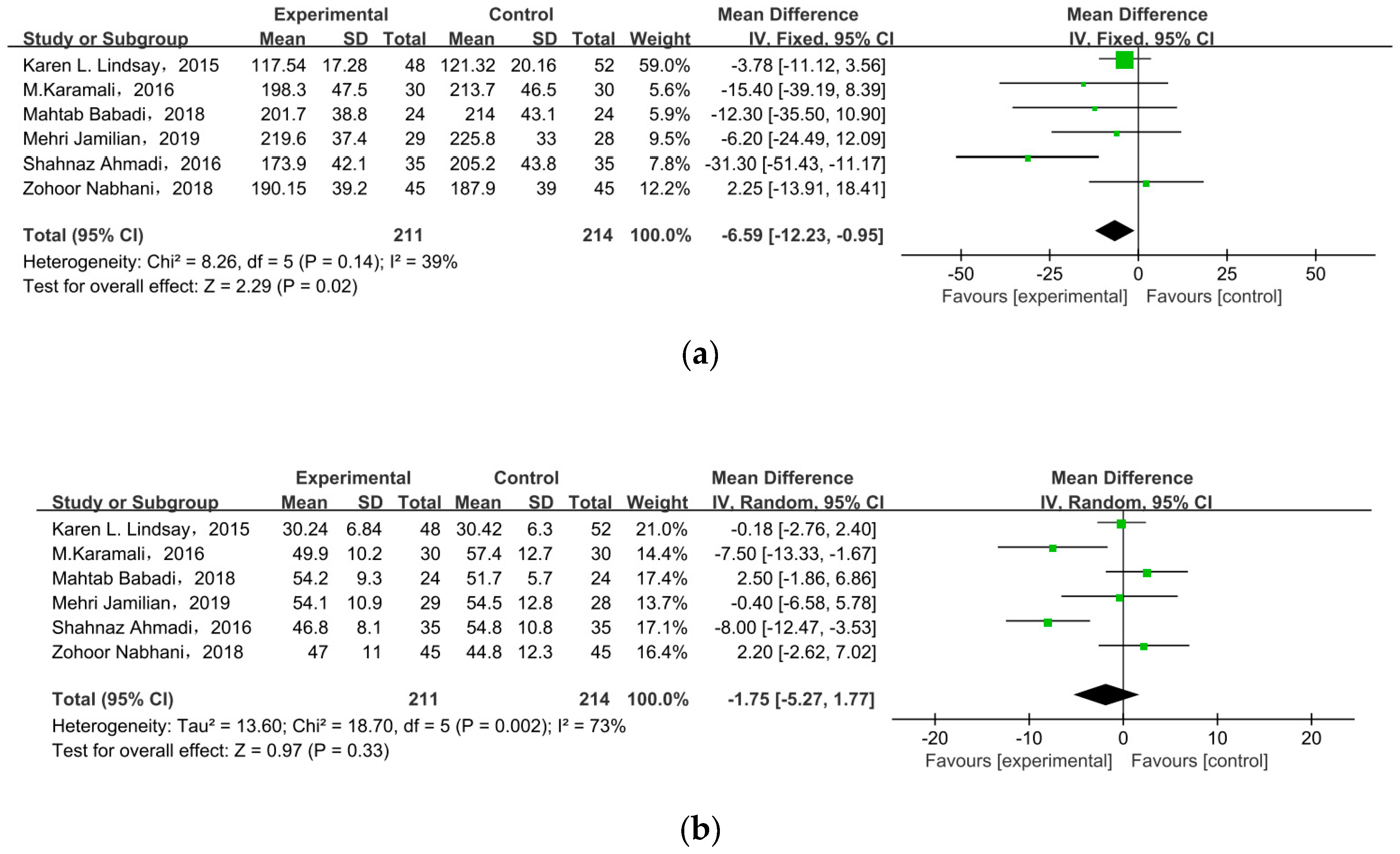
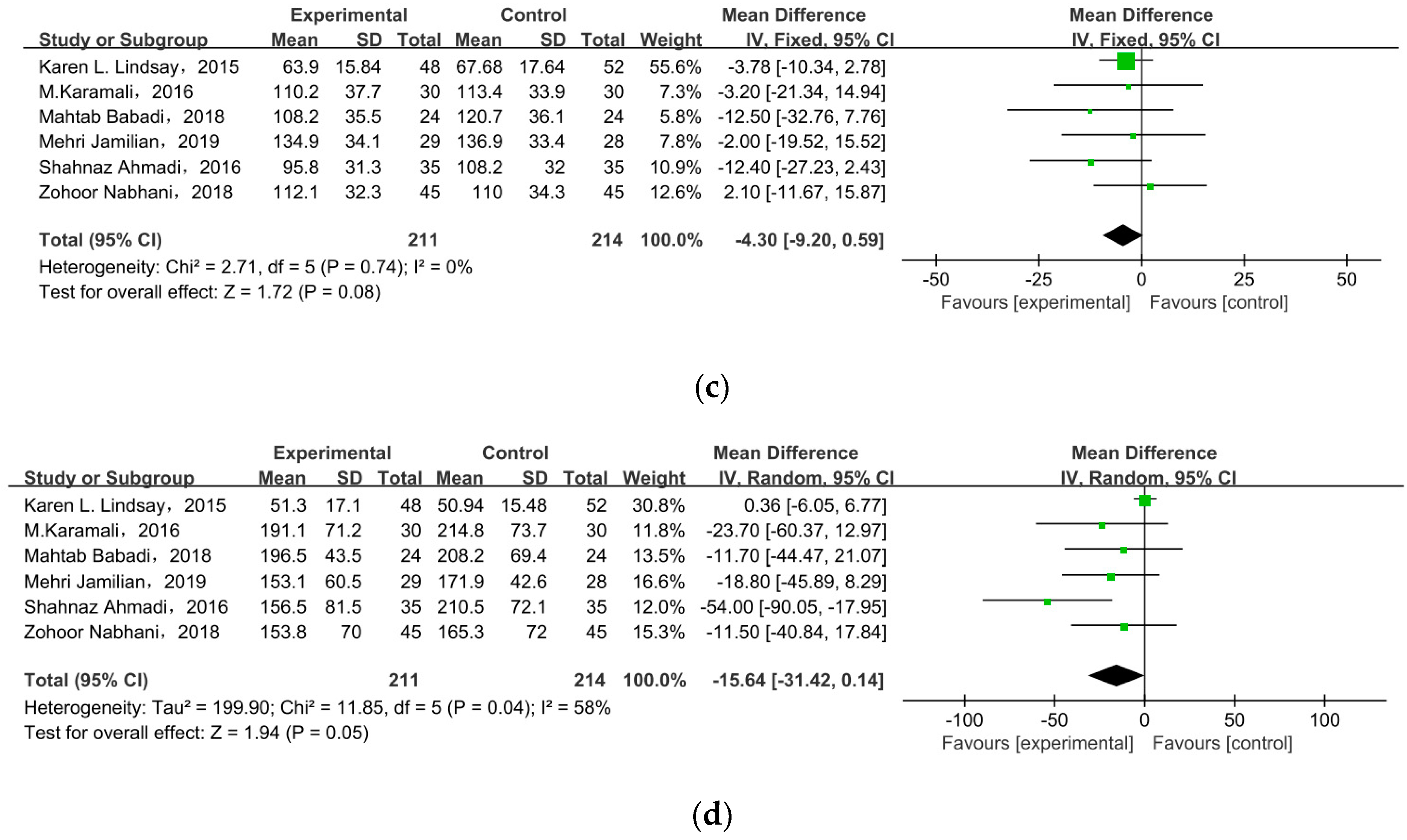
Six studies researched lipid profiles, including TC, HDL, LDL, and TG (Figure 5). All data were pooled for meta-analysis, and only TC was significantly reduced in pregnant women with GDM after receiving supplement therapy (MD = −6.59, 95% CI = −12.23, −0.95, p = 0.02) (Figure 5a). On the contrary, the other observed indexes described no obvious change between treatment groups and control groups: HDL cholesterol (MD = −1.75, 95% CI = −5.27, 1.77, p = 0.33) (Figure 5b), LDL cholesterol (MD = −4.30, 95% CI = −9.20, 0.59, p = 0.08), TG (MD = −15.64, 95% CI = −31.42, 0.14, p = 0.05) (Figure 5c).
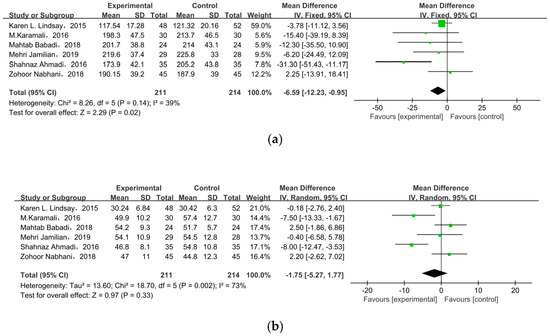
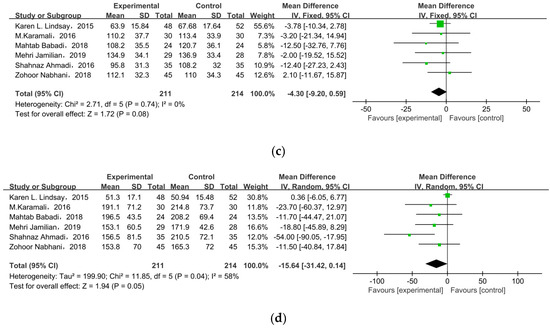
Figure 5.
Effect of probiotic supplementation on lipid profiles in pregnant women with GDM: (a) TC (mg/dL), (b) HDL cholesterol (mg/dL), (c) LDL cholesterol (mg/dL), and (d) TG (mg/dL). TC: total cholesterol; TG: triglycerides [22,29,30,31,34,35].
3.6. Body Weight
3.6.1. Weight at End of Trial
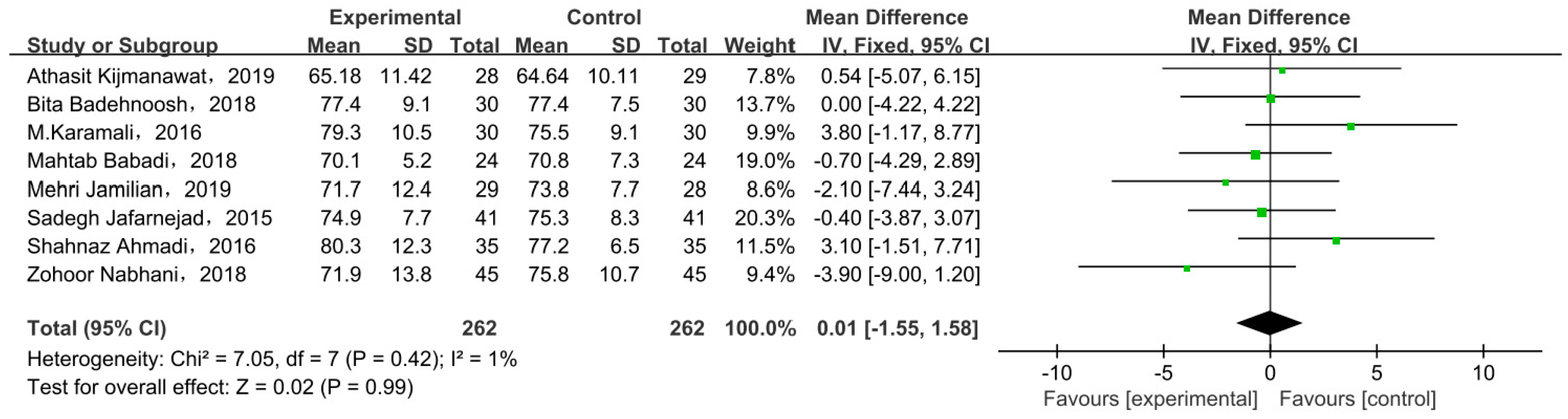
Eight studies compared body weight at end of trial with two types of intervention. There was no significant difference in body weight between the probiotic/synbiotic and placebo groups at the end of test in all included studies, as shown by the meta-analysis (Figure 6). The mean difference in body weight was 0.01 (95% CI = −1.55, 1.58, p = 0.99).
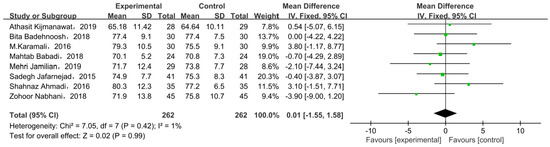
Figure 6.
Effect of probiotic supplementation on weight at end of trial in pregnant women with GDM [21,27,29,30,31,33,34,35].
3.6.2. Gestational Weight Gain
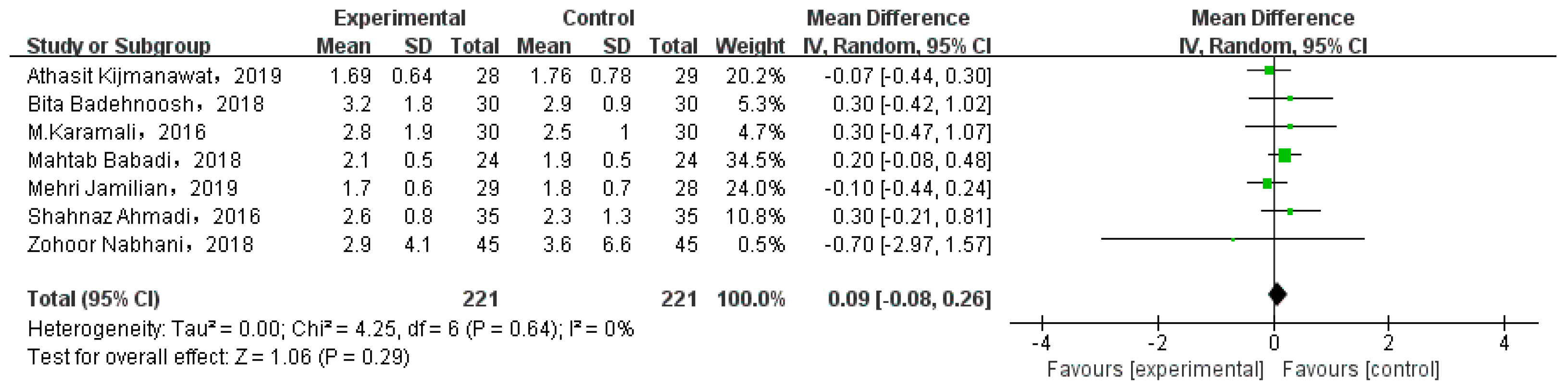
Eight studies involved GWG. While one of them compared the weight gain at different periods, and the data could not be extracted. Thus, we analyzed seven studies, as shown in Figure 7. There was no significant gestational weight gain between two types of intervention (MD = 0.09, 95% CI = −0.08, 0.26, p = 0.29).
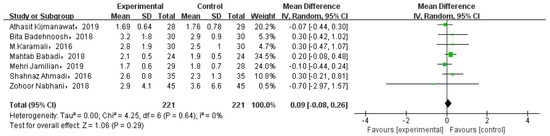
Figure 7.
Effect of probiotic supplementation on gestational weight gain in pregnant women with GDM [21,27,29,30,31,34,35].
3.7. Subgroup Analysis
Substantial heterogeneity was observed in the meta-analyses for FPG (I2 = 74%, p < 0.0001), FSI (I2 = 73%, p = 0.0003), HOMA-IR (I2 = 76%, p ≤ 0.0001), HDL cholesterol (I2 = 73%, p = 0.002), and TG (I2 = 58%, p = 0.04). We grouped duration and kind of supplement to investigate the potential reason for heterogeneity. The p-value and heterogeneity for different groups are shown in Table 3. The results showed that the kind of supplement led to heterogeneity for FPG and FSI, while we did not find the main cause of heterogeneity for others.

Table 3.
The results of subgroup analysis according to duration, intervention type, and dose.
4. Discussion
This review included 11 RCTs and 779 participants to assess the effect of probiotic/synbiotic supplements on improving glucose and lipid metabolism in women with GDM. We assessed the level of FPG, FSI, HOMA-IR, and QUICKI to explain the effect of supplements on glucose control. TC, HDL cholesterol, LDL cholesterol, and TG were used to investigate the effect of intervention on lipid metabolism. The weight at end of trial and gestational weight gain were used to reflect the maternal fat accumulation during pregnancy. This meta-analysis found the intervention improved glucose and lipid metabolism in terms of FPG (MD = −2.33, p = 0.02), FSI (MD = −2.47, p = 0.0003), HOMA-IR (MD = −0.40, p = 0.02), and TC (MD = −6.59, p = 0.02) in GDM women.
GDM is usually caused by β-cell impairment and insulin resistance. During the second and third trimesters of pregnancy, anti-insulin hormones such as estrogen, progesterone, and cortisol promote the development of insulin resistance [37]. In order to maintain the normal metabolism of glucose, the amount of insulin needs to be increased accordingly. During gestational diabetes, β-cells fail to secrete insulin for the demands of pregnancy, and, when combined with reduced insulin sensitivity, this results in hyperglycemia [38]. GDM is also related to reduced adipocyte differentiation and increased adipocyte size. Furthermore, this change is combined with insulin resistance, which leads to lipid deposition in the liver and skeletal muscle, thus aggravating the condition of GDM [38,39].
In GDM patients, a high level of body fat and intake of high-fat nutrition could change the normal gut microbial composition. This could increase Firmicutes and Faecalibacterium that produce butyrate, and result in overexpression of short chain fatty acids (SCFAs) [20]. Excess SCFAs increase the lipid storage in skeletal muscle and liver, thus glucose and lipid metabolism disorders develop [40]. Probiotics could perform a straightforward and important role in the intestinal mucosal barrier, which increases glucose tolerance, while reversing the gut flora imbalance induced by GDM [41]. Probiotics also regulate the level of SCFAs. When the SCFAs are within the normal range, they could regulate glucose metabolism, lowering the pH of the lumen while preventing pathogen growth. The intestinal epithelial cells could be activated by SCFAs through the cells’ G protein-coupled receptor 41 (GPR41) and GPR43, and the colonic epithelial intestinal expression of peptide YY and glucagon-like peptide-1 (GLP-1) hormones will thereby be increased [42,43].
Thus, transit time of food in the intestine could slow and insulin sensitivity could increase. By tight junction protein transcription regulation and glucagon-like peptide-2 (GLP-2) production enhancement, SCFAs decrease intestinal permeability and may reduce inflammation in colonic epithelial cells [44]. Moreover, SCFAs could be recognized by free fatty acid receptor-2 (FFA-2), and research has shown the synergistic effect between SCFAs, FFA-2, and gut microbiota in gestational glucose homeostasis [45]. Inulin is a common synbiotic. Taking inulin could improve glucose metabolism dysregulation and overweight, decrease the Firmicutes/Bacteroidetes ratio (F/B), as well as increase Bifidobacterium abundance [46,47]. Consistent with Shahnaz Ahmadi et al.’s [34]. Findings, we found that after 6 weeks of synbiotic supplements the FSI and HOMA-IR were significantly reduced. In this meta-analysis, six RCTs reported lipid profiles, and after intervention TC showed a significantly decrease. However, some other clinical studies have produced conflicting results, with taking probiotics or placebo in the first trimester of pregnancy having no effect on the lipid levels in the third trimester [22]. Beneficial gut bacteria are increased after taking probiotics/synbiotics. Thus, with the production of secondary bile acids that are not available for enterohepatic recirculation, those gut flora have a favorable impact on lipid metabolism. Next, the liver must produce new bile acids from circulating cholesterol [48]. The population with abnormal gut microbes and glucose and lipid metabolism may have insulin resistance, increasing the onset risk of GDM and the mother’s body weight. Continuous hyperglycemia in pregnant women can affect the fetal insulin levels, thereby accelerating fetal weight gain. Probiotics/synbiotics could lower maternal insulin resistance and restore gut flora to keep the mother’s weight in the normal range, as well as reduce the risk of eclampsia, shoulder dystocia, T2DM, and other GDM-related diseases [49]. The offspring absorbs nutrients through the placenta, further reducing the incidence of neonatal hypoglycemia, obesity, T2DM, and autism [50]. However, our included RCTs showed no difference between treatment and placebo on HDL cholesterol and LDL cholesterol. We hypothesized that a duration of intervention longer than 8 weeks may have generated outcomes with larger effect sizes.
In the current study, we also found substantial heterogeneity among FPG, FSI, HOMA-IR, HDL cholesterol, and TG. The subgroup analyses on FPG indicate that probiotic supplements may lead to great improvement in the GDM population. There was no significant difference in FSI with more than 8 weeks’ nutritional intervention, as only two relevant RCTs were included and the data were insufficient. In the subgroup analyses of intervention methods, synbiotics failed to reduce the HOMA-IR. For HDL and TC, the subgroup analysis revealed no significant differences. Prespecified subgroup analyses according to duration, intervention type, and dose showed that the heterogeneity was substantially reduced or even disappeared in a few subgroups, but persisted in most others. A possible reason is that the potential effect modification by the two subgroup factors could not be effectively investigated by our subgroup analyses. For example, the dose of the treatments varied from 1 × 109 CFU/capsule to 112.5 × 109 CFU/capsule, and some only had the range of the dose rather than the specific number. Another possible explanation is that the substantial heterogeneity may relate to the interaction of duration, intervention type, and dose. Additionally, this may be caused by other factors, e.g., the mean age and test method. In addition, most of the included studies are from Iran. Subsequent studies could increase duration (>8 weeks), include other racial backgrounds, and confirm the quantity of the dose, to reduce the interference factors.
5. Strength and Limitation
Our meta-analysis has several strengths. First, this study consists of multiple articles, to include more data for analysis and increase the reliability of the results. Second, most of the included RCTs were high quality with random sequence generation and double-blinding methods, so the data are reliable. Third, studies whose population was limited to women with GDM made the results more representative. However, the current study has a few limitations. First, the numbers of participants in the included RCTs were relatively small, ranging from 20 to 60. Second, eight RCTs were conducted in Iran, which means the research may show publication bias and the conclusions may not be applicable to populations from other racial backgrounds and countries. Third, we did not assess the effect of probiotic/synbiotic supplementation on infants and failed to capture the long-term effect for mothers and their offspring. Furthermore, the types and durations of probiotics/synbiotics were not consistent in the different studies that were included in this analysis. To identify the effect of intervention duration and type, more RCTs must be conducted. Overall, the glucose and lipid metabolism-related indicators were significantly improved, which can indicate that probiotic/synbiotic supplements may be considered as an adjunct treatment for GDM patients.
6. Conclusions
Probiotic/synbiotic could improve glucose and lipid metabolism in pregnant women with GDM. The use of specific probiotic supplementations containing Lactobacillus acidophilus and Bifidobacterium bifidum (>1 × 106 CFU/g) may be a promising prevention and therapeutic strategy for GDM, as they could directly act on the intestinal mucosal barrier and restore the gut flora balance. However, due to the heterogeneity among existing studies, further studies are warranted to address the limitations of existing evidence and better inform the management of GDM.
Author Contributions
Conceptualization, J.M. and X.G.; methodology, J.M., Y.Z. and G.C.; formal analysis, J.M. and Y.Z.; data curation, J.M. and Y.Z.; writing—original draft preparation, J.M.; writing—review and editing, J.M. and X.G.; supervision, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Fundamental Research Funds for the Central Universities (No. 2016YB030 and No. 2018GJ019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO): registration ID CRD42023387754.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wan, J.; Ma, J. Efficacy of dietary supplements targeting gut microbiota in the prevention and treatment of gestational diabetes mellitus. Front. Microbiol. 2022, 13, 927883. [Google Scholar] [CrossRef]
- Homayouni, A.; Bagheri, N.; Mohammad-Alizadeh-Charandabi, S.; Kashani, N.; Mobaraki-Asl, N.; Mirghafurvand, M.; Asgharian, H.; Ansari, F.; Pourjafar, H. Prevention of Gestational Diabetes Mellitus (GDM) and Probiotics: Mechanism of Action: A Review. Curr. Diabetes Rev. 2020, 16, 538–545. [Google Scholar]
- Quaresima, P.; Saccone, G.; Pellegrino, R.; Vaccarisi, S.; Taranto, L.; Mazzulla, R.; Bernardo, S.; Venturella, R.; Di Carlo, C.; Morelli, M. Incidental diagnosis of a pancreatic adenocarcinoma in a woman affected by gestational diabetes mellitus: Case report and literature review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100471. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Tocci, V.; Greco, E.; Foti, D.; Brunetti, A. Gestational diabetes: Implications for fetal growth, intervention timing, and treatment options. Curr. Opin. Pharmacol. 2021, 60, 1–10. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Melhorn, S.; Olerich, K.L.W.; Angelo, B.; Chow, T.; Xiang, A.; Schur, A.E.; Page, A.K. Exposure to Gestational Diabetes Mellitus Prior to 26 Weeks Is Related to the Presence of Mediobasal Hypothalamic Gliosis in Children. Diabetes 2022, 71, 2552–2556. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Nolan, C.J. The fetal glucose steal: An underappreciated phenomenon in diabetic pregnancy. Diabetologia 2016, 59, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Lowe, W.J.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, M.J.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380. [Google Scholar] [CrossRef]
- Huang, L.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, C.S. Impacts of gut microbiota on gestational diabetes mellitus: A comprehensive review. Eur. J. Nutr. 2021, 60, 2343–2360. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Hu, X.; Hu, X.; Zhang, J.; Zhang, H.; Wang, J.; Su, S.; Wang, Y.; Lyu, Z. The effect of gestational diabetes mellitus on fetal right heart growth in late-term pregnancy: A prospective study. Echocardiography 2022, 39, 1101–1112. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Li, Y.; Huang, S.; Zhang, L.; Cao, C.; Baker, N.P.; Tong, C.; Zheng, P.; Qi, H. Altered gut bacterial and metabolic signatures and their interaction in gestational diabetes mellitus. Gut Microbes 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, K.L.; Nitert, D.M. Probiotics for preventing gestational diabetes. Cochrane Database Syst. Rev. 2021, 4, D9951. [Google Scholar]
- Liu, H.; Pan, L.L.; Lv, S.; Yang, Q.; Zhang, H.; Chen, W.; Lv, Z.; Sun, J. Alterations of Gut Microbiota and Blood Lipidome in Gestational Diabetes Mellitus With Hyperlipidemia. Front. Physiol. 2019, 10, 1015. [Google Scholar] [CrossRef] [PubMed]
- Mahdizade, A.M.; Teymouri, S.; Fazlalian, T.; Asadollahi, P.; Afifirad, R.; Sabaghan, M.; Valizadeh, F.; Ghanavati, R.; Darbandi, A. The effect of probiotics on gestational diabetes and its complications in pregnant mother and newborn: A systematic review and meta-analysis during 2010–2020. J. Clin. Lab. Anal. 2022, 36, e24326. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Zhang, W. The Effect of Metformin Therapy for Preventing Gestational Diabetes Mellitus in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Exp. Clin. Endocrinol. Diabetes 2020, 128, 199–205. [Google Scholar] [CrossRef]
- Huifen, Z.; Yaping, X.; Meijing, Z.; Huibin, H.; Chunhong, L.; Fengfeng, H.; Yaping, Z. Effects of moderate-intensity resistance exercise on blood glucose and pregnancy outcome in patients with gestational diabetes mellitus: A randomized controlled trial. J. Diabetes Complications 2022, 36, 108186. [Google Scholar] [CrossRef]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, B.S.; Ovesen, G.P.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef]
- Rad, A.H.; Abbasalizadeh, S.; Vazifekhah, S.; Abbasalizadeh, F.; Hassanalilou, T.; Bastani, P.; Ejtahed, S.H.; Soroush, R.V.; Javadi, M.; Mortazavian, M.A. The Future of Diabetes Management by Healthy Probiotic Microorganisms. Curr. Diabetes Rev. 2017, 13, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Masulli, M.; Vitacolonna, E.; Fraticelli, F.; Pepa, D.G.; Mannucci, E.; Monami, M. Effects of probiotic supplementation during pregnancy on metabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2020, 162, 108111. [Google Scholar] [CrossRef] [PubMed]
- Kunasegaran, T.; Balasubramaniam, V.; Arasoo, V.; Palanisamy, U.; Ramadas, A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology 2021, 10, 1027. [Google Scholar] [CrossRef]
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2019, 10, 163–170. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, C.O.; Smith, T.; Curran, S.; Coffey, M.; Foley, E.M.; Hatunic, M.; Shanahan, F. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 491–496. [Google Scholar]
- Rethlefsen, M.L.; Page, M.J. PRISMA 2020 and PRISMA-S: Common questions on tracking records and the flow diagram. J. Med. Libr. Assoc. 2022, 110, 253–257. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, A.T.; Catalano, C.A.; Damm, P.; Dyer, R.A.; Leiva, A.; Hod, M.; Kitzmiler, L.J. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern.-Fetal Neonatal Med. 2018, 31, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Sahhaf Ebrahimi, F.; Homayouni Rad, A.; Mosen, M.; Abbasalizadeh, F.; Tabrizi, A.; Khalili, L. Effect of L. Effect of L. acidophilus and B. lactis on blood glucose in women with gestational diabetes mellitus: A randomized placebo-controlled trial. Diabetol. Metab. Syndr. 2019, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Mesgari Abbasi, M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015, 33, 25. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J. Nutr. Metab. 2016, 2016, 5190846. [Google Scholar] [CrossRef]
- Ahmadi, S.; Jamilian, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2016, 116, 1394–1401. [Google Scholar] [CrossRef]
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The effects of synbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: A randomized double-blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157. [Google Scholar] [CrossRef]
- Feng, C.; Adebero, T.; DePaul, G.V.; Vafaei, A.; Norman, E.K.; Auais, M. A Systematic Review and Meta-Analysis of Exercise Interventions and Use of Exercise Principles to Reduce Fear of Falling in Community-Dwelling Older Adults. Phys Ther. 2022, 102. [Google Scholar] [CrossRef]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am. J. Obstet. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Krssak, M.; Winzer, C.; Pacini, G.; Tura, A.; Farhan, S.; Wagner, O.; Brabant, G.; Horn, R.; Stingl, H. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes 2003, 52, 244–251. [Google Scholar] [CrossRef]
- Stefanovski, D.; Punjabi, N.M.; Boston, R.C.; Watanabe, R.M. Insulin Action, Glucose Homeostasis and Free Fatty Acid Metabolism: Insights From a Novel Model. Front. Endocrinol. 2021, 12, 625701. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Factories 2020, 19, 23. [Google Scholar] [CrossRef]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef]
- Fuller, M.; Priyadarshini, M.; Gibbons, S.M.; Angueira, A.R.; Brodsky, M.; Hayes, G.M.; Kovatcheva-Datchary, P.; Bäckhed, F.; Gilbert, J.A. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E840–E851. [Google Scholar] [CrossRef]
- Li, H.; Zhou, D.; Gan, R.; Huang, S.; Zhao, C.; Shang, A.; Xu, X.; Li, H. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Farhangi, M.A.; Tavakoli, F.; Aliasgarzadeh, A.; Akbari, A.M. Impact of prebiotic supplementation on T-cell subsets and their related cytokines, anthropometric features and blood pressure in patients with type 2 diabetes mellitus: A randomized placebo-controlled Trial. Complement. Ther. Med. 2016, 24, 6–102. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Fava, F.; Viola, R. ‘The way to a man’s heart is through his gut microbiota’--dietary pro- and prebiotics for the management of cardiovascular risk. Proc. Nutr. Soc. 2014, 73, 172–185. [Google Scholar] [CrossRef]
- Salles, B.; Cioffi, D.; Ferreira, S. Probiotics supplementation and insulin resistance: A systematic review. Diabetol. Metab. Syndr. 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Swartwout, B.; Luo, X.M. Implications of Probiotics on the Maternal-Neonatal Interface: Gut Microbiota, Immunomodulation, and Autoimmunity. Front. Immunol. 2018, 9, 2840. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).