Nuts, Energy Balance and Body Weight
Abstract
:1. Introduction
2. Energy Availability
2.1. Effect of Processing and Mastication on Almond Lipid Bioaccessibility
2.1.1. Effect of Roasting
2.1.2. Effect of Mastication
2.1.3. Observations with Walnuts and Pistachios
2.2. History of Determining the Energy Value of Foods
2.3. Recent Measures of the Metabolizable Energy Value of Nuts
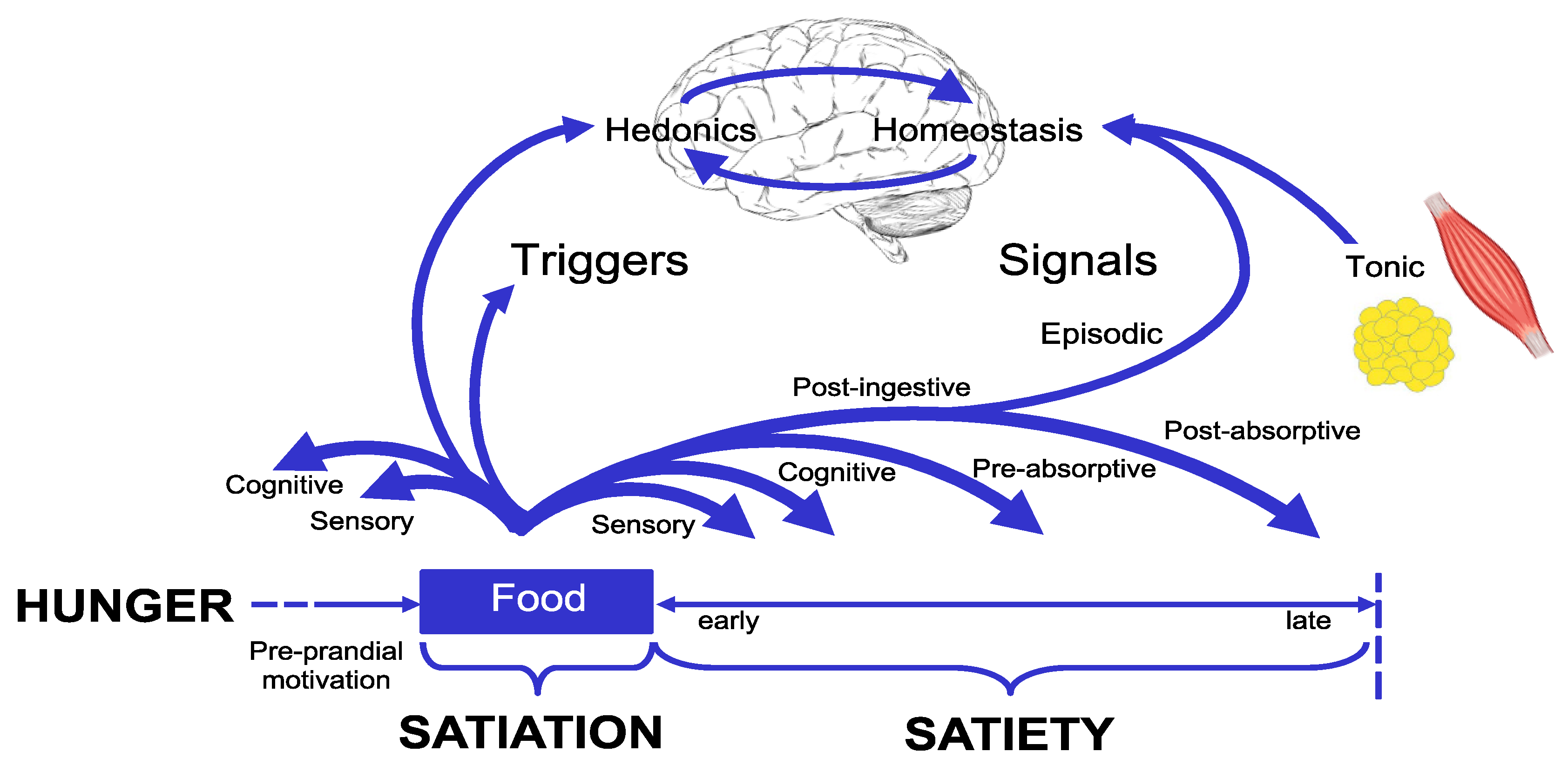
3. Appetite as a Complex System
3.1. Appetite and Satiety (and Satiation)
3.2. Foods and the Satiety Cascade
3.3. The Nature of Satiety Signals
3.4. A Note on (the Role of) Food Hedonics
3.5. Individual Variability in Appetite Control and the Low Satiety Phenotype
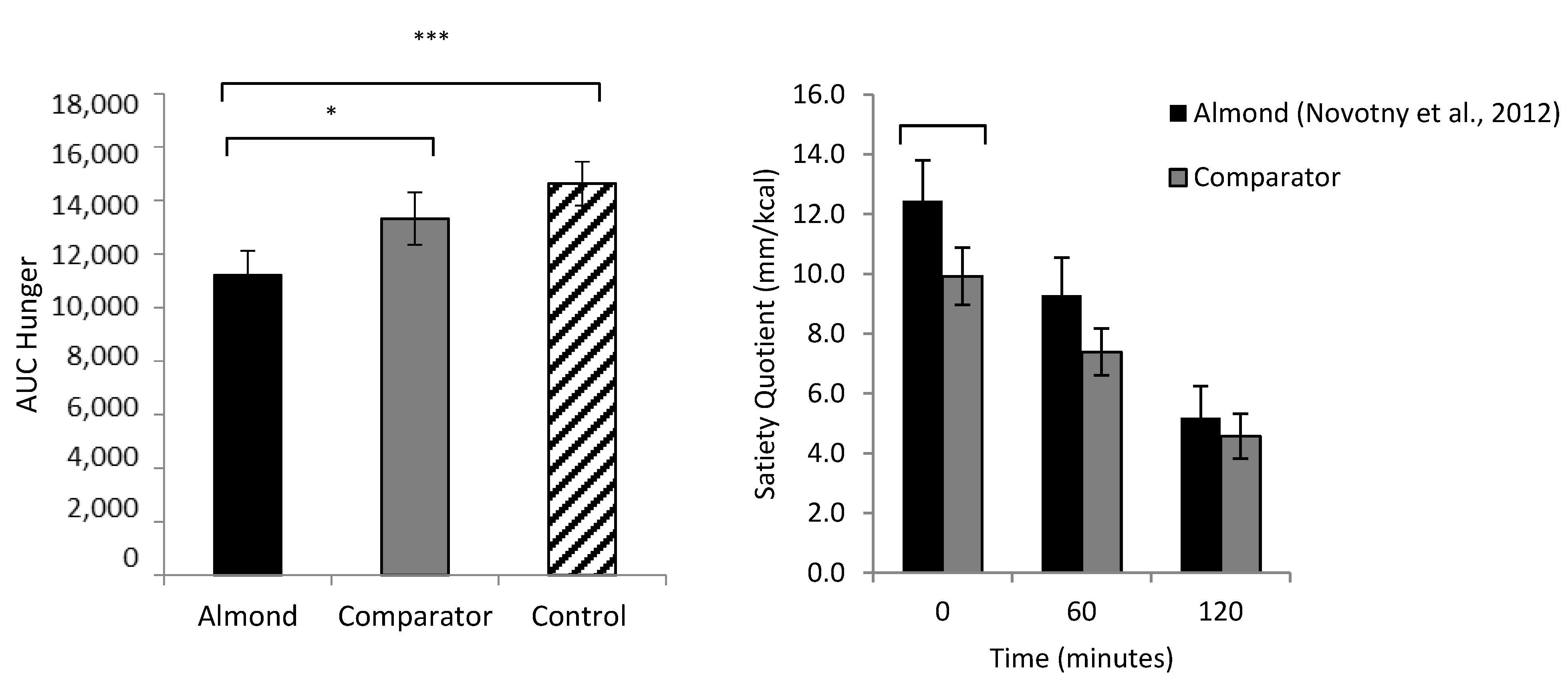
3.6. Nuts and Appetite Control: A Case Study with Almonds
3.7. Nuts and the Low Satiety Phenotype
4. Overview of Nut Consumption and Body Weight
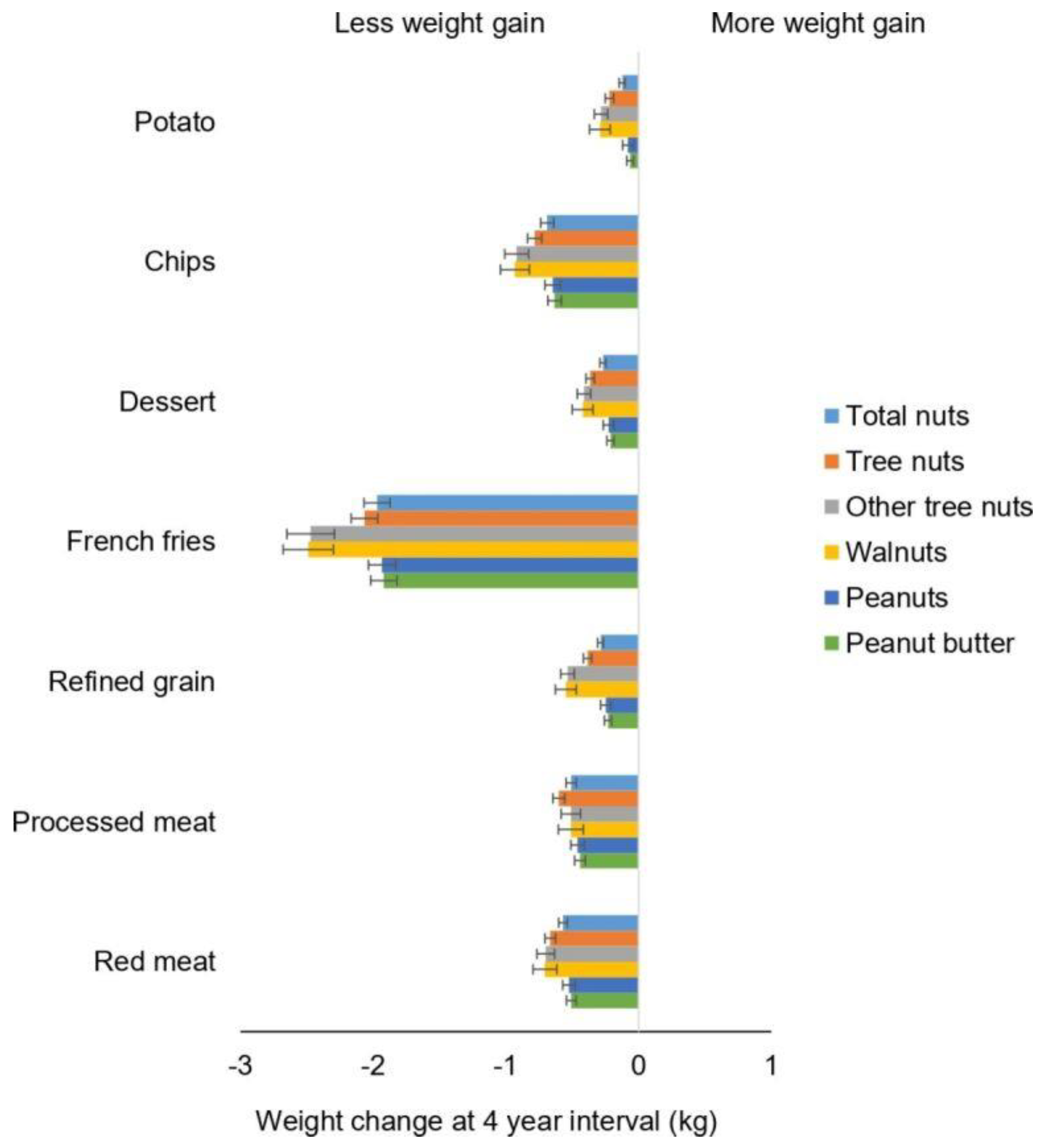
4.1. Evidence from Prospective Cohort Studies
4.2. Evidence from RCTs
4.3. Methodological Issues in Observational Studies and RCTs
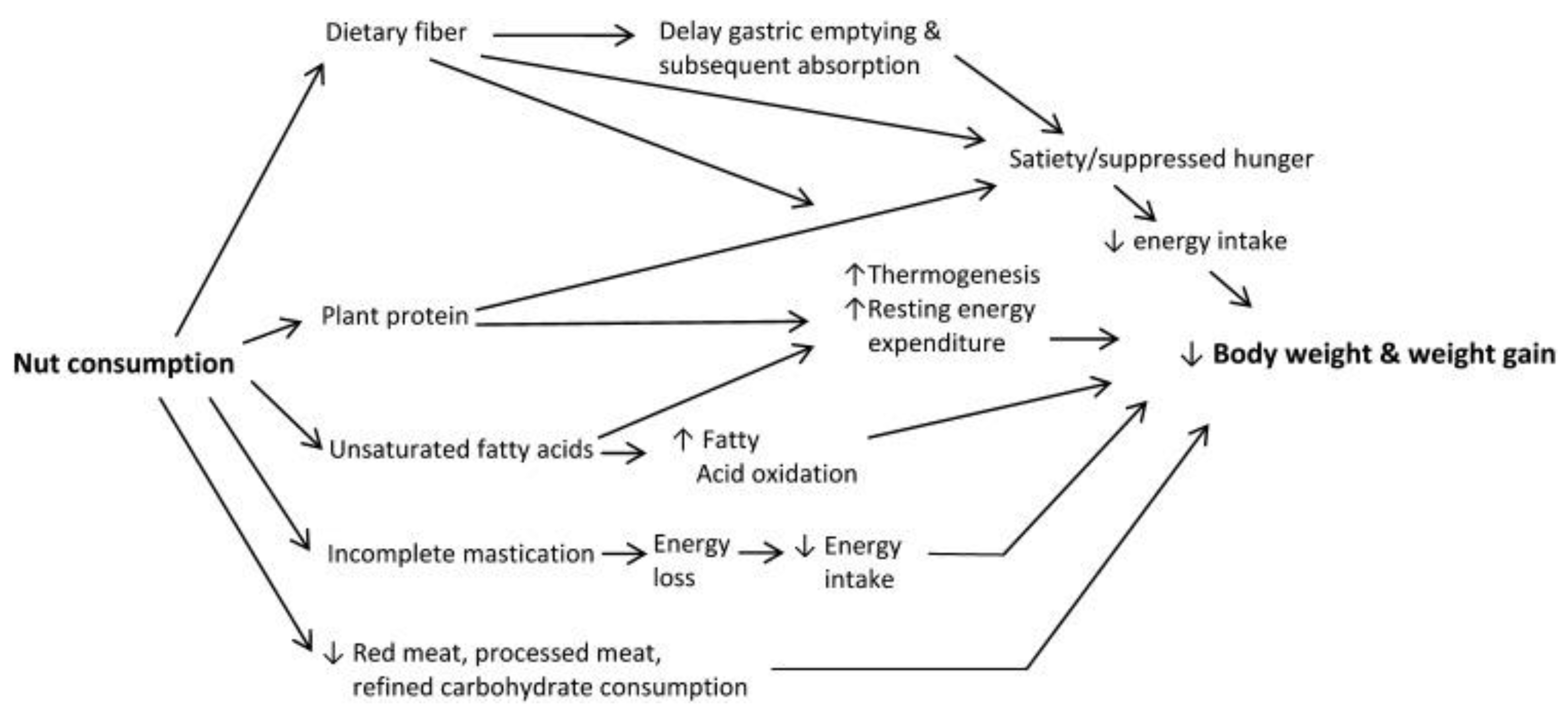
5. Potential Components of Nuts That Contribute to Weight Control
6. Clinical and Public Health Dietary Recommendations on Nuts and Weight Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Fraser, G.E.; Sabate, J.; Beeson, W.L.; Strahan, T.M. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch. Intern. Med. 1992, 152, 1416–1424. [Google Scholar] [CrossRef] [Green Version]
- United States. Dietary Guidelines Advisory Committee.; United States. Department of Agriculture.; United States. Department of Health and Human Services.; United States. Office of Disease Prevention and Health Promotion. Dietary guidelines for Americans. In USDA Publication Number: Home and Garden Bulletin No. 232; U.S. Dept. of Health and Human Services: U.S. Dept. of Agriculture: Washington, DC, USA, 1995; p. 45. [Google Scholar]
- Alberts, B. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Levine, A.S.; Silvis, S.E. Absorption of whole peanuts, peanut oil, and peanut butter. N. Engl. J. Med. 1980, 303, 917–918. [Google Scholar] [CrossRef]
- Baer, D.J.; Judd, J.T.; Kris-Etherton, P.M.; Zhao, G.; Emken, E.A. Stearic acid absorption and its metabolizable energy value are minimally lower than those of other fatty acids in healthy men fed mixed diets. J. Nutr. 2003, 133, 4129–4134. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.J.; Rumpler, W.V.; Miles, C.W.; Fahey, G.C., Jr. Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J. Nutr. 1997, 127, 579–586. [Google Scholar]
- Grundy, M.M.; Grassby, T.; Mandalari, G.; Waldron, K.W.; Butterworth, P.J.; Berry, S.E.; Ellis, P.R. Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. Am. J. Clin. Nutr. 2015, 101, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandalari, G.; Parker, M.L.; Grundy, M.M.; Grassby, T.; Smeriglio, A.; Bisignano, C.; Raciti, R.; Trombetta, D.; Baer, D.J.; Wilde, P.J. Understanding the effect of particle size and processing on almond lipid bioaccessibility through microstructural analysis: From mastication to faecal collection. Nutrients 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, S.K.; Novotny, J.A.; Bornhorst, G.M.; Baer, D.J. Food processing and structure impact the metabolizable energy of almonds. Food Funct. 2016, 7, 4231–4238. [Google Scholar] [CrossRef] [Green Version]
- Cassady, B.A.; Hollis, J.H.; Fulford, A.D.; Considine, R.V.; Mattes, R.D. Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. Am. J. Clin. Nutr. 2009, 89, 794–800. [Google Scholar] [CrossRef] [Green Version]
- McArthur, B.M.; Mattes, R.D. Energy extraction from nuts: Walnuts, almonds and pistachios. Br. J. Nutr. 2020, 123, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Merrill, A.L.; Watt, B.K. Energy Value of Foods: Basis and Derivation. Agriculture Handbook No. 74, ARS United States Department of Agriculture, Washington DC. 1973. Available online: https://www.ars.usda.gov/arsuserfiles/80400535/data/classics/usda%20handbook%2074.pdf (accessed on 15 November 2022).
- Jaffa, M.E. Further Investigations among Fruitarians at the California Agricultural Experiment Station, 1901–1902; Government Printing Office: Washington, DC, USA, 1903. [Google Scholar]
- Sanchez-Pena, M.J.; Marquez-Sandoval, F.; Ramirez-Anguiano, A.C.; Velasco-Ramirez, S.F.; Macedo-Ojeda, G.; Gonzalez-Ortiz, L.J. Calculating the metabolizable energy of macronutrients: A critical review of Atwater’s results. Nutr. Rev. 2017, 75, 37–48. [Google Scholar] [CrossRef]
- Novotny, J.A.; Gebauer, S.K.; Baer, D.J. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am. J. Clin. Nutr. 2012, 96, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Measured energy value of pistachios in the human diet. Br. J. Nutr. 2012, 107, 120–125. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.J.; Gebauer, S.K.; Novotny, J.A. Walnuts Consumed by Healthy Adults Provide Less Available Energy than Predicted by the Atwater Factors. J. Nutr. 2016, 146, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.J.; Novotny, J.A. Metabolizable energy from cashew nuts is less than that predicted by Atwater factors. Nutrients 2018, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Adminstration. Qualified Health Claims: Letter of Enforcement Discretion—Nuts and Coronary Heart Disease (Docket No 02P-0505). Available online: http://wayback.archive-it.org/7993/20171114183724/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072926.htm (accessed on 15 November 2022).
- US Food and Drug Adminstration. Qualified Health Claims: Letter of Enforcement Discretion—Walnuts and Coronary Heart Disease (Docket No 02P-0292). Available online: http://wayback.archive-it.org/7993/20171114183725/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072910.htm (accessed on 15 November 2022).
- Blundell, J.E.; Gibbons, C.; Beaulieu, K.; Casanova, N.; Duarte, C.; Finlayson, G.; Stubbs, R.J.; Hopkins, M. The drive to eat in homo sapiens: Energy expenditure drives energy intake. Physiol. Behav. 2020, 219, 112846. [Google Scholar] [CrossRef]
- Blundell, J. Pharmacological approaches to appetite suppression. Trends Pharmacol. Sci. 1991, 12, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, C.; Hopkins, M.; Beaulieu, K.; Oustric, P.; Blundell, J.E. Issues in measuring and interpreting human appetite (satiety/satiation) and its contribution to obesity. Curr. Obes. Rep. 2019, 8, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecil, J.; Dalton, M.; Finlayson, G.; Blundell, J.; Hetherington, M.; Palmer, C. Obesity and eating behaviour in children and adolescents: Contribution of common gene polymorphisms. Int. Rev. Psychiatry 2012, 24, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Blundell, J.E.; Stubbs, R.J.; Golding, C.; Croden, F.; Alam, R.; Whybrow, S.; Le Noury, J.; Lawton, C.L. Resistance and susceptibility to weight gain: Individual variability in response to a high-fat diet. Physiol. Behav. 2005, 86, 614–622. [Google Scholar] [CrossRef]
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfor, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity—An update. Biomed Pharm. 2021, 140, 111789. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Priefer, R. Anti-obesity weight loss medications: Short-term and long-term use. Life Sci. 2022, 306, 120825. [Google Scholar] [CrossRef]
- Blundell, J.E.; Finlayson, G. Is susceptibility to weight gain characterized by homeostatic or hedonic risk factors for overconsumption? Physiol. Behav. 2004, 82, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Finlayson, G. Mechanisms and biomarkers of appetite control. Agro Food Ind. Hi-Tech 2008, 19, 18–20. [Google Scholar]
- Buckland, N.J.; Camidge, D.; Croden, F.; Lavin, J.H.; Stubbs, R.J.; Hetherington, M.M.; Blundell, J.E.; Finlayson, G. A low energy–dense diet in the context of a weight-management program affects appetite control in overweight and obese women. J. Nutr. 2018, 148, 798–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V. Ultra-processed diets cause excess calorie intake and weight gain: An inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019, 30, 67–77.e63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peciña, S.; Smith, K.S.; Berridge, K.C. Hedonic hot spots in the brain. Neurosci 2006, 12, 500–511. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef] [Green Version]
- Finlayson, G.; King, N.; Blundell, J.E. Liking vs. wanting food: Importance for human appetite control and weight regulation. Neurosci. Biobehav. Rev. 2007, 31, 987–1002. [Google Scholar] [CrossRef] [Green Version]
- Oustric, P.; Thivel, D.; Dalton, M.; Beaulieu, K.; Gibbons, C.; Hopkins, M.; Blundell, J.; Finlayson, G. Measuring food preference and reward: Application and cross-cultural adaptation of the Leeds Food Preference Questionnaire in human experimental research. Food Qual. Prefer. 2020, 80, 103824. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Münzberg, H.; Morrison, C.D. Blaming the brain for obesity: Integration of hedonic and homeostatic mechanisms. Gastroenterology 2017, 152, 1728–1738. [Google Scholar] [CrossRef] [Green Version]
- Schachter, S. Obesity and eating: Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science 1968, 161, 751–756. [Google Scholar] [CrossRef]
- Barkeling, B.; King, N.; Näslund, E.; Blundell, J. Characterization of obese individuals who claim to detect no relationship between their eating pattern and sensations of hunger or fullness. Int. J. Obes. 2007, 31, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Drapeau, V.; Hetherington, M.; Tremblay, A. Impact of eating and lifestyle behaviors on body weight: Beyond energy value. In Handbook of Behavior, Food and Nutrition; Springer: Berlin/Heidelberg, Germany, 2011; pp. 693–706. [Google Scholar]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drapeau, V.; Blundell, J.; Gallant, A.; Arguin, H.; Després, J.-P.; Lamarche, B.; Tremblay, A. Behavioural and metabolic characterisation of the low satiety phenotype. Appetite 2013, 70, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Dalton, M.; Hollingworth, S.; Blundell, J.; Finlayson, G. Weak satiety responsiveness is a reliable trait associated with hedonic risk factors for overeating among women. Nutrients 2015, 7, 7421–7436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckland, N.J.; Camidge, D.; Croden, F.; Myers, A.; Lavin, J.H.; Stubbs, R.J.; Blundell, J.E.; Finlayson, G. Women with a low-satiety phenotype show impaired appetite control and greater resistance to weight loss. Br. J. Nutr. 2019, 122, 951–959. [Google Scholar] [CrossRef]
- Arguin, H.; Tremblay, A.; Blundell, J.E.; Després, J.-P.; Richard, D.; Lamarche, B.; Drapeau, V. Impact of a non-restrictive satiating diet on anthropometrics, satiety responsiveness and eating behaviour traits in obese men displaying a high or a low satiety phenotype. Br. J. Nutr. 2017, 118, 750–760. [Google Scholar] [CrossRef] [Green Version]
- Drapeau, V.; Jacob, R.; Panahi, S.; Tremblay, A. Effect of energy restriction on eating behavior traits and psychobehavioral factors in the low satiety phenotype. Nutrients 2019, 11, 245. [Google Scholar] [CrossRef] [Green Version]
- Piernas, C.; Popkin, B.M. Snacking increased among US adults between 1977 and 2006. J. Nutr. 2010, 140, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Duffey, K.J.; Popkin, B.M. Energy density, portion size, and eating occasions: Contributions to increased energy intake in the United States, 1977–2006. PLoS Med. 2011, 8, e1001050. [Google Scholar] [CrossRef] [Green Version]
- Zizza, C.A.; Xu, B. Snacking is associated with overall diet quality among adults. J. Acad. Nutr. Diet. 2012, 112, 291–296. [Google Scholar]
- Leidy, H.J.; Campbell, W.W. The effect of eating frequency on appetite control and food intake: Brief synopsis of controlled feeding studies. J. Nutr. 2011, 141, 154–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, C.; Siegrist, M.; van der Horst, K. Snack frequency: Associations with healthy and unhealthy food choices. Public Health Nutr. 2013, 16, 1487–1496. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, M.; Ghobadi, S.; Zare, M.; Foshati, S. Effect of nuts on energy intake, hunger, and fullness, a systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Grundy, M.M.-L.; Grassby, T.; Parker, M.L.; Cross, K.L.; Chessa, S.; Bisignano, C.; Barreca, D.; Bellocco, E.; Lagana, G. The effects of processing and mastication on almond lipid bioaccessibility using novel methods of in vitro digestion modelling and micro-structural analysis. Br. J. Nutr. 2014, 112, 1521–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, M.J.; Slavin, J.L. The effect of fiber on satiety and food intake: A systematic review. J. Am. Coll. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef]
- Fromentin, G.; Darcel, N.; Chaumontet, C.; Marsset-Baglieri, A.; Nadkarni, N.; Tomé, D. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr. Res. Rev. 2012, 25, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Kendall, C.W.; Josse, A.R.; Salvatore, S.; Brighenti, F.; Augustin, L.S.; Ellis, P.R.; Vidgen, E.; Rao, A.V. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J. Nutr. 2006, 136, 2987–2992. [Google Scholar] [CrossRef] [Green Version]
- Josse, A.R.; Kendall, C.W.; Augustin, L.S.; Ellis, P.R.; Jenkins, D.J. Almonds and postprandial glycemia—A dose-response study. Metabolism 2007, 56, 400–404. [Google Scholar] [CrossRef]
- Mori, A.M.; Considine, R.V.; Mattes, R.D. Acute and second-meal effects of almond form in impaired glucose tolerant adults: A randomized crossover trial. Nutr. Metab. 2011, 8, 6. [Google Scholar]
- Tan, S.Y.; Mattes, R. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, S.; Re, R.; Chambers, L.; Echaniz, A.; Wickham, M.S. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur. J. Nutr. 2015, 54, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollingworth, S.; Dalton, M.; Blundell, J.E.; Finlayson, G. Evaluation of the influence of raw almonds on appetite control: Satiation, satiety, hedonics and consumer perceptions. Nutrients 2019, 11, 2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, G.E.; Bennett, H.W.; Jaceldo, K.B.; Sabaté, J. Effect on body weight of a free 76 kilojoule (320 calorie) daily supplement of almonds for six months. J. Am. Coll. Nutr. 2002, 21, 275–283. [Google Scholar] [CrossRef]
- Sabaté, J. Nut consumption and body weight. Am. J. Clin. Nutr. 2003, 78, 647S–650S. [Google Scholar] [CrossRef] [Green Version]
- Hollis, J.; Mattes, R. Effect of chronic consumption of almonds on body weight in healthy humans. Br. J. Nutr. 2007, 98, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Halford, J.C.; Harrold, J.A. Satiety-enhancing products for appetite control: Science and regulation of functional foods for weight management. Proc. Nutr. Soc. 2012, 71, 350–362. [Google Scholar] [CrossRef]
- Holt, S.H.; Brand Miller, J.C.; Petocz, P.; Farmakalidis, E. A satiety index of common foods. Eur. J. Clin. Nutr. 1995, 49, 675–690. [Google Scholar]
- Blundell, J.E.; MacDiarmid, J.I. Fat as a risk factor for overconsumption: Satiation, satiety, and patterns of eating. J. Am. Diet. Assoc. 1997, 97, S63–S69. [Google Scholar] [CrossRef]
- Rolls, B.J. Dietary energy density: Applying behavioural science to weight management. Nutr. Bull. 2017, 42, 246–253. [Google Scholar] [CrossRef]
- Buckland, N.J.; Stubbs, R.J.; Finlayson, G. Towards a satiety map of common foods: Associations between perceived satiety value of 100 foods and their objective and subjective attributes. Physiol. Behav. 2015, 152, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, S.L. Biopsychological Investigation of Satiety Responsiveness and Its Implications for Appetite Control; University of Leeds: Leeds, UK, 2020. [Google Scholar]
- Forde, C.G.; Almiron-Roig, E.; Brunstrom, J.M. Expected satiety: Application to weight management and understanding energy selection in humans. Curr. Obes. Rep. 2015, 4, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Hogenkamp, P.S.; Schiöth, H.B. Effect of oral processing behaviour on food intake and satiety. Trends Food Sci. Technol. 2013, 34, 67–75. [Google Scholar] [CrossRef]
- Dalton, M.; Buckland, N.; Blundell, J. Psychobiology of Obesity: Eating Behavior and Appetite Control. In Clinical Obesity in Adults and Children; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 99–112. [Google Scholar]
- Bes-Rastrollo, M.; Wedick, N.M.; Martinez-Gonzalez, M.A.; Li, T.Y.; Sampson, L.; Hu, F.B. Prospective study of nut consumption, long-term weight change, and obesity risk in women. Am. J. Clin. Nutr. 2009, 89, 1913–1919. [Google Scholar] [CrossRef] [Green Version]
- Mozzaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [Green Version]
- Bes-Rastrollo, M.; Sabate, J.; Gomez-Gracia, E.; Alonso, A.; Martinez, J.A.; Martinez-Gonzalez, M.A. Nut consumption and weight gain in a Mediterranean cohort: The SUN study. Obes. (Silver Spring Md.) 2007, 15, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.Y.; Steffen, L.M.; Zhou, X.; Shikany, J.M.; Jacobs, D.R., Jr. Association of nut consumption with CVD risk factors in young to middle-aged adults: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Nutr. Metab. Cardiovasc. Dis. NMCD 2022, 32, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Guasch-Ferre, M.; Willett, W.C.; Drouin-Chartier, J.P.; Bhupathiraju, S.N.; Tobias, D.K. Changes in nut consumption influence long-term weight change in US men and women. BMJ Nutr. Prev. Health 2019, 2, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishi, S.K.; Viguiliouk, E.; Blanco Mejia, S.; Kendall, C.W.C.; Bazinet, R.P.; Hanley, A.J.; Comelli, E.M.; Salas Salvado, J.; Jenkins, D.J.A.; Sievenpiper, J.L. Are fatty nuts a weighty concern? A systematic review and meta-analysis and dose-response meta-regression of prospective cohorts and randomized controlled trials. Obes. Rev. 2021, 22, e13330. [Google Scholar] [CrossRef]
- Wien, M.A.; Sabate, J.M.; Ikle, D.N.; Cole, S.E.; Kandeel, F.R. Almonds vs complex carbohydrates in a weight reduction program. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, 1365–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Rodriguez, R.; Mesas, A.E.; Garrido-Miguel, M.; Martinez-Ortega, I.A.; Jimenez-Lopez, E.; Martinez-Vizcaino, V. The Relationship of Tree Nuts and Peanuts with Adiposity Parameters: A Systematic Review and Network Meta-Analysis. Nutrients 2021, 13, 2251. [Google Scholar] [CrossRef] [PubMed]
- Lee-Bravatti, M.A.; Wang, J.; Avendano, E.E.; King, L.; Johnson, E.J.; Raman, G. Almond Consumption and Risk Factors for Cardiovascular Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Adv. Nutr. 2019, 10, 1076–1088. [Google Scholar] [CrossRef]
- Guarneiri, L.L.; Cooper, J.A. Intake of Nuts or Nut Products Does Not Lead to Weight Gain, Independent of Dietary Substitution Instructions: A Systematic Review and Meta-Analysis of Randomized Trials. Adv. Nutr. 2021, 12, 384–401. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Fito, M.; Chiva-Blanch, G.; Fiol, M.; Gomez-Gracia, E.; Aros, F.; Lapetra, J.; et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet. Diabetes Endocrinol. 2019, 7, e6–e17. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Obesity Epidemiology; Oxford University Press: Oxford, NY, USA, 2008; p. xiii. [Google Scholar]
- Jackson, C.L.; Hu, F.B. Long-term associations of nut consumption with body weight and obesity. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 408S–411S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alasalvar, C.; Salas-Salvadó, J.; Ros, E.; Sabaté, J. Health Benefits of Nuts and Dried Fruits; CRC Press: Boca Raton, FL, USA, 2020; p. xxi. [Google Scholar]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baer, D.J.; Dalton, M.; Blundell, J.; Finlayson, G.; Hu, F.B. Nuts, Energy Balance and Body Weight. Nutrients 2023, 15, 1162. https://doi.org/10.3390/nu15051162
Baer DJ, Dalton M, Blundell J, Finlayson G, Hu FB. Nuts, Energy Balance and Body Weight. Nutrients. 2023; 15(5):1162. https://doi.org/10.3390/nu15051162
Chicago/Turabian StyleBaer, David J., Michelle Dalton, John Blundell, Graham Finlayson, and Frank B. Hu. 2023. "Nuts, Energy Balance and Body Weight" Nutrients 15, no. 5: 1162. https://doi.org/10.3390/nu15051162




