The Effect of a Vegan Diet on the Coverage of the Recommended Dietary Allowance (RDA) for Iodine among People from Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. The Survey Questionnaire
2.2. The Group of Respondents
2.3. Statistical Analysis
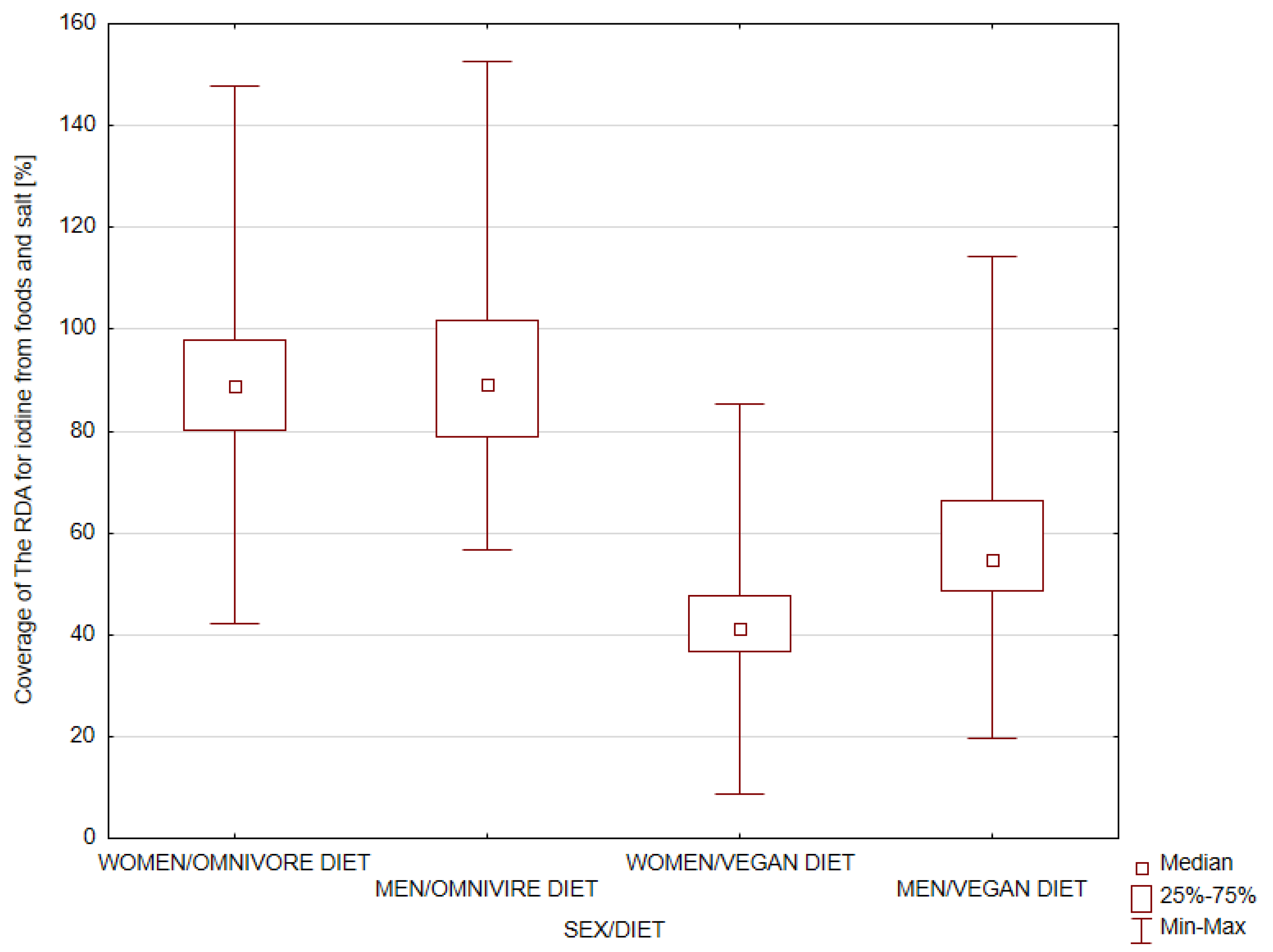
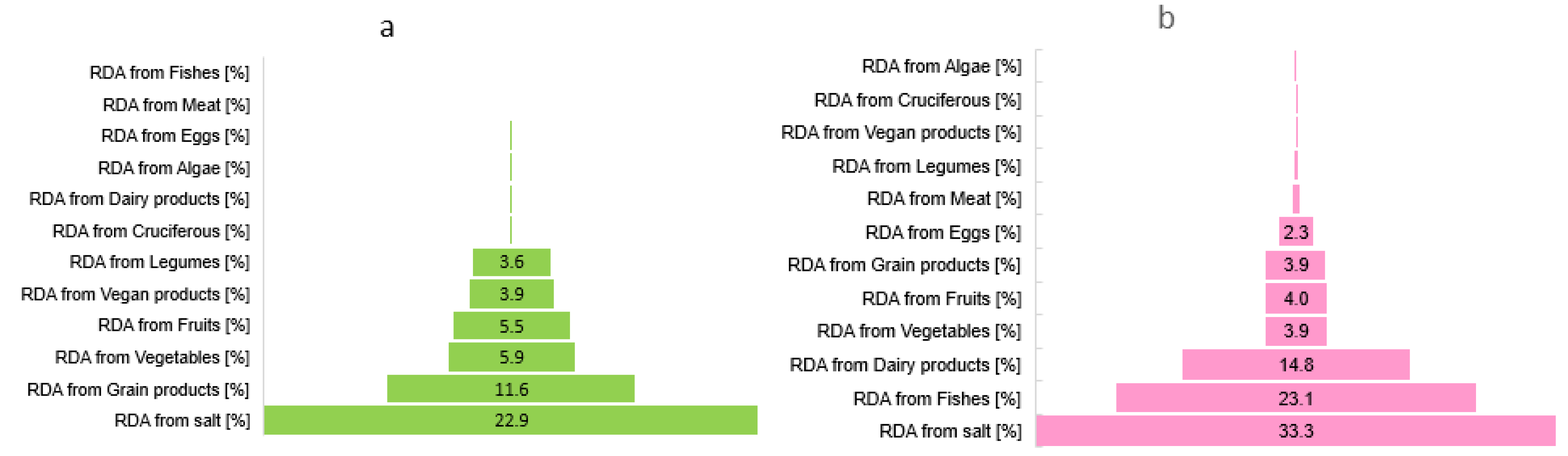
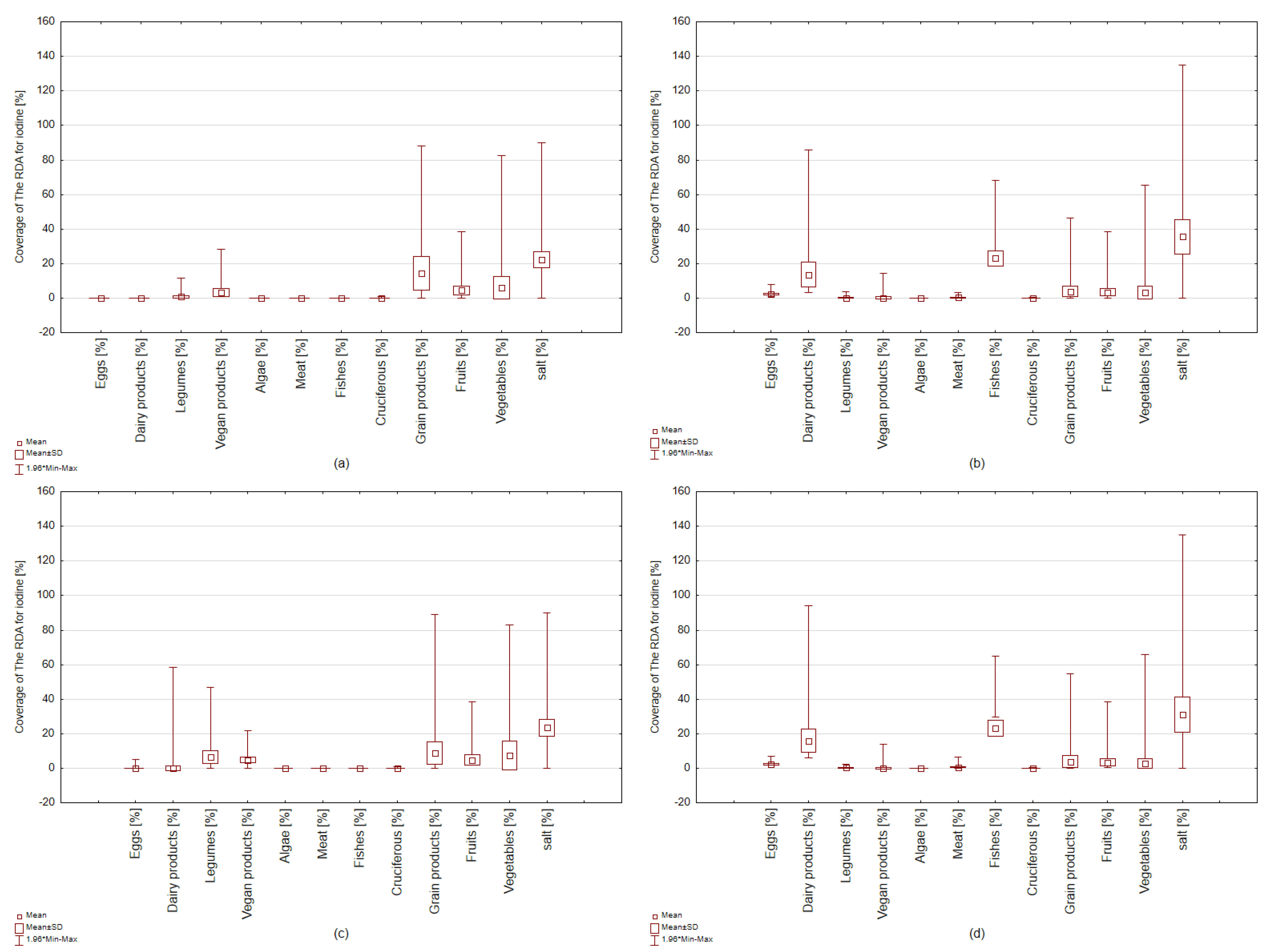
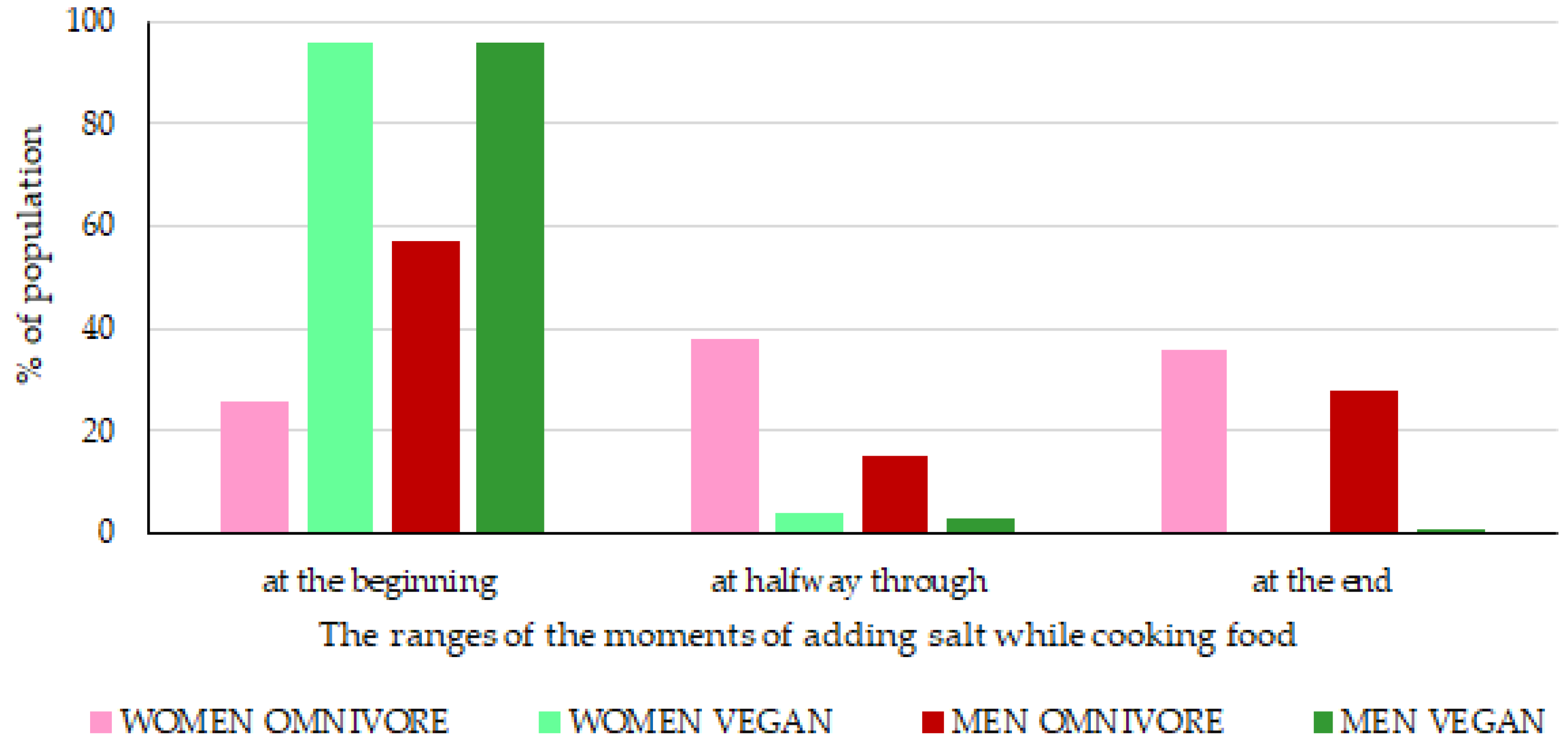
3. Results
3.1. Iodine Intake
3.2. Iodine Sources
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tso, R.; Forde, C.G. Unintended Consequences: Nutritional Impact and Potential Pitfalls of Switching from Animal to Plant-based Foods. Nutrients 2021, 13, 2527. [Google Scholar] [CrossRef]
- Mandel, A. Record Interest in Veganism. Available online: https://www.rp.pl/przemysl-spozywczy/art523671-rekordowe-zainteresowanie-weganizmem (accessed on 16 September 2020).
- Selinger, E.; Neuenschwander, M.; Koller, A.; Gojda, J.; Kühn, T.; Schwingshackl, L.; Barbaresko, J.; Schlesinger, S. Evidence of a Vegan Diet for Health Benefits and Risks—An Umbrella Review of Meta-Analyses of Observational and Clinical Studies. Crit. Rev. Food Sci. Nutr. 2022, 1–11. [Google Scholar] [CrossRef]
- Gvion, L. Generation V: Millennial Vegans in Israel. J. Contemp. Ethnogr. 2020, 49, 564–586. [Google Scholar] [CrossRef]
- Mazurkiewicz, P. Poles Turn Their Backs on Meat. More and More Plant Substitutes on the Shelves. Available online: https://www.rp.pl/przemysl-spozywczy/art36804141-polacy-odwracaja-sie-od-miesa-na-polkach-coraz-wiecej-roslinnych-zamiennikow (accessed on 2 August 2022).
- Janssen, M.; Busch, C.; Rödiger, M.; Hamm, U. Motives of Consumers Following a Vegan Diet and Their Attitudes towards Animal Agriculture. Appetite 2016, 105, 643–651. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional Status and the Influence of the Vegan Dieton the Gut Microbiota and Human Health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Tetens, I.; Bügel, S.G.; Felby, C.; Schacht, S.R.; Hill, J.O.; Ravussin, E.; Astrup, A. Perspective: A Perspective on the Transition to Plant-Based Diets: A Diet Change May Attenuate Climate Change, but Can It Also Attenuate Obesityand Chronic Disease Risk? Adv. Nutr. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J. Health Effects of Vegan Diets. Am. J. Clin. Nutr. 2009, 89, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, K. Adherence to a Vegetarian Diet and Diabetes Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 603. [Google Scholar] [CrossRef]
- Krela-Kaźmierczak, I.; Czarnywojtek, A.; Skoracka, K.; Rychter, A.M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Ruchała, M.; Dobrowolska, A. Is There an Ideal Diet to Protect against Iodine Deficiency? Nutrients 2021, 13, 513. [Google Scholar] [CrossRef]
- Medawar, E.; Huhn, S.; Villringer, A.; Veronica Witte, A. The Effects of Plant-Based Diets on the Body and the Brain: A Systematic Review. Transl. Psychiatry 2019, 9, 226. [Google Scholar] [CrossRef]
- Villette, C.; Vasseur, P.; Lapidus, N.; Debin, M.; Hanslik, T.; Blanchon, T.; Steichen, O.; Rossignol, L. Vegetarian and Vegan Diets: Beliefs and Attitudes of General Practitioners and Pediatricians in France. Nutrients 2022, 14, 3101. [Google Scholar] [CrossRef]
- Ahad, F.; Ganie, S.A. Iodine, Iodine Metabolism and Iodine Deficiency Disorders Revisited. Indian J. Endocrinol. Metab. 2010, 14, 13–17. [Google Scholar]
- Zimmermann, M.B. The Role of Iodine in Human Growth and Development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Brodowski, J.; Pokorska-Niewiada, K.; Szczuko, M. The Change in the Content of Nutrients in Diets Eliminating Products of Animal Origin in Comparison to a Regular Diet from the Area of Middle-Eastern Europe. Nutrients 2020, 12, 2986. [Google Scholar] [CrossRef] [PubMed]
- Winder, M.; Kosztyła, Z.; Boral, A.; Kocełak, P.; Chudek, J. The Impact of Iodine Concentration Disorders on Health and Cancer. Nutrients 2022, 14, 2209. [Google Scholar] [CrossRef] [PubMed]
- WHO Radiation Emergency: Iodine Thyroid Blocking. Available online: https://cdn.who.int/media/docs/default-source/radiation/1_ki_final.pdf?sfvrsn=d7d94d88_3 (accessed on 20 December 2022).
- WHO. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2007; ISBN 978924 1595827.
- Zava, T.T.; Zava, D.T. Assessment of Japanese Iodine Intake Based on Seaweed Consumption in Japan: A Literature-Based Analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, S.; Mikeda, T.; Okada, T.; Nakamura, K.; Kotani, T.; Hishinuma, A. Transient Hypothyroidism or Persistent Hyperthyrotropinemia in Neonates Born to Mothers with Excessive Iodine Intake. Thyroid 2004, 14, 1077–1083. [Google Scholar] [CrossRef]
- Arasaki, S.; Arasaki, T. Vegetables from the Sea; Japan Publications Inc.: Tokyo, Japan, 1983. [Google Scholar]
- Andersson, M.; Takkouche, B.; Egli, I.; Allen, H.E.; De Benoist, B. Current Global Iodine Status and Progress over the Last Decade towards the Elimination of Iodine Deficiency. Bull. World Health Organ. 2005, 83, 518–525. [Google Scholar] [PubMed]
- Hatch-McChesney, A.; Lieberman, H.R. Iodine and Iodine Deficiency: A Comprehensive Review of a Re-Emerging Issue. Nutrients 2022, 14, 3474. [Google Scholar] [CrossRef] [PubMed]
- Szymandera-Buszka, K.; Waszkowiak, K.; Woźniak, P. The Estimation of Consumption of Food Products Being Iodine Sources among Women of the Wielkopolska Region. Probl. Hig. Epidemiol. 2011, 92, 73–76. [Google Scholar]
- Yeliosof, O.; Silverman, L.A. Veganism as a Cause of Iodine Deficient Hypothyroidism. J. Pediatr. Endocrinol. Metab. 2018, 31, 91–94. [Google Scholar] [CrossRef]
- Blikra, M.J.; Henjum, S.; Aakre, I. Iodine from Brown Algae in Human Nutrition, with an Emphasis on Bioaccessibility, Bioavailability, Chemistry, and Effects of Processing: A Systematic Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1517–1536. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative proteins and EU food law. Food Control 2021, 130, 108336. [Google Scholar] [CrossRef]
- Szybiński, Z. Iodine prophylaxis in Poland in the lights of the WHO recommendation on reduction of the daily salt intake. Pediatr. Endocrinol. Diabetol. Metab. 2009, 15, 103–107. [Google Scholar]
- WHO. Universal Salt Iodization and Sodium Intake Reduction Compatible, Cost–Effective Strategies of Great Public Health Benefit; World Health Organization: Geneva, Switzerland, 2022. Available online: https://www.who.int/publications/i/item/9789240053717 (accessed on 22 August 2022).
- Jezewska-Zychowicz, M.; Gawecki, J.; Wadolowska, L.; Czarnocinska, J.; Galinski, G.; Kollajtis-Dolowy, A.; Roszkowski, W.; Wawrzyniak, A.; Przybylowicz, K.; Krusinska, B.; et al. Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing of Nutritional Data; The Committee of Human Nutrition, Polish Academy of Sciences. 2017. Available online: http://www.knozc.pan.pl/images/stories/MLonnie/EN_Kwestionariusz_KomPAN_i_PROCEDURA_versja_2_znak_tow_2019_2.pdf (accessed on 10 June 2019).
- Kurosad, A.; Nicpoń, J.; Kubiak, K.; Jankowski, M.; Kungl, K. Iodine Occurrence, Circulation, Deficiency Region and the Main Iodine Sources in Human and Animal Nutrition. Adv. Clin. Exp. Med. 2005, 14, 1019–1025. [Google Scholar]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tables of Food Composition and Nutritional Value; PZWL: Warszawa, Poland, 2020. [Google Scholar]
- Smyth, P.P.A. Iodine, Seaweed, and the Thyroid. Eur. Thyroid J. 2021, 10, 101–108. [Google Scholar] [CrossRef]
- Truong, T.; Baron-Dubourdieu, D.; Rougier, Y.; Guénel, P. Role of dietary iodine and cruciferous vegetables in thyroid cancer: A countrywide case-control study in New Caledonia. Cancer Causes Control 2010, 21, 1183–1192. [Google Scholar] [CrossRef]
- Szymandera-Buszka, K.; Waszkowiak, K.; Kaczmarek, A.; Zaremba, A. Wheat Dietary Fibre and Soy Protein as New Carriers of Iodine Compounds for Food Fortification—The Effect of Storage Conditions on the Stability of Potassium Iodide and Potassium Iodate. LWT—Food Sci. Technol. 2021, 137, 110424. [Google Scholar] [CrossRef]
- Li, M.; Eastman, C.J. The Changing Epidemiology of Iodine Deficiency. Nat. Rev. Endocrinol. 2012, 8, 434–440. [Google Scholar] [CrossRef]
- Szymandera-Buszka, K.; Waszkowiak, K. Iodine Retention in Ground Pork Burgers Fried in Fat Free Conditions. Acta Sci. Pol. Technol. Aliment. 2004, 3, 157–162. [Google Scholar]
- Waszkowiak, K.; Szymandera-Buszka, K. Effect of Collagen Preparations Used as Carriers of Potassium Iodide on Retention of Iodine and Thiamine during Cooking and Storage of Pork Meatballs. J. Sci. Food Agric. 2007, 87, 1473–1479. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Szymandera-Buszka, K. Effect of Storage Conditions on Potassium Iodide Stability in Iodised Table Salt and Collagen Preparations. Int. J. Food Sci. Technol. 2008, 43, 895–899. [Google Scholar] [CrossRef]
- Rana, R.; Raghuvanshi, R.S. Effect of Different Cooking Methods on Iodine Losses. J. Food Sci. Technol. 2013, 50, 1212–1216. [Google Scholar] [CrossRef]
- Eveleigh, E.; Coneyworth, L.; Zhou, M.; Burdett, H.; Malla, J.; Nguyen, V.H.; Welham, S. Vegans and Vegetarians Living in Nottingham (UK) Continue to Be at Risk of Iodine Deficiency. Br. J. Nutr. 2022, 1–46. [Google Scholar] [CrossRef]
- Nyström, H.F.; Brantsæter, A.L.; Erlund, I.; Gunnarsdottir, I.; Hulthén, L.; Laurberg, P.; Mattisson, I.; Rasmussen, L.B.; Virtanen, S.; Meltzer, H.M. Iodine Status in the Nordic Countries Past and Present. Food Nutr. Res. 2016, 60, 31969. [Google Scholar] [CrossRef] [PubMed]
- Granfors, M.; Andersson, M.; Stinca, S.; Åkerud, H.; Skalkidou, A.; SundströmPoromaa, I.; Wikström, A.K.; FilipssonNyström, H. Iodine Deficiency in a Study Population of Pregnant Women in Sweden. Acta Obstet. Gynecol. Scand. 2015, 94, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Lindorfer, H.; Krebs, M.; Kautzky-Willer, A.; Bancher-Todesca, D.; Sager, M.; Gessl, A. Iodine Deficiency in Pregnant Women in Austria. Eur. J. Clin. Nutr. 2015, 69, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, Y.S.; Arbelle, J.E.; Gefel, D.; Brik, H.; Wolf, T.; Nadler, V.; Hunziker, S.; Zimmermann, M.B.; Troen, A.M. First Israeli National Iodine Survey Demonstrates Iodine Deficiency among School-Aged Children and Pregnant Women. Thyroid 2017, 27, 1083–1091. [Google Scholar] [CrossRef]
- Groufh-Jacobsen, S.; Hess, S.Y.; Aakre, I.; Gjengedal, E.L.F.; Pettersen, K.B.; Henjum, S. Vegans, Vegetarians and Pescatarians Are at Risk of Iodine Deficiency in Norway. Nutrients 2020, 12, 3555. [Google Scholar] [CrossRef]
- Krajcovicová-Kudlácková, M.; Bucková, K.; Klimes, I.; Seboková, E. Iodine Deficiency in Vegetarians and Vegans. Ann. Nutr. Metab. 2003, 47, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Dahl, L.; Johansson, L.; Julshamn, K.; Meltzer, H.M. The Iodine Content of Norwegian Foods and Diets. Public Health Nutr. 2004, 7, 569–576. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Abel, M.H.; Haugen, M.; Meltzer, H.M. Risk of Suboptimal Iodine Intake in Pregnant Norwegian Women. Nutrients 2013, 5, 424–440. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Knutsen, H.K.; Johansen, N.C.; Nyheim, K.A.; Erlund, I.; Meltzer, H.M.; Henjum, S. Inadequate Iodine Intake in Population Groups Defined by Age, Life Stage and Vegetarian Dietary Practice in a Norwegian Convenience Sample. Nutrients 2018, 10, 230. [Google Scholar] [CrossRef]
- Henjum, S.; Aakre, I.; Lilleengen, A.M.; Garnweidner-holme, L.; Borthne, S.; Id, Z.P.; Blix, E.; Lovise, E.; Gjengedal, F.; Lise, A.; et al. Suboptimal Iodine Status among Pregnant Women in the Oslo Area, Norway. Nutrients 2018, 10, 280. [Google Scholar] [CrossRef]
- Manousou, S.; Dahl, L.; Heinsbaek Thuesen, B.; Hulthén, L.; NyströmFilipsson, H. Iodine Deficiency and Nutrition in Scandinavia. Minerva Med. 2017, 108, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Henjum, S.; Lilleengen, A.M.; Aakre, I.; Dudareva, A.; Gjengedal, E.L.F.; Meltzer, H.M.; Brantsæter, A.L. Suboptimal Iodine Concentration in Breastmilk and Inadequate Iodine Intake among Lactating Women in Norway. Nutriens 2017, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Andersen, L.F.; Dahl, L.; Norberg, N.; Hjartåker, A. New Iodine Food Composition Database and Updated Calculations of Iodine Intake among Norwegians. Nutrients 2018, 10, 930. [Google Scholar] [CrossRef]
- Witard, O.C.; Bath, S.C.; Dineva, M.; Sellem, L.; Mulet-Cabero, A.I.; van Dongen, L.H.; Zheng, J.S.; Valenzuela, C.; Smeuninx, B. Dairy as a Source of Iodine and Protein in the UK: Implications for Human Health Across the Life Course, and Future Policy and Research. Front Nutr. 2022, 9, 64. [Google Scholar] [CrossRef]
- WHO. Scientific Update on the Iodine Content of Portuguese Foods; World Health Organization: Geneva, Switzerland, 2018. Available online: https://www.euro.who.int/__data/assets/pdf_file/0009/392877/iodine-portugal.pdf (accessed on 4 April 2022).
- Sakai, N.; Esho, O.; Mukai, M. Iodine Concentrations in Conventional and Organic Milk in the Northeastern US. Dairy 2022, 3, 211–219. [Google Scholar] [CrossRef]
- Brzóska, F.; Szybiński, Z.; Śiwiński, B. Iodine in consumer milk in Poland and its role in disease prevention in humans. Wiadomości Zootech. 2015, 53, 41–49. [Google Scholar]
- Mikláš, Š.; Tancin, V.; Toman, R.; Trávnícek, J. Iodine Concentration in Milk and Human Nutrition: A Review. Czech J. Anim. Sci. 2021, 66, 189–199. [Google Scholar] [CrossRef]
- van der Reijden, O.L.; Zimmermann, M.B.; Galetti, V. Iodine in Dairy Milk: Sources, Concentrations and Importance to Human Health. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, Y.S.; Gefel, D.; Weizmann, N.; Raizman, M.; Goldsmith, R.; Mabjeesh, S.J.; Dahl, L.; Troen, A.M. Low Iodine Intake from Dairy Foods Despite High Milk Iodine Content in Israel. Thyroid 2018, 28, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Kardas, M.; Grochowska-Niedworok, E.; Calyniuk, B.; Kolasa, I.; Grajek, M.; Bielaszka, A.; Kiciak, A.; Muc-Wierzgoń, M. Consumption of Milk and Milk Products in the Population of the Upper Silesian Agglomeration Inhabitants. Food Nutr. Res. 2016, 60, 28976. [Google Scholar] [CrossRef] [PubMed]
- Totland, T.H.; Melnæs, B.K.; Lundberg-Hallen, N.; Helland-Kigen, M.K.; Lund-Blix, N.A.; Myhre, J.B.; Johansen, A.M.W.; Løken, E.B.; Andersen, L. Norkost 3. En Landsomfattende Kostholdsundersøkelse Blant Menn Og Kvinner i Norge i Alderen 18–70 År, 2010–2011. Available online: https://www.helsedirektoratet.no/rapporter/norkost-3-en-landsomfattende-kostholdsundersokelse-blant-menn-og-kvinner-i-norge-i-alderen-18-70-ar-2010-11/Norkost%203%20en%20landsomfattende%20kostholdsundersokelse%20blant%20menn%20og%20kvinner%20i%20Norge%20i%20alderen-18-70%20%C3%A5r%202010-11.pdf/_/attachment/inline/b7bafaab-6059-4450-8d76-c3ed9f3eaf3f:be251cd1153cf1ae8e4c46eedddc13b36da3d11d/Norkost%203%20en%20landsomfattende%20kostholdsundersokelse%20blant%20menn%20og%20kvinner%20i%20Norge%20i%20alderen-18-70%20%C3%A5r%202010-11.pdf; (accessed on 15 December 2022).
- Ozdogan, Y.; Yardimci, H.; Ozcelik, A.O. Young Adults’ Milk Consumption Habits and Knowledge about Milk. Stud. Ethno-Med. 2017, 11, 106–113. [Google Scholar] [CrossRef]
- Sritharan, A. Do People in Europe Still Want Dairy? Available online: https://www.newfoodmagazine.com/news/164605/do-people-in-europe-still-want-dairy/ (accessed on 5 May 2022).
- Kuchler, F.; Stewart, H. Fluid Milk Consumption Continues Downward Trend, Proving Difficult to Reverse. Available online: https://www.ers.usda.gov/amber-waves/2022/june/fluid-milk-consumption-continues-downward-trend-proving-difficult-to-reverse/ (accessed on 21 June 2022).
- Dineva, M.; Rayman, M.P.; Bath, S.C. Iodine Status of Consumers of Milk-Alternative Drinks v. Cows’ Milk: Data from the UK National Diet and Nutrition Survey. Br. J. Nutr. 2021, 126, 28–36. [Google Scholar] [CrossRef]
- Bath, S.C.; Hill, S.; Infante, H.G.; Elghul, S.; Nezianya, C.J.; Rayman, M.P. Iodine Concentration of Milk-Alternative Drinks Available in the UK in Comparison with Cows’ Milk. Br. J. Nutr. 2017, 118, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-Based Milk Substitutes: Bioactive Compounds, Conventional and Novel Processes, Bioavailability Studies, and Health Effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Nerhus, I.; Markhus, M.W.; Nilsen, B.M.; Øyen, J.; Maage, A.; Ødegård, E.R.; Midtbø, L.K.; Frantzen, S.; Kögel, T.; Graff, I.E.; et al. Iodine Content of Six Fish Species, Norwegian Dairy Products and Hen’s Egg. Food Nutr. Res. 2018, 62, 1–13. [Google Scholar] [CrossRef]
- Szymandera-Buszka, K.; Jędrusek-Golińska, A. Estimation of fish consumption as iodine source. Bromatol. Chem. Toksykol. 2008, 41, 319–322. [Google Scholar]
- Sprague, M.; Chau, T.C.; Givens, D.I. Iodine Content of Wild and Farmed Seafood and Its Estimated Contribution to UK Dietary Iodine Intake. Nutrients 2021, 14, 195. [Google Scholar] [CrossRef]
- Goldberg, G.R.; Black, A.E. Assessment of the Validity of Reported Energy Intakes—Review and Recent Developments. Scand. J. Nutr. 1998, 42, 6–9. [Google Scholar] [CrossRef]
- Beverley, B.; David, C.; Kerry, S.J.; Polly, P.; Caireen, R.; Toni, S.; Gillian, S. National Diet and Nutrition Survey Roling Programme Years 9 to 11 (2016/2017 to 2018/2019). 2020. Available online: https://doi.org/10.17863/CAM.81787 (accessed on 15 December 2022). [CrossRef]
- Allès, B.; Baudry, J.; Méjean, C.; Touvier, M.; Péneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of Sociodemographic and Nutritional Characteristics between Self-Reported Vegetarians, Vegans, and Meat-Eaters from the Nutrinet-Santé Study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and Adequacy of the Vegan Diet. A Systematic Review of the Evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Remer, T.; Neubert, A.; Manz, F. Increased Risk of Iodine Deficiency with Vegetarian Nutrition. Br. J. Nutr. 1999, 81, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.; Borak, J. Iodine Deficiency in Vegetarian and Vegan Diets: Evidence-Based Review of the World’s Literature on Iodine Content in Vegetarian Diets. In Comprehensive Handbook of Iodine. Nutritional, Biochemical, Pathological and Therapeutic Aspects; Preedy, V.R., Burrow, G.N., Watson, R., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2009; pp. 521–531. [Google Scholar]
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef]
- Iodine, D.J. Modern Nutrition in Health and Disease; Lippincott Williams &Wilkins: New York, NY, USA, 2006. [Google Scholar]
- Mente, A.; Dagenais, G.; Wielgosz, A.; Lear, S.A.; McQueen, M.J.; Zeidler, J.; Fu, L.; DeJesus, J.; Rangarajan, S.; Bourlaud, A.-S.; et al. Assessment of Dietary Sodium and Potassium in Canadians Using 24-Hour Urinary Collection. Can. J. Cardiol. 2016, 32, 319–326. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Brown, M.; Tan, M.; MacGregor, G.A. Reducing population salt intake—An update on latest evidence and global action. J. Clin. Hypertens. 2019, 21, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.M.; LaMar, A.; He, X.; Braverman, L.E.; Pearce, E.N. Iodine Status and Thyroid Function of Boston-Area Vegetarians and Vegans. J. Clin. Endocrinol. Metab. 2011, 96, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Fallon, N.; Dillon, S.A. Low Intakes of Iodine and Selenium Amongst Vegan and Vegetarian Women Highlight a Potential Nutritional Vulnerability. Front. Nutr. 2020, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Minh Duc, N.T.; Luu Lam Thang, T.; Nam, N.H.; Ng, S.J.; Abbas, K.S.; Huy, N.T.; Marušić, A.; Paul, C.L.; Kwok, J.; et al. A Consensus-Based Checklist for Reporting of Survey Studies (CROSS). J. Gen. Intern. Med. 2021, 36, 3179–3187. [Google Scholar] [CrossRef] [PubMed]

| Diet | Sex | Educational Level | Age | Distribution | Diet | Sex | Educational Level | Age | Distribution |
|---|---|---|---|---|---|---|---|---|---|
| Omnivorous | Women n = 541 p < 0.05 | Vocational n = 151 | 18–30 | 21 | Vegan | Women n = 564 p < 0.05 | Vocational n = 160 | 18–30 | 20 |
| 31–40 | 25 | 31–40 | 25 | ||||||
| 41–50 | 27 | 41–50 | 27 | ||||||
| 51–60 | 23 | 51–60 | 29 | ||||||
| 61–70 | 24 | 61–70 | 27 | ||||||
| 71–80 | 31 | 71–80 | 32 | ||||||
| Secondary n= 150 | 18–30 | 25 | Secondary n = 171 | 18–30 | 27 | ||||
| 31–40 | 25 | 31–40 | 26 | ||||||
| 41–50 | 24 | 41–50 | 28 | ||||||
| 51–60 | 20 | 51–60 | 23 | ||||||
| 61–70 | 31 | 61–70 | 40 | ||||||
| 71–80 | 25 | 71–80 | 27 | ||||||
| Higher n = 240 | 18–30 | 31 | Higher n = 233 | 18–30 | 32 | ||||
| 31–40 | 29 | 31–40 | 29 | ||||||
| 41–50 | 34 | 41–50 | 33 | ||||||
| 51–60 | 42 | 51–60 | 40 | ||||||
| 61–70 | 50 | 61–70 | 49 | ||||||
| 71–80 | 54 | 71–80 | 50 | ||||||
| Men n = 531 p < 0.05 | Vocational n = 175 | 18–30 | 34 | Men n = 564 p < 0.05 | Vocational n = 182 | 18–30 | 33 | ||
| 31–40 | 29 | 31–40 | 32 | ||||||
| 41–50 | 12 | 41–50 | 11 | ||||||
| 51–60 | 52 | 51–60 | 35 | ||||||
| 61–70 | 13 | 61–70 | 31 | ||||||
| 71–80 | 35 | 71–80 | 40 | ||||||
| Secondary n = 135 | 18–30 | 18 | Secondary n = 161 | 18–30 | 20 | ||||
| 31–40 | 27 | 31–40 | 31 | ||||||
| 41–50 | 9 | 41–50 | 16 | ||||||
| 51–60 | 35 | 51–60 | 28 | ||||||
| 61–70 | 14 | 61–70 | 30 | ||||||
| 71–80 | 32 | 71–80 | 36 | ||||||
| Higher n = 221 | 18–30 | 29 | Higher n = 221 | 18–30 | 23 | ||||
| 31–40 | 35 | 31–40 | 38 | ||||||
| 41–50 | 28 | 41–50 | 23 | ||||||
| 51–60 | 61 | 51–60 | 35 | ||||||
| 61–70 | 24 | 61–70 | 52 | ||||||
| 71–80 | 44 | 71–80 | 50 |
| Predictors | SS | df | MSE | F-Value | p-Value | Post Hoc Power α = 0.05 |
|---|---|---|---|---|---|---|
| Diet | 8.448 × 105 | 1 | 8.448 × 105 | 3.550 × 103 | <0.05 | 1.000 |
| Sex | 3.832 × 104 | 1 | 3.832 × 104 | 6.335 × 101 | <0.05 | 1.000 |
| Age | 2.635 × 104 | 5 | 5.269 × 103 | 8.618 × 100 | <0.05 | 0.999 |
| Education | 3.457 × 103 | 2 | 1.729 × 103 | 2.783 × 100 | 0.063 | 0.549 |
| Type of Group | Test Value | The Degrees of Freedom (df) | p-Value |
|---|---|---|---|
| Diet | 1.478 × 103 | 5 | <0.05 |
| Sex | 2.267 × 101 | 5 | <0.05 |
| Age | 1.164 × 101 | 25 | <0.05 |
| Education | 2.451 × 10−2 | 10 | 0.094 |
| Level | Odd Ratio (OR) | 95% CI |
|---|---|---|
| TOTAL GROUP | ||
| <20 | 9.848 × 105 | 13.801–31.622 |
| 21–40 | 2.887 × 107 | 0.444–17.171 |
| 41–60 | 6.597 × 101 | 0.348–4.189 |
| 61–80 | 6.656 × 10−1 | −0.407–0.642 |
| 81–100 | 4.283 × 10−2 | −3.150–1.991 |
| >100 | 1.970 × 10−2 | 0.000–0.015 |
| WOMEN | ||
| <20 | 1.805 × 106 | 14.412–31.623 |
| 21–40 | 7.195 × 107 | 0.444–11.809 |
| 41–60 | 3.457 × 101 | 0.348–3.543 |
| 61–80 | 3.042 × 10−1 | −1.190–0.642 |
| 81–100 | 4.459 × 10−3 | −5.413–1.991 |
| >100 | 0.000 × 100 | −17.082–1.471 |
| MEN | ||
| <20 | 1.776 × 105 | 12.091–31.623 |
| 21–40 | 2.920 × 106 | 0.444–14.887 |
| 41–60 | 1.633 × 102 | 0.348–5.095 |
| 61–80 | 1.039 × 100 | 0.038–0.642 |
| 81–100 | 0.101 × 10−1 | −2.294–1.990 |
| >100 | 3.314 × 10−2 | −3.407–1.471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaremba, A.; Gramza-Michalowska, A.; Pal, K.; Szymandera-Buszka, K. The Effect of a Vegan Diet on the Coverage of the Recommended Dietary Allowance (RDA) for Iodine among People from Poland. Nutrients 2023, 15, 1163. https://doi.org/10.3390/nu15051163
Zaremba A, Gramza-Michalowska A, Pal K, Szymandera-Buszka K. The Effect of a Vegan Diet on the Coverage of the Recommended Dietary Allowance (RDA) for Iodine among People from Poland. Nutrients. 2023; 15(5):1163. https://doi.org/10.3390/nu15051163
Chicago/Turabian StyleZaremba, Agata, Anna Gramza-Michalowska, Kunal Pal, and Krystyna Szymandera-Buszka. 2023. "The Effect of a Vegan Diet on the Coverage of the Recommended Dietary Allowance (RDA) for Iodine among People from Poland" Nutrients 15, no. 5: 1163. https://doi.org/10.3390/nu15051163
APA StyleZaremba, A., Gramza-Michalowska, A., Pal, K., & Szymandera-Buszka, K. (2023). The Effect of a Vegan Diet on the Coverage of the Recommended Dietary Allowance (RDA) for Iodine among People from Poland. Nutrients, 15(5), 1163. https://doi.org/10.3390/nu15051163








