Mediterranean Diet and Physical Activity Nudges versus Usual Care in Women with Rheumatoid Arthritis: Results from the MADEIRA Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol
2.2. Ethical Clearance
2.3. Participants
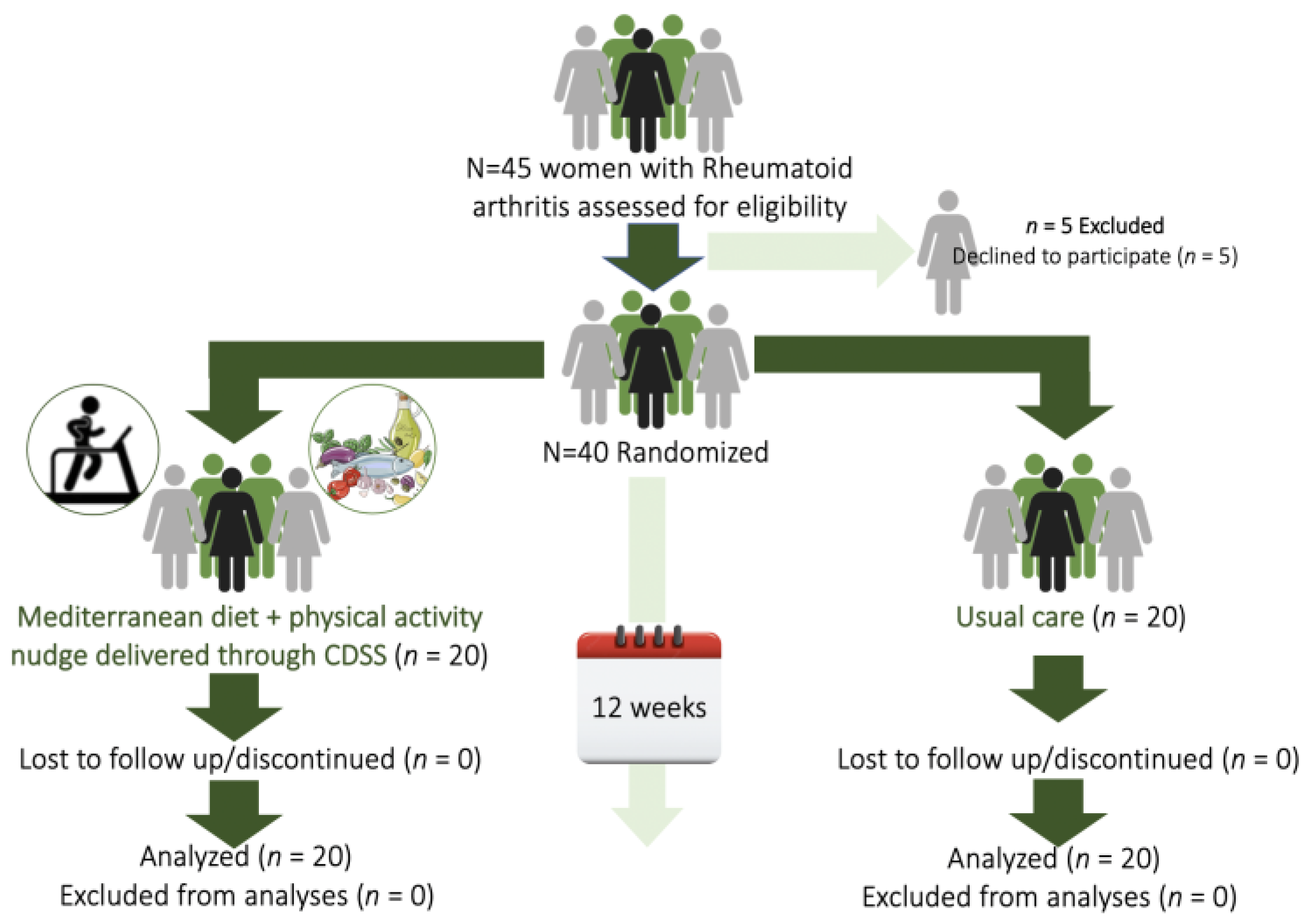
2.4. Randomization Procedure
2.5. Procedures and Tools
2.5.1. Medical History and RA Specificities
2.5.2. Dietary Intake and MD Adherence
2.5.3. Physical Activity (PA) Levels
2.5.4. Anthropometric Indices
2.5.5. Blood Samples: Collection and Assays
2.6. Intervention and Comparator
2.6.1. Intervention
2.6.2. Comparator (Control Arm)
2.6.3. Treatment Adherence
2.6.4. Study Timepoints
2.7. Primary and Secondary Outcomes
2.8. Sample Size Calculation
2.9. Statistical Analyses
3. Results
3.1. Participants
3.2. Mediterranean Diet Adherence and Dietary Intake
3.3. Disease Activity
3.4. Anthropometrics and PA Levels
3.5. Dietary Intake
3.6. Blood Glucose, Serum Lipids, CRP and Vitamin D Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, K.; Jandu, J.S.; Goyal, A.; Al-Dhahir, M.A. Rheumatoid Arthritis; StatPearls Publishing: Tampa, FL, USA, 2022; ISBN 9781469889351. [Google Scholar]
- Fang, Q.; Zhou, C.; Nandakumar, K.S. Molecular and Cellular Pathways Contributing to Joint Damage in Rheumatoid Arthritis. Mediators Inflamm. 2020, 2020, 3830212. [Google Scholar] [CrossRef] [PubMed]
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Kvien, T.K.; Uhlig, T.; Ødegård, S.; Heiberg, M.S. Epidemiological aspects of rheumatoid arthritis: The sex ratio. Ann. NY Acad. Sci. 2006, 1069, 212–222. [Google Scholar] [CrossRef]
- Gerosa, M.; De Angelis, V.; Riboldi, P.; Meroni, P.L. Rheumatoid arthritis: A female challenge. Womens Health 2008, 4, 195–201. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2019, 27, 501. [Google Scholar] [CrossRef]
- Cutolo, M.; Nikiphorou, E. Nutrition and Diet in Rheumatoid Arthritis. Nutrients 2022, 14, 888. [Google Scholar] [CrossRef] [PubMed]
- Cassotta, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Zabaleta, M.E.; Cano, S.S.; Dominguez, I.; Bullon, B.; Regolo, L.; Alvarez-Suarez, J.M.; Giampieri, F.; et al. Nutrition and Rheumatoid Arthritis in the ‘Omics’ Era. Nutrients 2021, 13, 763. [Google Scholar] [CrossRef]
- Alexandropoulou, I.; Grammatikopoulou, M.G.; Gkouskou, K.K.; Pritsa, A.A.; Vassilakou, T.; Rigopoulou, E.; Lindqvist, H.M.; Bogdanos, D.P. Ceramides in Autoimmune Rheumatic Diseases: Existing Evidence and Therapeutic Considerations for Diet as an Anticeramide Treatment. Nutrients 2023, 15, 229. [Google Scholar] [CrossRef]
- Gkiouras, K.; Grammatikopoulou, M.G.; Myrogiannis, I.; Papamitsou, T.; Rigopoulou, E.I.; Sakkas, L.I.; Bogdanos, D.P. Efficacy of n-3 fatty acid supplementation on rheumatoid arthritis’ disease activity indicators: A systematic review and meta-analysis of randomized placebo-controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124.e4. [Google Scholar] [CrossRef] [PubMed]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; Dort, S.; Calcagno, M.; Burgess, N.; Crosby, L.; Barnard, N.D. Nutrition Interventions in Rheumatoid Arthritis: The Potential Use of Plant-Based Diets. A Review. Front. Nutr. 2019, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Marakis, G.; Gkiouras, K.; Athanatou, D.; Maraki, M.I.; Bogdanos, D.P. Fly me to the immune: Immunonutrition in rheumatic diseases. Mediterr. J. Rheumatol. 2023, 33. [Google Scholar]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 2383. [Google Scholar] [CrossRef] [PubMed]
- Mosalmanzadeh, N.; Jandari, S.; Soleimani, D.; Shadmand Foumani Moghadam, M.R.; Khorramrouz, F.; Araste, A.; Molavi, S.F.; Fakhlaie, R.; Jokar, M.; Rezvani, R. Major dietary patterns and food groups in relation to rheumatoid arthritis in newly diagnosed patients. Food Sci. Nutr. 2020, 8, 6477. [Google Scholar] [CrossRef] [PubMed]
- Nezamoleslami, S.; Ghiasvand, R.; Feizi, A.; Salesi, M.; Pourmasoumi, M. The relationship between dietary patterns and rheumatoid arthritis: A case-control study. Nutr. Metab. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Espinoza, G.; Maldonado, G.; Narvaez, J.; Guerrero, R.; Citera, G.; Rios, C. Beyond Rheumatoid Arthritis Evaluation: What are We Missing? Open Access Rheumatol. Res. Rev. 2021, 13, 45–55. [Google Scholar] [CrossRef]
- Badsha, H. Role of Diet in Influencing Rheumatoid Arthritis Disease Activity. Open Rheumatol. J. 2018, 12, 19–28. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Markaki, A.G.; Gkiouras, K.; Papakitsos, C.; Grammatikopoulou, M.G.; Papatsaraki, A.; Ioannou, R.; Tsagkari, A.; Papamitsou, T.; Bogdanos, D.P. Disease Activity, Functional Ability and Nutritional Status in Patients with Rheumatoid Arthritis: An Observational Study in Greece. Mediterr. J. Rheumatol. 2020, 31, 406. [Google Scholar] [CrossRef]
- Vranou, P.; Gkoutzourelas, A.; Athanatou, D.; Zafiriou, E.; Grammatikopoulou, M.G.; Bogdanos, D.P. Let Food Be Thy Medicine: The Case of The Mediterranean Diet in Rheumatoid Arthritis. Med. J. Rheumatol. 2020, 31, 20–24. [Google Scholar] [CrossRef]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The effect of the mediterranean diet on metabolic health: A systematic review and meta-analysis of controlled trials in adults. Nutrients 2020, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Klonizakis, M.; Bugg, A.; Hunt, B.; Theodoridis, X.; Bogdanos, D.P.; Grammatikopoulou, M.G. Assessing the Physiological Effects of Traditional Regional Diets Targeting the Prevention of Cardiovascular Disease: A Systematic Review of Randomized Controlled Trials Implementing Mediterranean, New Nordic, Japanese, Atlantic, Persian and Mexican Dietary Interventions. Nutrients 2021, 13, 3034. [Google Scholar] [CrossRef] [PubMed]
- Gioxari, A.; Grammatikopoulou, M.G.; Katsarou, C.; Panagiotakos, D.B.; Toutouza, M.; Kavouras, S.A.; Sidossis, L.S.; Maraki, M.I. A Modified Mediterranean Diet Improves Fasting and Postprandial Glucoregulation in Adults with Overweight and Obesity: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 15347. [Google Scholar] [CrossRef] [PubMed]
- Hansildaar, R.; Vedder, D.; Baniaamam, M.; Tausche, A.K.; Gerritsen, M.; Nurmohamed, M.T. Cardiovascular risk in inflammatory arthritis: Rheumatoid arthritis and gout. Lancet. Rheumatol. 2021, 3, e58–e70. [Google Scholar] [CrossRef]
- Crowson, C.S.; Liao, K.P.; Davis, J.M.; Solomon, D.H.; Matteson, E.L.; Knutson, K.L.; Hlatky, M.A.; Gabriel, S.E. Rheumatoid Arthritis and Cardiovascular Disease. Am. Heart J. 2013, 166, 622. [Google Scholar] [CrossRef] [PubMed]
- Nicola, P.J.; Maradit-Kremers, H.; Roger, V.L.; Jacobsen, S.J.; Crowson, C.S.; Ballman, K.V.; Gabriel, S.E. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005, 52, 412–420. [Google Scholar] [CrossRef]
- Chodara, A.M.; Wattiaux, A.; Bartels, C.M. Managing cardiovascular disease risk in rheumatoid arthritis: Clinical updates and three strategic approaches. Curr. Rheumatol. Rep. 2017, 19, 16. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Wieczorek, M.; Balanescu, A.; Bischoff-Ferrari, H.A.; Boonen, A.; Cavalli, G.; de Souza, S.; de Thurah, A.; Dorner, T.E.; Moe, R.H.; et al. 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2022, 82, 48–56. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.L.; Van’T Hof, M.A.; Kuper, H.H.; Van Leeuwen, M.A.; Van De Putte, L.B.A.; Van Riel, P.L.C.M. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Mediterranean diet and survival among patients with coronary heart disease in Greece. Arch. Intern. Med. 2005, 165, 929–935. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hell. J. Cardiol. 2009, 50, 282–294. [Google Scholar]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.L.; McCarthy, H.D. Evaluation of factors determining the precision of body composition measurements by air displacement plethysmography. Eur. J. Clin. Nutr. 2003, 57, 770–776. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 1998.
- Papandreou, P.; Gioxari, A.; Nimee, F.; Skouroliakou, M. Application of Clinical Decision Support System to Assist Breast Cancer Patients with Lifestyle Modifications during the COVID-19 Pandemic: A Randomised Controlled Trial. Nutrients 2021, 13, 2115. [Google Scholar] [CrossRef]
- Papandreou, P.; Gioxari, A.; Daskalou, E.; Vasilopoulou, A.; Skouroliakou, M. Personalized Nutritional Intervention to Improve Mediterranean Diet Adherence in Female Patients with Multiple Sclerosis: A Randomized Controlled Study. Diet 2022, 1, 25–38. [Google Scholar] [CrossRef]
- Papandreou, P.; Amerikanou, C.; Vezou, C.; Gioxari, A.; Kaliora, A.C.; Skouroliakou, M. Improving Adherence to the Mediterranean Diet in Early Pregnancy Using a Clinical Decision Support System; A Randomised Controlled Clinical Trial. Nutrients 2023, 15, 432. [Google Scholar] [CrossRef]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An overview of clinical decision support systems: Benefits, risks, and strategies for success. NPJ Digit. Med. 2020, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prolepsis Institute. National Nutritional Guide for Adults; Prolepsis Institute: Athens, Greece, 2014. [Google Scholar]
- Jamovi. The Jamovi Project; Jamovi: Sydney, Australia, 2020. [Google Scholar]
- Comee, L.; Taylor, C.A.; Nahikian-Nelms, M.; Ganesan, L.P.; Krok-Schoen, J.L. Dietary patterns and nutrient intake of individuals with rheumatoid arthritis and osteoarthritis in the United States. Nutrition 2019, 67–68, 110533. [Google Scholar] [CrossRef]
- Skoczyńska, M.; Swierkot, J. The role of diet in rheumatoid arthritis. Reumatologia 2018, 56, 259–267. [Google Scholar] [CrossRef]
- Berube, L.T.; Kiely, M.; Yazici, Y.; Woolf, K. Diet quality of individuals with rheumatoid arthritis using the Healthy Eating Index (HEI)-2010. Nutr. Health 2017, 23, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Grimstvedt, M.E.; Woolf, K.; Milliron, B.J.; Manore, M.M. Lower Healthy Eating Index-2005 dietary quality scores in older women with rheumatoid arthritis v. healthy controls. Public Health Nutr. 2010, 13, 1170–1177. [Google Scholar] [CrossRef]
- Turesson Wadell, A.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Inadequate Dietary Nutrient Intake in Patients With Rheumatoid Arthritis in Southwestern Sweden: A Cross-Sectional Study. Front. Nutr. 2022, 9, 915064. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Schioppo, T.; Scotti, I.; Ubiali, T.; De Lucia, O.; Murgo, A.; Marano, G.; Boracchi, P.; Caporali, R. Adherence to Mediterranean diet and patient perception of rheumatoid arthritis. Complement. Ther. Med. 2020, 52, 102519. [Google Scholar] [CrossRef]
- Skoldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Schönenberger, K.A.; Schüpfer, A.C.; Gloy, V.L.; Hasler, P.; Stanga, Z.; Kaegi-braun, N.; Reber, E. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4221. [Google Scholar] [CrossRef]
- McKellar, G.; Morrison, E.; McEntegart, A.; Hampson, R.; Tierney, A.; Mackle, G.; Scoular, J.; Scott, J.A.; Capell, H.A. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis. 2007, 66, 1239–1243. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sugioka, Y.; Tada, M.; Okano, T.; Mamoto, K.; Inui, K.; Habu, D.; Koike, T. Monounsaturated fatty acids might be key factors in the Mediterranean diet that suppress rheumatoid arthritis disease activity: The TOMORROW study. Clin. Nutr. 2018, 37, 675–680. [Google Scholar] [CrossRef]
- Ricker, M.A.; Haas, W.C. Anti-Inflammatory Diet in Clinical Practice: A Review. Nutr. Clin. Pract. 2017, 32, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Häger, J.; Bang, H.; Hagen, M.; Frech, M.; Träger, P.; Sokolova, M.V.; Stéen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. [Google Scholar] [CrossRef]
- Elahi, N.; Elahi, H.; Navashenaq, J.G.; Abdollahzad, H.; Mahaki, B.; Soleimani, D.; Mostafaei, R.; Samadi, M.; Bagheri, A.; Nachvak, S.M. The relationship between major dietary patterns and disease activity of rheumatoid arthritis. Clin. Nutr. ESPEN 2022, 51, 274–279. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Norton, S.; Young, A.; Dixey, J.; Walsh, D.; Helliwell, H.; Kiely, P.; Davies, P.; Hill, L.; Gough, A.; et al. The association of obesity with disease activity, functional ability and quality of life in early rheumatoid arthritis: Data from the Early Rheumatoid Arthritis Study/Early Rheumatoid Arthritis Network UK prospective cohorts. Rheumatology 2018, 57, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Costello, R.E.; Ray, D.W.; Dixon, W.G. How Do Glucocorticoids Used in Rheumatic Disease Affect Body Weight? A Narrative Review of the Evidence. Arthritis Care Res. 2020, 72, 489–497. [Google Scholar] [CrossRef]
- Poudel, D.; George, M.D.; Baker, J.F. The Impact of Obesity on Disease Activity and Treatment Response in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2020, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, G.; Nurmohamed, M.T.; González-Gay, M.A.; Seres, I.; Paragh, G.; Kardos, Z.; Baráth, Z.; Tamási, L.; Soltész, P.; Szekanecz, Z. Rheumatoid arthritis and metabolic syndrome. Nat. Rev. Rheumatol. 2014, 10, 691–696. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Chiodini, P.; Panagiotakos, D.; Giugliano, D. A journey into a Mediterranean diet and type 2 diabetes: A systematic review with meta-analyses. BMJ Open 2015, 5, e008222. [Google Scholar] [CrossRef]
- Heidari, B.; Hajian-Tilaki, K.; Babaei, M. Vitamin D Deficiency and Rheumatoid Arthritis: Epidemiological, Immunological, Clinical and Therapeutic Aspects. Mediterr. J. Rheumatol. 2019, 30, 94–102. [Google Scholar] [CrossRef]
- Guan, Y.; Hao, Y.; Guan, Y.; Bu, H.; Wang, H. The Effect of Vitamin D Supplementation on Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 596007. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, M.; Barchetta, I.; Iannuccelli, C.; Gerardi, M.C.; Frisenda, S.; Ceccarelli, F.; Valesini, G.; Cavallo, M.G. Hypovitaminosis D in recent onset rheumatoid arthritis is predictive of reduced response to treatment and increased disease activity: A 12 month follow-up study. BMC Musculoskelet. Disord. 2015, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Hollan, I.; Dessein, P.H.; Ronda, N.; Wasko, M.C.; Svenungsson, E.; Agewall, S.; Cohen-Tervaert, J.W.; Maki-Petaja, K.; Grundtvig, M.; Karpouzas, G.A.; et al. Prevention of cardiovascular disease in rheumatoid arthritis. Autoimmun. Rev. 2015, 14, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Lagunova, Z.; Porojnicu, A.C.; Vieth, R.; Lindberg, F.A.; Hexeberg, S.; Moan, J. Serum 25-hydroxyvitamin D is a predictor of serum 1,25-dihydroxyvitamin D in overweight and obese patients. J. Nutr. 2011, 141, 112–117. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Alsafar, H.; Hyrich, K.L.; Lunt, M.; Barton, A.; Verstappen, S.M.M. Do people with rheumatoid arthritis maintain their physical activity level at treatment onset over the first year of methotrexate therapy? Rheumatology 2021, 60, 4633–4642. [Google Scholar] [CrossRef]
- Bremander, A.; Malm, K.; Andersson, M.L. Physical activity in established rheumatoid arthritis and variables associated with maintenance of physical activity over a seven-year period—A longitudinal observational study. BMC Rheumatol. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Swärdh, E.; Opava, C.; Brodin, N. Physical activity in patients with rheumatoid arthritis—An agile lifelong behaviour: A qualitative meta-synthesis. RMD Open 2021, 7, e001635. [Google Scholar] [CrossRef]
- Forberger, S.; Wichmann, F.; Comito, C.N. Nudges used to promote physical activity and to reduce sedentary behaviour in the workplace: Results of a scoping review. Prev. Med. 2022, 155, 106922. [Google Scholar] [CrossRef]
- Björk, M.; Dragioti, E.; Alexandersson, H.; Esbensen, B.A.; Boström, C.; Friden, C.; Hjalmarsson, S.; Hörnberg, K.; Kjeken, I.; Regardt, M.; et al. Inflammatory Arthritis and the Effect of Physical Activity on Quality of Life and Self-Reported Function: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2022, 74, 31–43. [Google Scholar] [CrossRef]
- Ye, H.; Weng, H.; Xu, Y.; Wang, L.; Wang, Q.; Xu, G. Effectiveness and safety of aerobic exercise for rheumatoid arthritis: A systematic review and meta-analysis of randomized controlled trials. BMC Sports Sci. Med. Rehabil. 2022, 14, 1–15. [Google Scholar] [CrossRef]
- Rausch Osthoff, A.K.; Juhl, C.B.; Knittle, K.; Dagfinrud, H.; Hurkmans, E.; Braun, J.; Schoones, J.; Vliet Vlieland, T.P.M.; Niedermann, K. Effects of exercise and physical activity promotion: Meta-analysis informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis. RMD Open 2018, 4, e000713. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, K.; Wong, A. Effects of exercise training on inflammatory and cardiometabolic health markers in overweight and obese adults: A systematic review and meta-analysis of randomized controlled trials. J. Sports Med. Phys. Fitness 2022. [Google Scholar] [CrossRef] [PubMed]
- Batrakoulis, A.; Jamurtas, A.Z.; Metsios, G.S.; Perivoliotis, K.; Liguori, G.; Feito, Y.; Riebe, D.; Thompson, W.R.; Angelopoulos, T.J.; Krustrup, P.; et al. Comparative Efficacy of 5 Exercise Types on Cardiometabolic Health in Overweight and Obese Adults: A Systematic Review and Network Meta-Analysis of 81 Randomized Controlled Trials. Circ. Cardiovasc. Qual. Outcomes 2022, 15, E008243. [Google Scholar] [CrossRef] [PubMed]
- Laiou, E.; Rapti, I.; Schwarzer, R.; Fleig, L.; Cianferotti, L.; Ngo, J.; Rizos, E.C.; Wetle, T.F.; Kahlmeier, S.; Vigilanza, A.; et al. Review: Nudge interventions to promote healthy diets and physical activity. Food Policy 2021, 102, 102103. [Google Scholar] [CrossRef]
- Ensaff, H. A nudge in the right direction: The role of food choice architecture in changing populations’ diets. Proc. Nutr. Soc. 2021, 80, 195–206. [Google Scholar] [CrossRef]
- Chen, Y.; Harris, S.; Rogers, Y.; Ahmad, T.; Asselbergs, F.W. Nudging within learning health systems: Next generation decision support to improve cardiovascular care. Eur. Heart J. 2022, 43, 1296. [Google Scholar] [CrossRef]
- Kwan, J.L.; Lo, L.; Ferguson, J.; Goldberg, H.; Diaz-Martinez, J.P.; Tomlinson, G.; Grimshaw, J.M.; Shojania, K.G. Computerised clinical decision support systems and absolute improvements in care: Meta-analysis of controlled clinical trials. BMJ 2020, 370, m3216. [Google Scholar] [CrossRef]
- Hazlewood, G.S.; Marshall, D.A.; Barber, C.E.H.; Li, L.C.; Barnabe, C.; Bykerk, V.; Tugwell, P.; Hull, P.M.; Bansback, N. Using a Discrete-Choice Experiment in a Decision Aid to Nudge Patients Towards Value-Concordant Treatment Choices in Rheumatoid Arthritis: A Proof-of-Concept Study. Patient Prefer. Adherence 2020, 14, 829–838. [Google Scholar] [CrossRef]
- Umaefulam, V.; Fox, T.L.; Hazlewood, G.; Bansback, N.; Barber, C.E.H.; Barnabe, C. Adaptation of a Shared Decision-Making Tool for Early Rheumatoid Arthritis Treatment Decisions with Indigenous Patients. Patient 2022, 15, 233–243. [Google Scholar] [CrossRef]
- Vamvakis, A.; Gkaliagkousi, E.; Lazaridis, A.; Grammatikopoulou, M.G.; Triantafyllou, A.; Nikolaidou, B.; Koletsos, N.; Anyfanti, P.; Tzimos, C.; Zebekakis, P.; et al. Impact of intensive lifestyle treatment (Diet plus exercise) on endothelial and vascular function, arterial stiffness and blood pressure in stage 1 hypertension: Results of the Hintreat randomized controlled trial. Nutrients 2020, 12, 1326. [Google Scholar] [CrossRef]
- Franz, M.J.; Monk, A.; Barry, B.; McCllain, K.; Weaver, T.; Cooper, N.; Upham, P.; Bergerstall, R.; Mazze, R.S. Effectiveness of Medical Nutrition Therapy Provided by Dietitians in the Management of Non-Insulin-Dependent Diabetes Mellitus. A Randomized, Controlled Clinical Trial. J. Am. Diet. Assoc. 1995, 95, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Briggs Early, K.; Stanley, K. Position of the Academy of Nutrition and Dietetics: The Role of Medical Nutrition Therapy and Registered Dietitian Nutritionists in the Prevention and Treatment of Prediabetes and Type 2 Diabetes. J. Acad. Nutr. Diet. 2018, 118, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Larson, E. Disease management, registered dietitians and medical nutrition therapy. J. Am. Diet. Assoc. 2002, 102, 190–191. [Google Scholar] [CrossRef]
- Johansen, M.Y.; Karstoft, K.; MacDonald, C.S.; Hansen, K.B.; Ellingsgaard, H.; Hartmann, B.; Wewer Albrechtsen, N.J.; Vaag, A.A.; Holst, J.J.; Pedersen, B.K.; et al. Effects of an intensive lifestyle intervention on the underlying mechanisms of improved glycaemic control in individuals with type 2 diabetes: A secondary analysis of a randomised clinical trial. Diabetologia 2020, 63, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.Y.; Macdonald, C.S.; Hansen, K.B.; Karstoft, K.; Christensen, R.; Pedersen, M.; Hansen, L.S.; Zacho, M.; Wedell-Neergaard, A.S.; Nielsen, S.T.; et al. Effect of an intensive lifestyle intervention on glycemic control in patients with type 2 diabetes: A randomized clinical trial. JAMA 2017, 318, 637–646. [Google Scholar] [CrossRef]
- MacDonald, C.S.; Nielsen, S.M.; Bjørner, J.; Johansen, M.Y.; Christensen, R.; Vaag, A.; Lieberman, D.E.; Pedersen, B.K.; Langberg, H.; Ried-Larsen, M.; et al. One-year intensive lifestyle intervention and improvements in health-related quality of life and mental health in persons with type 2 diabetes: A secondary analysis of the U-TURN randomized controlled trial. BMJ Open Diabetes Res. Care 2021, 9, 1840. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Houlihan, C.A.; Balas, E.A.; Lobach, D.F. Improving clinical practice using clinical decision support systems: A systematic review of trials to identify features critical to success. Br. Med. J. 2005, 330, 765–768. [Google Scholar] [CrossRef]
- Alonso-Molero, J.; Prieto-Peña, D.; Mendoza, G.; Atienza-Mateo, B.; Corrales, A.; González-Gay, M.; Llorca, J. Misperception of the Cardiovascular Risk in Patients with Rheumatoid Arthritis. Int. J. Environ. Res. Public Health 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Metsios, G.S.; Kitas, G.D. Physical activity, exercise and rheumatoid arthritis: Effectiveness, mechanisms and implementation. Best Pract. Res. Clin. Rheumatol. 2018, 32, 669–682. [Google Scholar] [CrossRef]
- Khanna, S.; Jaiswal, K.S.; Gupta, B. Managing Rheumatoid Arthritis with Dietary Interventions. Front. Nutr. 2017, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perdoni, F.; Peroni, G.; Caporali, R.; Gasparri, C.; Riva, A.; Petrangolini, G.; Faliva, M.A.; Infantino, V.; Naso, M.; et al. Ideal food pyramid for patients with rheumatoid arthritis: A narrative review. Clin. Nutr. 2021, 40, 661–689. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| (1) Adult women (≥ 18 years of age) | (1) Adolescent women |
| with an RA diagnosis according to the ACR/EULAR criteria [33] for >2 years | (2) Patients with altered treatment regime ≤ 6 months before, or during the trial |
| (2) With mild-moderate disease activity based on the DAS28 (DAS28 < 3.2) | (3) Women with RA with active disease (DAS28 > 5.1) [34] |
| (4) People unable to read and comprehend the consent form | |
| (3) On an unchanged treatment regime for >6 months | (5) Patients with psychiatric conditions |
| (4) Who provided consent for participation | (6) Pregnant or lactating women |
| (7) Women on weight loss medication | |
| (8) Women following a vegan diet ≤ 5 years prior to screening | |
| (9) Those who did not consent or were unable to provide consent | |
| (10) Women with allergies, food intolerances, serious or life-threating illness, e.g., malignancy; infections; heart, liver, or renal failure; congenital metabolic diseases; malabsorption; or cognitive disorders | |
| (11) Alcoholism or drug addiction | |
| (12) Women taking vitamin or mineral supplementation during or ≤6 months prior to screening |
| Characteristics | Enrolled (N = 40) | Intervention Arm (n = 20) | Control Arm (n = 20) | |
|---|---|---|---|---|
| Age (years) | 34.03 ± 5.45 | 34.15 ± 5.95 | 33.9 ± 5.06 | |
| Anthropometry: | BW (kg) | 78.86 ± 19.46 | 79.19 ± 21.38 | 78.52 ± 17.88 |
| BMI (kg/m2) | 25.76 ± 5.22 | 26.13 ± 5.89 | 25.40 ± 4.59 | |
| FM (% of BW) | 21.13 ± 12.07 | 20.63 ± 11.86 | 21.64 ± 12.56 | |
| FFM (% of BW) | 57.68 ± 11.92 | 57.53 ± 13.33 | 57.82 ± 10.66 | |
| Weight status: | Normoweight/Overweight/Obese (n) | 22/14/4 | 12/6/2 | 10/8/2 |
| Blood assays: | Glucose (mg/dL) | 108.13 ± 17.16 | 110.61 ± 7.34 | 105.65 ± 23.19 |
| TC (mg/dL) | 213.21 ± 50.11 | 209.22 ± 53.02 | 217.2 ± 48.07 | |
| HDL (mg/dL) | 69.33 ± 21.16 | 71.78 ± 17.62 | 66.88 ± 24.42 | |
| LDL (mg/dL) | 129.20 ± 42.31 | 130.02 ± 45.87 | 128.37 ± 39.61 | |
| TG (mg/dL) | 113.34 ± 67.12 | 112.23 ± 75.76 | 114.45 ± 59.20 | |
| CRP (mg/dL) | 0.74 ± 0.12 | 0.71 ± 0.11 | 0.77 ± 0.12 | |
| 1,25(OH)2D (ng/mL) | 32.97 ± 4.10 | 33.79 ± 3.94 | 32.14 ± 4.19 | |
| Physical activity (IPAQ): | METs-min/week | 647.46 ± 308.01 | 580.78 ± 322.39 | 714.14 ± 284.24 |
| Adherence to the MD: | MedDietScore | 38.25 ± 3.21 | 38.36 ± 3.46 | 38.14 ± 3.03 |
| Daily dietary intake: | Total fat (g) | 68.76 ± 9.49 | 67.02 ± 7.15 | 70.50 ± 11.29 |
| Dietary cholesterol (mg) | 199.19 ± 45.49 | 188.94 ± 58.45 | 209.44 ± 24.70 | |
| Fiber (g) | 21.24 ± 4.85 | 19.98± 4.28 | 17.95 ± 4.24 | |
| SFA (g) | 18.98± 3.64 | 19.87 ± 2.16 | 18.09 ± 4.58 | |
| MUFA (g) | 29.63 ± 5.85 | 30.84 ± 5.47 | 28.42 ± 6.11 | |
| Disease activity: | DAS28 | 2.82 ± 0.19 | 2.83 ± 0.19 | 2.81 ± 0.20 |
| Characteristics | Intervention Arm (n = 20) | p-Values within Timepoints † | Control Arm (n = 20) | p-Values within Timepoints † | p-Values between Groups † | p-Values between Groups † | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| before | after | before | after | Baseline | 12 Weeks | Δ | ||||
| Anthropometry: | BW (kg) | 79.2 ± 21.4 | 77.1 ± 20.8 | <0.001 | 78.5 ± 17.9 | 80.6 ± 18.3 | <0.001 | 0.916 | 0.576 | <0.001 |
| BMI (kg/m2) | 26.13 ± 5.89 | 25.42 ± 5.63 | <0.001 | 25.40 ± 4.59 | 26.04 ± 4.58 | <0.001 | 0.663 | 0.702 | <0.001 | |
| FM (% of BW) | 20.6 ± 11.9 | 20.3 ± 12.1 | <0.001 | 21.6 ± 12.6 | 22.1 ± 12.1 | 0.002 | 0.794 | 0.665 | <0.001 | |
| FFM (% of BW) | 57.5 ± 13.3 | 56.7 ± 13.3 | 0.001 | 57.8 ± 10.7 | 57.8 ± 11.1 | 0.954 | 0.940 | 0.774 | 0.348 | |
| Blood assays: | Glucose (mg/dL) | 110.6 ± 7.3 | 104.8 ± 5.3 | <0.001 | 105.7 ± 23.2 | 104.7 ± 23.2 | 0.401 | 0.368 | 0.964 | 0.005 |
| TC (mg/dL) | 209.2 ± 53.0 | 200.6 ± 56.5 | 0.183 | 217.2 ± 48.1 | 228.8 ± 53.0 | 0.209 | 0.621 | 0.111 | 0.071 | |
| HDL (mg/dL) | 71.8 ± 17.6 | 69.7 ± 19.2 | 0.680 | 66.9 ± 24.4 | 73.4 ± 27.3 | 0.133 | 0.472 | 0.625 | 0.193 | |
| LDL (mg/dL) | 130.0 ± 45.9 | 126.5 ± 52.0 | 0.637 | 128.4 ± 39.6 | 132.3 ± 45.3 | 0.623 | 0.904 | 0.708 | 0.493 | |
| TG (mg/dL) | 112.2 ± 75.8 | 97.5 ± 57.7 | 0.057 | 114.5 ± 59.2 | 126.3 ± 83.6 | 0.380 | 0.918 | 0.212 | 0.086 | |
| CRP (mg/dL) | 0.71 ± 0.11 | 0.72 ± 0.11 | 0.408 | 0.77 ± 0.12 | 0.75 ± 0.11 | 0.367 | 0.084 | 0.460 | 0.240 | |
| 1,25(OH)2D (ng/mL) | 33.8 ± 3.9 | 51.6 ± 11.8 | <0.001 | 32.1 ± 4.2 | 40.4 ± 6.4 | <0.001 | 0.208 | <0.001 | 0.001 | |
| Physical activity (IPAQ): | METs (min/week) | 580.8 ± 322.4 | 616.5 ± 315.9 | 0.002 | 714.1 ± 284.2 | 684.7 ± 251.1 | 0.065 | 0.174 | 0.454 | 0.002 |
| MD adherence: | MedDietScore | 38.36 ± 3.46 | 42.11 ± 2.48 | <0.001 | 38.14 ± 3.03 | 38.36 ± 2.74 | 0.359 | 0.829 | <0.001 | <0.001 |
| Daily dietary intake: | Total fat (g) | 67.02 ± 7.15 | 61.19 ± 7.90 | <0.001 | 70.50 ± 11.29 | 73.42 ± 11.57 | <0.001 | 0.251 | <0.001 | <0.001 |
| Cholesterol (mg) | 188.9 ± 58.5 | 169.2 ± 50.3 | <0.001 | 209.4 ± 24.7 | 224.6 ± 28.3 | <0.001 | 0.157 | <0.001 | <0.001 | |
| Fiber (g) | 19.98 ± 4.28 | 26.09 ± 5.00 | <0.001 | 17.95 ± 4.24 | 23.73 ± 4.24 | 0.067 | 0.140 | 0.116 | <0.001 | |
| SFA (g) | 19.87 ± 2.16 | 16.73 ± 3.12 | 0.004 | 18.09 ± 4.58 | 18.48 ± 4.77 | 0.132 | 0.125 | 0.177 | 0.001 | |
| MUFA (g) | 30.84 ± 5.47 | 36.98 ± 5.82 | <0.001 | 28.42 ± 6.11 | 28.73 ± 5.38 | 0.433 | 0.195 | <0.001 | <0.001 | |
| Disease activity: | DAS28 | 2.83 ± 0.19 | 2.71 ± 0.14 | <0.001 | 2.81 ± 0.20 | 2.80 ± 0.17 | 0.804 | 0.744 | 0.054 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papandreou, P.; Gioxari, A.; Daskalou, E.; Grammatikopoulou, M.G.; Skouroliakou, M.; Bogdanos, D.P. Mediterranean Diet and Physical Activity Nudges versus Usual Care in Women with Rheumatoid Arthritis: Results from the MADEIRA Randomized Controlled Trial. Nutrients 2023, 15, 676. https://doi.org/10.3390/nu15030676
Papandreou P, Gioxari A, Daskalou E, Grammatikopoulou MG, Skouroliakou M, Bogdanos DP. Mediterranean Diet and Physical Activity Nudges versus Usual Care in Women with Rheumatoid Arthritis: Results from the MADEIRA Randomized Controlled Trial. Nutrients. 2023; 15(3):676. https://doi.org/10.3390/nu15030676
Chicago/Turabian StylePapandreou, Panos, Aristea Gioxari, Efstratia Daskalou, Maria G. Grammatikopoulou, Maria Skouroliakou, and Dimitrios P. Bogdanos. 2023. "Mediterranean Diet and Physical Activity Nudges versus Usual Care in Women with Rheumatoid Arthritis: Results from the MADEIRA Randomized Controlled Trial" Nutrients 15, no. 3: 676. https://doi.org/10.3390/nu15030676
APA StylePapandreou, P., Gioxari, A., Daskalou, E., Grammatikopoulou, M. G., Skouroliakou, M., & Bogdanos, D. P. (2023). Mediterranean Diet and Physical Activity Nudges versus Usual Care in Women with Rheumatoid Arthritis: Results from the MADEIRA Randomized Controlled Trial. Nutrients, 15(3), 676. https://doi.org/10.3390/nu15030676







