Effects of a Dulaglutide plus Calorie-Restricted Diet versus a Calorie-Restricted Diet on Visceral Fat and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Ethics Statements
2.2. Eligibility Criteria
2.3. Randomization and Intervention Programs
2.4. Trial Outcomes
2.5. Sample Size Estimation and Statistical Analysis
3. Results
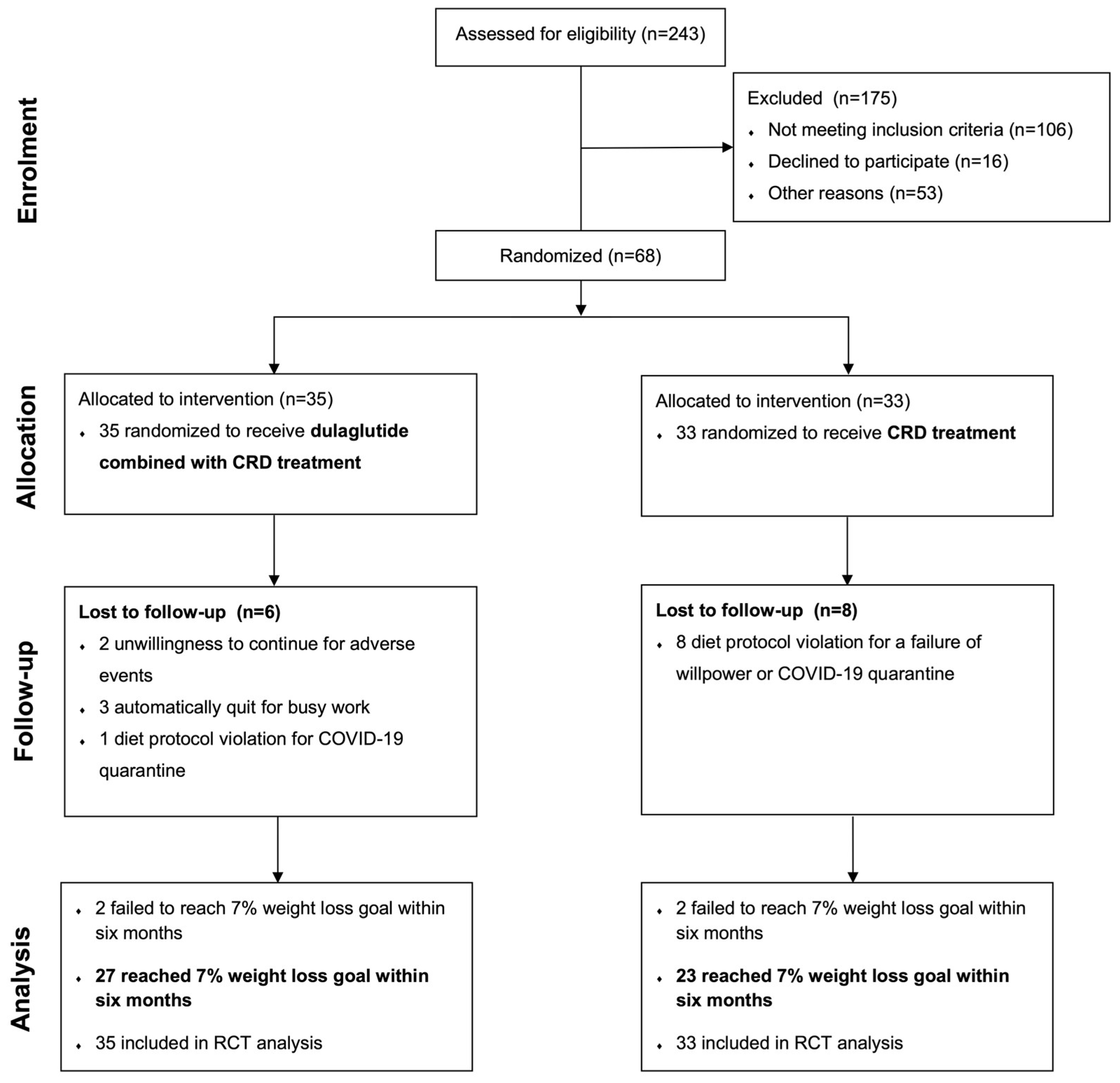
3.1. Trial Participants
3.2. Baseline Characteristics and Adherence
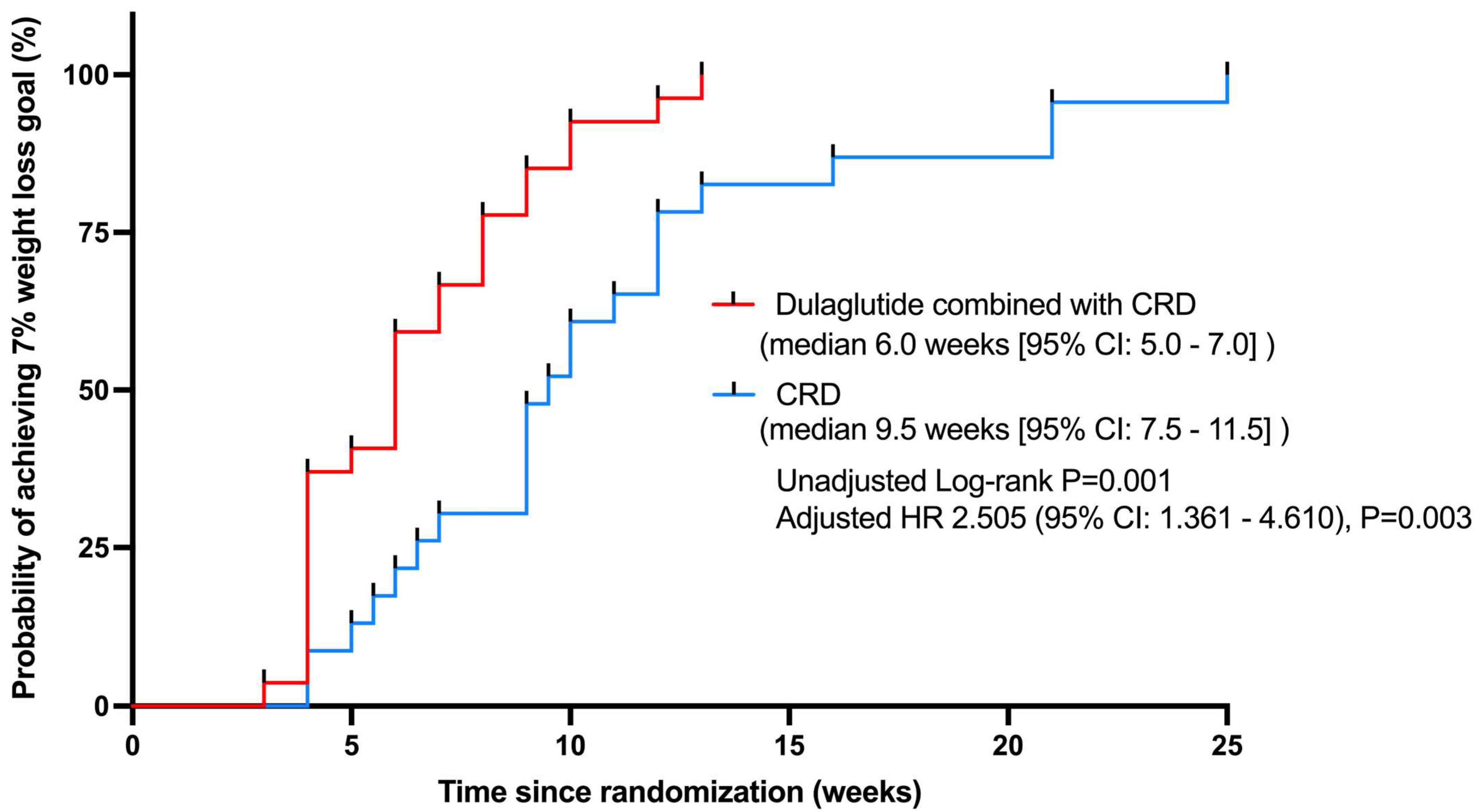
3.3. Weight Loss
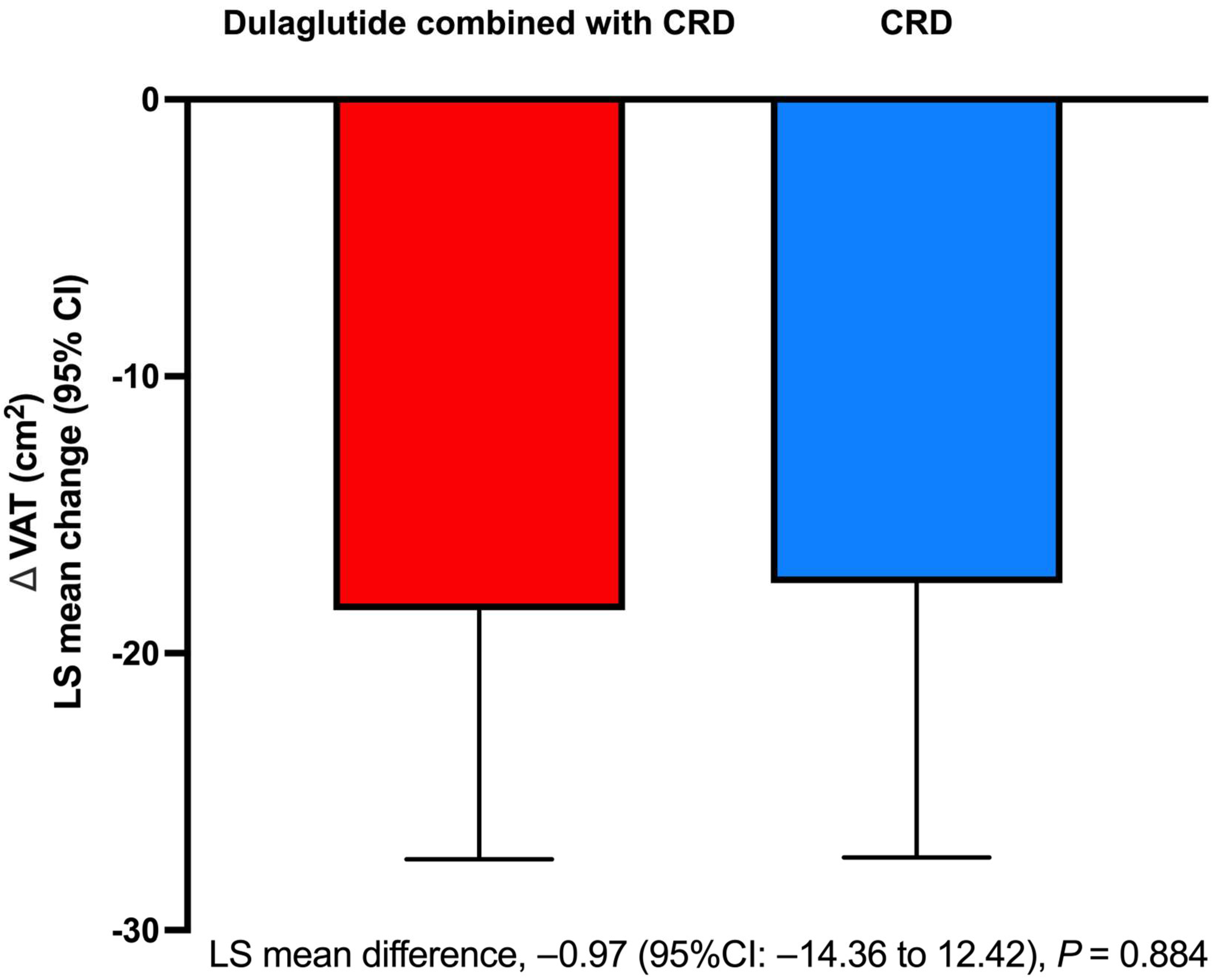
3.4. Body Composition and Fat Distribution
3.5. Metabolic Risk Factors
3.6. Menstrual Frequency and Reproductive Hormones
3.7. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escobar-Morreale, H. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; La Marca, A.; Petraglia, F. Insulin-Lowering Agents in the Management of Polycystic Ovary Syndrome. Endocr. Rev. 2003, 24, 633–667. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Millán, J.L.S. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol. Metab. 2007, 18, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Akopians, A.L.; Madrigal, V.K.; Ramirez, E.; Margolis, D.J.; Sarma, M.K.; Thomas, A.M.; Grogan, T.R.; Haykal, R.; Schooler, T.A.; et al. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J. Clin. Endocrinol. Metab. 2016, 101, 4178–4188. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-H.; Li, X.-L. Visceral adiposity index as a predictor of clinical severity and therapeutic outcome of PCOS. Gynecol. Endocrinol. 2016, 32, 177–183. [Google Scholar] [CrossRef]
- Hoeger, K.M. Role of lifestyle modification in the management of polycystic ovary syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 293–310. [Google Scholar] [CrossRef]
- Lim, S.; Smith, C.A.; Costello, M.F.; MacMillan, F.; Moran, L.; Ee, C. Barriers and facilitators to weight management in overweight and obese women living in Australia with PCOS: A qualitative study. BMC Endocr. Disord. 2019, 19, 106. [Google Scholar] [CrossRef]
- Yoshimura, E.; Kumahara, H.; Tobina, T.; Matsuda, T.; Ayabe, M.; Kiyonaga, A.; Anzai, K.; Higaki, Y.; Tanaka, H. Lifestyle Intervention Involving Calorie Restriction with or without Aerobic Exercise Training Improves Liver Fat in Adults with Visceral Adiposity. J. Obes. 2014, 2014, 197216. [Google Scholar] [CrossRef]
- Huffman, K.M.; Redman, L.M.; Landerman, L.R.; Pieper, C.F.; Stevens, R.D.; Muehlbauer, M.J.; Wenner, B.R.; Bain, J.R.; Kraus, V.B.; Newgard, C.B.; et al. Caloric Restriction Alters the Metabolic Response to a Mixed-Meal: Results from a Randomized, Controlled Trial. PLoS ONE 2012, 7, e28190. [Google Scholar] [CrossRef]
- Johnson, L.K.; Holven, K.B.; Nordstrand, N.; Mellembakken, J.R.; Tanbo, T.; Hjelmesæth, J. Fructose content of low calorie diets: Effect on cardiometabolic risk factors in obese women with polycystic ovarian syndrome: A randomized controlled trial. Endocr. Connect. 2015, 4, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 Agonism Stimulates Brown Adipose Tissue Thermogenesis and Browning Through Hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Frøssing, S.; Nylander, M.; Chabanova, E.; Frystyk, J.; Holst, J.J.; Kistorp, C.; Skouby, S.O.; Faber, J. Effect of liraglutide on ectopic fat in polycystic ovary syndrome: A randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.J.; Sathyapalan, T.; Vince, R.; Coady, A.-M.; Ajjan, R.A.; Kilpatrick, E.S.; Atkin, S.L. The Effect of Exenatide on Cardiovascular Risk Markers in Women with Polycystic Ovary Syndrome. Front. Endocrinol. 2019, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Zheng, S.-Y.; Lin, R.; Xie, Y.-J.; Chen, H.; Zheng, Y.-X.; Liu, E.; Chen, L.; Yan, J.-H.; et al. Efficacy of exenatide on weight loss, metabolic parameters and pregnancy in overweight/obese polycystic ovary syndrome. Clin. Endocrinol. 2017, 87, 767–774. [Google Scholar] [CrossRef]
- Amblee, A. Mode of administration of dulaglutide: Implications for treatment adherence. Patient Prefer. Adherence 2016, 10, 975–982. [Google Scholar] [CrossRef]
- Eleni, K.; George, T.; Stephen, F. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Huang, M.A.; Greenson, J.K.; Chao, C.; Anderson, L.; Peterman, D.; Jacobson, J.; Emick, D.; Lok, A.S.; Conjeevaram, H.S. One-Year Intense Nutritional Counseling Results in Histological Improvement in Patients with Nonalcoholic Steatohepatitis: A Pilot Study. Am. J. Gastroenterol. 2005, 100, 1072–1081. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S76–S99. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, R.; Shepherd, J.A.; El Ghormli, L.; Copeland, K.C.; Geffner, M.E.; Higgins, J.; Levitsky, L.L.; Nadeau, K.J.; Weinstock, R.S.; White, N.H. Changes in Visceral and Subcutaneous Fat in Youth with Type 2 Diabetes in the Today Study. Diabetes Care 2019, 42, 1549–1559. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Catarig, A.-M.; Frias, J.P.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Lingvay, I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: A substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia 2020, 63, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Gill, C.M.; Keating, L.K.; Torriani, M.; Anderson, E.J.; Punyanitya, M.; Wilson, K.E.; Kelly, T.L.; Miller, K.K. Assessment of Abdominal Fat Compartments Using DXA in Premenopausal Women from Anorexia Nervosa to Morbid Obesity: DXA for Assessment of Abdominal Fat. Obesity 2013, 21, 2458–2464. [Google Scholar] [CrossRef]
- Panizza, C.E.; Lim, U.; Yonemori, K.M.; Cassel, K.D.; Wilkens, L.R.; Harvie, M.N.; Maskarinec, G.; Delp, E.J.; Lampe, J.W.; Shepherd, J.A.; et al. Effects of Intermittent Energy Restriction Combined with a Mediterranean Diet on Reducing Visceral Adiposity: A Randomized Active Comparator Pilot Study. Nutrients 2019, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Nylander, M.; Frøssing, S.; Clausen, H.V.; Kistorp, C.; Faber, J.; Skouby, S.O. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: A randomized clinical trial. Reprod. Biomed. Online 2017, 35, 121–127. [Google Scholar] [CrossRef]
- Santilli, F.; Simeone, P.G.; Guagnano, M.T.; Leo, M.; Maccarone, M.T.; Di Castelnuovo, A.; Sborgia, C.; Bonadonna, R.C.; Angelucci, E.; Federico, V.; et al. Effects of Liraglutide on Weight Loss, Fat Distribution, and β-Cell Function in Obese Subjects With Prediabetes or Early Type 2 Diabetes. Diabetes Care 2017, 40, 1556–1564. [Google Scholar] [CrossRef]
- Weiss, E.P.; Jordan, R.C.; Frese, E.M.; Albert, S.G.; Villareal, D.T. Effects of Weight Loss on Lean Mass, Strength, Bone, and Aerobic Capacity. Med. Sci. Sports Exerc. 2017, 49, 206–217. [Google Scholar] [CrossRef]
- Swora-Cwynar, E.; Kujawska-Łuczak, M.; Suliburska, J.; Reguła, J.; Kargulewicz, A.; Kręgielska-Narożna, M.; Marcinkowska, E.; Kanikowska, A.; Bielas, M.; Grzymisławski, M.; et al. The effects of a low-calorie diet or an isocaloric diet combined with metformin on sex hormones in obese women of child-bearing age. Acta Sci. Pol. Technol. Aliment. 2016, 15, 213–220. [Google Scholar] [CrossRef]
- Marzouk, T.M.; Ahmed, W.A.S. Effect of Dietary Weight Loss on Menstrual Regularity in Obese Young Adult Women with Polycystic Ovary Syndrome. J. Pediatr. Adolesc. Gynecol. 2015, 28, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.; Von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Heilbronn, L.K.; Martin, C.K.; Alfonso, A.; Smith, S.R.; Ravussin, E. Effect of Calorie Restriction with or without Exercise on Body Composition and Fat Distribution. J. Clin. Endocrinol. Metab. 2007, 92, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.; Batterham, M.; Huang, X.; Tan, S.-Y.; Teuss, G.; Charlton, K.; Oshea, J.; Warensjö, E. Short term effects of energy restriction and dietary fat sub-type on weight loss and disease risk factors. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Kravos, N.A.; Goričar, K.; Janez, A. Short-term effectiveness of low dose liraglutide in combination with metformin versus high dose liraglutide alone in treatment of obese PCOS: Randomized trial. BMC Endocr. Disord. 2017, 17, 5. [Google Scholar] [CrossRef]
- Chang, K.-C.; Shao, S.-C.; Kuo, S.; Yang, C.-Y.; Chen, H.-Y.; Chan, Y.-Y.; Ou, H.-T. Comparative effectiveness of dulaglutide versus liraglutide in Asian type 2 diabetes patients: A multi-institutional cohort study and meta-analysis. Cardiovasc. Diabetol. 2020, 19, 172. [Google Scholar] [CrossRef]
- Ferdinand, K.C.; White, W.B.; Calhoun, D.A.; Lonn, E.M.; Sager, P.T.; Brunelle, R.; Jiang, H.H.; Threlkeld, R.J.; Robertson, K.E.; Geiger, M.J. Effects of the Once-Weekly Glucagon-Like Peptide-1 Receptor Agonist Dulaglutide on Ambulatory Blood Pressure and Heart Rate in Patients with Type 2 Diabetes Mellitus. Hypertension 2014, 64, 731–737. [Google Scholar] [CrossRef]
- Kraus, W.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Das, S.K.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; Ravussin, E.; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683. [Google Scholar] [CrossRef]
- Bagheri, M.J.; Talebpour, M.; Sharifi, A.; Talebpour, A.; Mohseni, A.; Mohseni, A. Lipid profile change after bariatric surgeries: Laparoscopic gastric plication versus mini gastric bypass. Acta Chir. Belg. 2018, 119, 146–151. [Google Scholar] [CrossRef]
- Stern, L.; Iqbal, N.; Seshadri, P.; Chicano, K.L.; Daily, D.A.; McGrory, J.; Williams, M.; Gracely, E.J.; Samaha, F.F. The Effects of Low-Carbohydrate versus Conventional Weight Loss Diets in Severely Obese Adults: One-Year Follow-up of a Randomized Trial. Ann. Intern. Med. 2004, 140, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol. Res. 2017, 47, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Choudhary, N.S.; Singh, M.K.; Wasir, J.S.; Kaur, P.; Gill, H.K.; Bano, T.; Farooqui, K.J.; et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: Randomised controlled trial (D-LIFT trial). Diabetologia 2020, 63, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Guzman, G.; De Mello, L.L.C.; Trein, B.; Spina, L.; Bussade, I.; Prata, J.M.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients with Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Jafari-Sfidvajani, S.; Ahangari, R.; Hozoori, M.; Mozaffari-Khosravi, H.; Fallahzadeh, H.; Nadjarzadeh, A. The effect of vitamin D supplementation in combination with low-calorie diet on anthropometric indices and androgen hormones in women with polycystic ovary syndrome: A double-blind, randomized, placebo-controlled trial. J. Endocrinol. Investig. 2018, 41, 597–607. [Google Scholar] [CrossRef]
- Dungan, K.M.; Povedano, S.T.; Forst, T.; González, J.G.G.; Atisso, C.; Sealls, W.; Fahrbach, J.L. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): A randomised, open-label, phase 3, non-inferiority trial. Lancet 2014, 384, 1349–1357. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, B.; Hou, J.; Zhou, Z. Evaluation of Characteristics of Gastrointestinal Adverse Events with Once-Weekly Dulaglutide Treatment in Chinese Patients with Type 2 Diabetes: A Post Hoc Pooled Analysis of Two Randomized Trials. Diabetes Ther. 2020, 11, 1821–1833. [Google Scholar] [CrossRef]
- Ahrén, B.; Atkin, S.L.; Charpentier, G.; Warren, M.L.; Wilding, J.P.H.; Birch, S.; Holst, A.G.; Leiter, L.A. Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes Obes. Metab. 2018, 20, 2210–2219. [Google Scholar] [CrossRef]
- Sundfør, T.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
- Keenan, S.; Cooke, M.B.; Chen, W.S.; Wu, S.; Belski, R. The Effects of Intermittent Fasting and Continuous Energy Restriction with Exercise on Cardiometabolic Biomarkers, Dietary Compliance, and Perceived Hunger and Mood: Secondary Outcomes of a Randomised, Controlled Trial. Nutrients 2022, 14, 3071. [Google Scholar] [CrossRef]
- Micklesfield, L.; Goedecke, J.; Punyanitya, M.; Wilson, K.E.; Kelly, T.L. Dual-Energy X-Ray Performs as Well as Clinical Computed Tomography for the Measurement of Visceral Fat. Obesity 2012, 20, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Frøssing, S.; Nylander, M.C.; Chabanova, E.; Kistorp, C.; Skouby, S.O.; Faber, J. Quantification of visceral adipose tissue in polycystic ovary syndrome: Dual-energy X-ray absorptiometry versus magnetic resonance imaging. Acta Radiol. 2018, 59, 13–17. [Google Scholar] [CrossRef] [PubMed]
| Variables | Dulaglutide Combined with CRD Therapy (n = 35) | CRD Therapy (n = 33) |
|---|---|---|
| Age (years) | 30.31 (28.58 to 32.05) | 28.64 (27.09 to 30.18) |
| Weight (kg) | 77.62 (74.19 to 81.06) | 78.84 (74.24 to 83.43) |
| WC (cm) | 96.76 (93.61 to 99.92) | 94.89 (91.45 to 98.33) |
| BMI (kg/m2) | 29.68 (28.44 to 30.93) | 29.71 (28.39 to 31.04) |
| Menstrual Cycles (no./yr) | 7.44 (6.07 to 8.81) | 7.90 (6.83 to 8.96) |
| SBP (mmHg) | 124.14 (119.77 to 128.52) | 127.69 (121.16 to 134.22) |
| DBP (mmHg) | 82.06 (78.55 to 85.56) | 84.38 (79.81 to 88.94) |
| FPG (mmol/L) | 4.96 (4.77 to 5.14) | 5.03 (4.85 to 5.21) |
| PPG (mmol/L) | 7.53 (6.57 to 8.50) | 7.58 (6.81 to 8.34) |
| FINS (mU/L) | 21.54 (17.19 to 25.89) | 22.59 (14.44 to 30.75) |
| PINS (mU/L) | 139.41 (99.15 to 179.67) | 149.16 (111.19 to 187.14) |
| HbA1c (%) | 5.72 (5.61 to 5.84) | 5.64 (5.48 to 5.80) |
| HOMA-IR | 4.85 (3.79 to 5.91) | 5.18 (3.26 to 7.10) |
| ALT (U/L) | 47.99 (35.45 to 60.52) | 35.06 (27.58 to 42.54) |
| AST (U/L) | 27.91 (22.83 to 33.00) | 22.08 (18.48 to 25.68) |
| TC (mmol/L) | 4.81 (4.49 to 5.13) | 4.74 (4.46 to 5.02) |
| TG (mmol/L) | 1.64 (1.34 to 1.94) | 1.64 (1.36 to 1.93) |
| LDL-c (mmol/L) | 3.10 (2.77 to 3.44) | 3.04 (2.76 to 3.32) |
| HDL-c (mmol/L) | 1.28 (1.21 to 1.35) | 1.31 (1.22 to 1.41) |
| Cr (umol/L) | 62.06 (60.24 to 63.88) | 60.94 (58.68 to 63.19) |
| SUA (umol/L) | 396.27 (371.54 to 421.00) | 372.60 (340.88 to 404.32) |
| LH (IU/L) | 9.06 (7.34 to 10.79) | 11.62 (9.73 to 13.50) |
| FSH (IU/L) | 5.45 (4.69 to 6.20) | 5.54 (5.08 to 5.99) |
| PRL (mIU/L) | 379.84 (322.33 to 437.36) | 356.00 (304.60 to 407.39) |
| TT (nmol/L) | 1.76 (1.48 to 2.04) | 1.84 (1.60 to 2.07) |
| FT (pg/mL) | 2.53 (2.13 to 2.93) | 2.40 (1.99 to 2.81) |
| AD (ng/mL) | 4.16 (3.55 to 4.77) | 4.61 (3.46 to 5.76) |
| DHEAS (ug/dl) | 227.83 (193.64 to 262.02) | 225.00 (182.71 to 267.29) |
| SHBG (nmol/L) | 29.74 (16.80 to 42.67) | 23.96 (14.59 to 33.33) |
| FAI | 0.09 (0.07 to 0.11) | 0.11 (0.08 to 0.13) |
| CAP (dB/m) | 332.69 (314.13 to 351.25) | 318.70 (300.50 to 336.89) |
| LSM (kPa) | 5.95 (5.29 to 6.61) | 5.82 (5.00 to 6.64) |
| Total body fat (%) | 43.27 (42.08 to 44.46) | 43.57 (42.25 to 44.89) |
| Total body lean (%) | 53.50 (52.37 to 54.64) | 52.75 (51.44 to 54.07) |
| Total fat mass (kg) | 33.03 (30.94 to 35.11) | 33.50 (31.20 to 35.80) |
| Total lean mass (kg) | 40.68 (39.13 to 42.23) | 40.29 (37.85 to 42.73) |
| VAT area (cm2) | 163.03 (146.19 to 179.88) | 156.74 (140.42 to 173.05) |
| SAT mass (kg) | 1.96 (1.79 to 2.13) | 1.92 (1.73 to 2.12) |
| Variables | Dulaglutide Combined with CRD Therapy, LS Mean Change (95% CI) (n = 35) | CRD Therapy, LS Mean Change (95% CI) (n = 33) | LS Mean Difference between Groups (95% CI) | p |
|---|---|---|---|---|
| Weight (kg) | −5.42 (−5.79 to −5.05) | −5.44 (−5.84 to −5.04) | 0.02 (−0.53 to 0.57) | 0.945 |
| WC (cm) | −4.34 (−5.55 to −3.14) | −3.61 (−5.05 to −2.17) | −0.73 (−2.61 to 1.15) | 0.435 |
| BMI (kg/m2) | −2.06 (−2.25 to −1.87) | −2.23 (−2.43 to −2.02) | 0.17 (−0.11 to 0.45) | 0.232 |
| Menstrual Cycles (no./yr) | 0.42 (0.01 to 0.83) | 0.79 (0.32 to 1.26) | −0.37 (−0.99 to 0.25) | 0.236 |
| SBP (mmHg) | −9.29 (−13.27 to −5.30) | −6.04 (−10.58 to −1.51) | −3.24 (−9.30 to 2.81) | 0.284 |
| DBP (mmHg) | −7.30 (−10.36 to −4.23) | −6.44 (−9.93 to −2.95) | −0.86 (−5.52 to 3.80) | 0.711 |
| FPG (mmol/L) | −0.30 (−0.50 to −0.10) | −0.07 (−0.29 to 0.15) | −0.23 (−0.53 to 0.07) | 0.131 |
| PPG (mmol/L) | −2.12 (−2.82 to −1.41) | −0.86 (−1.65 to −0.08) | −1.26 (−2.31 to −0.20) | 0.021 |
| FINS (mU/L) | −7.33 (−12.14 to −2.51) | −7.37 (−12.57 to −2.17) | 0.04 (−7.05 to 7.13) | 0.990 |
| PINS (mU/L) | −44.11 (−89.04 to 0.82) | −23.37 (−75.98 to 29.25) | −20.74 (−91.08 to 49.60) | 0.552 |
| HbA1c (%) | −0.28 (−0.41 to −0.15) | −0.06 (−0.20 to 0.09) | −0.23 (−0.42 to −0.03) | 0.027 |
| HOMA-IR | −1.84 (−2.95 to −0.74) | −1.63 (−2.83 to −0.44) | −0.21 (−1.84 to 1.42) | 0.794 |
| ALT (U/L) | −16.61 (−21.49 to −11.73) | −14.09 (−19.24 to −8.94) | −2.52 (−9.74 to 4.70) | 0.484 |
| AST (U/L) | −6.38 (−8.14 to −4.62) | −6.54 (−8.46 to −4.62) | 0.16 (−2.52 to 2.84) | 0.904 |
| TC (mmol/L) | −0.62 (−0.83 to −0.41) | −0.52 (−0.75 to −0.28) | −0.11 (−0.42 to 0.21) | 0.509 |
| TG (mmol/L) | −0.59 (−0.73 to −0.45) | −0.46 (−0.61 to −0.31) | −0.13 (−0.34 to 0.08) | 0.205 |
| LDL-c (mmol/L) | −0.37 (−0.54 to −0.21) | −0.38 (−0.56 to −0.21) | 0.01 (−0.23 to 0.25) | 0.949 |
| HDL-c (mmol/L) | −0.12 (−0.21 to −0.04) | −0.06 (−0.14 to 0.03) | −0.07 (−0.19 to 0.05) | 0.259 |
| Cr (umol/L) | −0.29 (−2.22 to 1.63) | 1.49 (−0.54 to 3.53) | −1.78 (−4.62 to 1.05) | 0.209 |
| SUA (umol/L) | −24.27 (−53.04 to 4.50) | −44.73 (−76.75 to −12.70) | 20.46 (−22.91 to 63.82) | 0.345 |
| LH (IU/L) | −0.56 (−4.66 to 3.54) | 3.33 (−1.10 to 7.75) | −3.89 (−9.98to 2.21) | 0.205 |
| FSH (IU/L) | −0.56 (−1.50 to 0.39) | 0.26 (−0.74 to 1.26) | −0.82 (−2.19 to 0.56) | 0.237 |
| PRL (mIU/L) | 57.91 (−27.24 to 143.06) | 7.76 (−85.04 to 100.55) | 50.15 (−75.82 to 176.13) | 0.423 |
| TT (nmol/L) | −0.06 (−0.26 to 0.14) | −0.22 (−0.43 to −0.01) | 0.16 (−0.13 to 0.45) | 0.274 |
| FT (pg/mL) | −0.04 (−0.57 to 0.48) | −0.35 (−0.93 to 0.22) | 0.31 (−0.48 to 1.10) | 0.427 |
| AD (ng/mL) | 0.04 (−0.63 to 0.71) | −0.62 (−1.23 to −0.02) | 0.67 (−0.24 to 1.57) | 0.143 |
| DHEAS (ug/dl) | 4.75 (−24.39 to 33.89) | 18.02 (−13.02 to 49.07) | −13.27 (−56.05 to 29.51) | 0.531 |
| SHBG (nmol/L) | −1.60 (−9.14 to 5.93) | 1.52 (−6.01 to 9.06) | −3.13 (−13.96 to 7.70) | 0.557 |
| FAI | 0.03 (0.01 to 0.04) | 0.04 (0.02 to 0.05) | −0.01 (−0.03 to 0.01) | 0.351 |
| CAP (dB/m) | −12.46 (−30.56 to 5.64) | −22.35 (−43.91 to −0.79) | 9.89 (−18.32 to 38.10) | 0.478 |
| LSM (kPa) | −0.83 (−1.53 to −0.13) | −0.35 (−1.18 to 0.48) | −0.49 (−1.57 to 0.60) | 0.368 |
| Total body fat (%) | −0.09 (−1.21 to 1.04) | 0.09 (−1.15 to 1.33) | −0.18 (−1.86 to 1.51) | 0.833 |
| Total body lean (%) | −0.20 (−1.33 to 0.92) | −0.36 (−1.63 to 0.91) | 0.16 (−1.55 to 1.87) | 0.852 |
| Total fat mass (kg) | −2.11 (−2.98 to −1.23) | −1.95 (−2.93 to −0.96) | −0.16 (−1.48 to 1.17) | 0.810 |
| Total lean mass (kg) | −3.14 (−3.98 to −2.31) | −2.77 (−3.71 to −1.82) | −0.38 (−1.64 to 0.88) | 0.548 |
| SAT mass (kg) | −0.25 (−0.35 to −0.14) | −0.17 (−0.29 to −0.06) | −0.07 (−0.23 to 0.08) | 0.351 |
| Event | Dulaglutide Combined with CRD Therapy (n = 35) | CRD Therapy (n = 33) |
|---|---|---|
| No. of Participants (%) | No. of Participants (%) | |
| Adverse events-related discontinuation | 2 (5.71) | 0 (0) |
| Patients with ≥1 GI TEAE | 13 (37.14) | 0 (0) |
| Nausea | 8 (22.86) | 0 (0) |
| Vomiting | 7 (20.00) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) |
| Constipation | 4 (11.43) | 0 (0) |
| Loss of appetite | 4 (11.43) | 0 (0) |
| Abdominal distension | 2 (5.71) | 0 (0) |
| Abdominal pain | 1 (2.86) | 0 (0) |
| Eructation | 1 (2.86) | 0 (0) |
| Sensations of hunger | 0(0) | 3(9.09) |
| Hypoglycemia | 0 (0) | 0 (0) |
| Dizziness | 3 (8.57) | 1 (3.03) |
| Injection site reaction | 0 (0) | 0 (0) |
| Upper respiratory tract infection | 0 (0) | 0 (0) |
| Headache | 1 (2.86) | 0 (0) |
| Nasopharyngitis | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qu, Z.; Lu, T.; Shao, X.; Cai, M.; Dilimulati, D.; Gao, X.; Mao, W.; Hu, F.; Su, L.; et al. Effects of a Dulaglutide plus Calorie-Restricted Diet versus a Calorie-Restricted Diet on Visceral Fat and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial. Nutrients 2023, 15, 556. https://doi.org/10.3390/nu15030556
Zhang Y, Qu Z, Lu T, Shao X, Cai M, Dilimulati D, Gao X, Mao W, Hu F, Su L, et al. Effects of a Dulaglutide plus Calorie-Restricted Diet versus a Calorie-Restricted Diet on Visceral Fat and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial. Nutrients. 2023; 15(3):556. https://doi.org/10.3390/nu15030556
Chicago/Turabian StyleZhang, Yuqin, Zhihua Qu, Ting Lu, Xiaowen Shao, Meili Cai, Diliqingna Dilimulati, Xinxin Gao, Weiqing Mao, Fan Hu, Lili Su, and et al. 2023. "Effects of a Dulaglutide plus Calorie-Restricted Diet versus a Calorie-Restricted Diet on Visceral Fat and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial" Nutrients 15, no. 3: 556. https://doi.org/10.3390/nu15030556
APA StyleZhang, Y., Qu, Z., Lu, T., Shao, X., Cai, M., Dilimulati, D., Gao, X., Mao, W., Hu, F., Su, L., Liao, Q., Han, T., Zhang, M., & Qu, S. (2023). Effects of a Dulaglutide plus Calorie-Restricted Diet versus a Calorie-Restricted Diet on Visceral Fat and Metabolic Profiles in Women with Polycystic Ovary Syndrome: A Randomized Controlled Trial. Nutrients, 15(3), 556. https://doi.org/10.3390/nu15030556







