Dietary Liberalization in Tetrahydrobiopterin-Treated PKU Patients: Does It Improve Outcomes?
Abstract
1. Introduction
2. Materials and Methods
2.1. Use of Guidelines
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Collection and Data Items
2.6. Data Analysis
2.7. Risk of Bias and Certainty of Evidence
3. Results
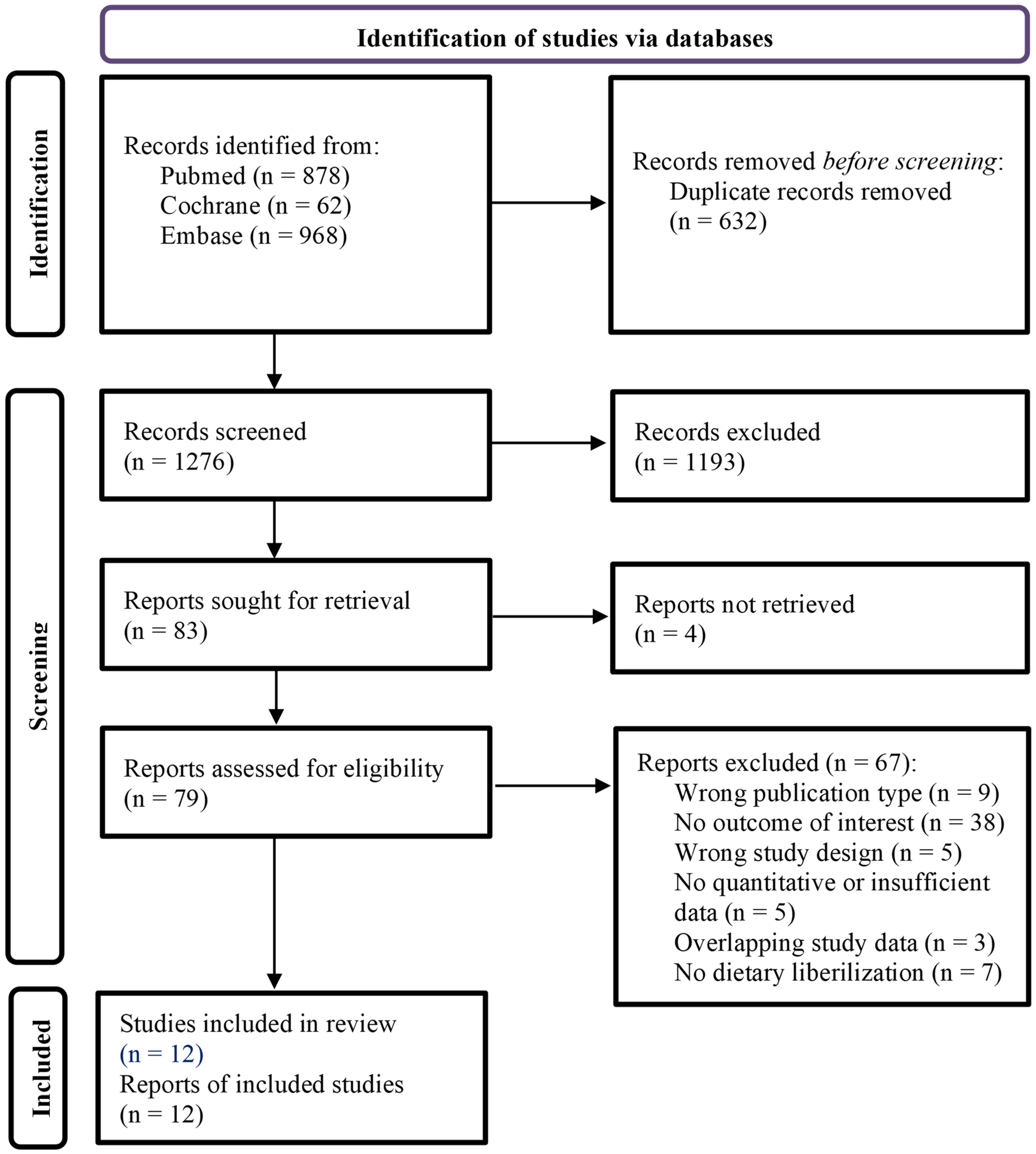
3.1. Study Selection
3.2. Study Characteristics
| Study (Reference) | Main Study Characteristics | Characteristics of BH4-Treated Patients at Baseline | Direct Effects of BH4 Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Country | Study Design | Type of Control Group | Patient Number | Gender (% Female) | Age, Mean ± SD (Years) | Follow-Up Time (Months) | Change 1 in Blood Phe Concentrations | Change 1 in Dietary Phe Intake 2 | Change 1 in Protein Substitute Intake | |
| Lambruschini 2005 [27] | Spain | P | None | 11 | 64 | 5.0 ± 4.2 | 12 | 16% increase | 4.3-fold increase 3 | 100% decrease |
| Singh 2010 [28] | USA | P | None | 6 | 0 | n/r | 24 | 10% decrease 5 | 3.3-fold increase 4 | 84% decrease |
| Ziesch 2012 [29] | Germany | P | Non-BH4-treated PKU patients | 8 | 50 | 11.1 ± 4.4 | 3 | 7% increase | 3.4-fold increase 3 | n/r |
| Aldámiz-Echevarría 2013 (1) [25] | Spain | R | Non-BH4-treated PKU patients | 36 | 50 | 5.0 ± 4.6 | 24 | 43% increase | 1.4-fold increase 4 | 44% decrease |
| Aldámiz-Echevarría 2013 (2) [25] | 10 | 40 | 5.2 ± 3.1 | 60 | 42% increase | 1.2-fold increase 4 | 57% decrease | |||
| Demirdas 2013 [30] | The Netherlands | P | Non-BH4-treated PKU patients | 10 | n/r | 13.8 ± 9.7 | n/r | n/r | 4.1-fold increase 3 | n/r |
| Douglas 2013 [31] | USA | P | Non-BH4-treated PKU patients | 11 | n/r | n/r | 12 | 33% decrease 5 | 3.8-fold increase 3,5 | 85% decrease5 |
| Scala 2015 [32] | Italy | P | None | 17 | n/r | n/r | 62 6 | 53% increase | 1.7-fold increase 3 | n/r |
| Tansek 2016 [33] | Slovenia | P | None | 9 | n/r | 6.2 ± 3.1 | 24 | 5% decrease | 3.2-fold increase 3 | 93% decrease |
| Feldmann 2017 [34] | Germany | P | Non-BH4-treated PKU patients | 20 | 35 | 12.5 | 6 | n/r | 2.6-fold increase 4 | 42% decrease |
| Brantley 2018 [35] | USA | P | Healthy controls and non-BH4-treated PKU patients | 18 | 44 | 16.6 ± 10.3 | 12 | 23% decrease | 1.5-fold increase | 66% decrease |
| Evers 2018 [36] | The Netherlands | R | Non-BH4-treated PKU patients | 21 | 67 | 13.1 ± 9.2 | 60 | 3% decrease 5 | 1.5-fold increase 4 | 68% decrease |
| Muntau 2021 (1) [26] | Austria, Belgium, Czech Republic, Germany, Italy, The Netherlands, Slovakia, Turkey, UK | P | None | 25 | 40 | 1.7 ± 1.0 | 36 | 6% decrease 5 | 2.0-fold increase5 | n/r |
| Muntau 2021 (2) [26] | 26 | 46 | 1.7 ± 1.0 | 36 | 12% decrease 5 | 1.1-fold increase 5 | n/r | |||
3.3. Results of the Systematic Review and Meta-Analyses
3.3.1. Anthropometric Measurements
Results of Individual Studies
Results from Meta-Analyses
3.3.2. Nutritional Biomarkers
Results of Individual Studies
Results from Meta-Analyses
3.3.3. Quality of Life
Results of Individual Studies
Results from Meta-Analyses
3.3.4. Bone Density, Mental Health and Psychosocial Functioning, and Burden of Care
3.4. Assessment of Risk of Bias Assessment and Certainty of Evidence
3.4.1. Risk of Bias in Individual Studies
3.4.2. Risk of Reporting Bias
3.4.3. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Prim. 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Burlina, A.P.; Lachmann, R.H.; Manara, R.; Cazzorla, C.; Celato, A.; van Spronsen, F.J.; Burlina, A. The neurological and psychological phenotype of adult patients with early-treated phenylketonuria: A systematic review. J. Inherit. Metab. Dis. 2019, 42, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Montoya Parra, G.A.; Singh, R.H.; Cetinyurek-Yavuz, A.; Kuhn, M.; MacDonald, A. Status of nutrients important in brain function in phenylketonuria: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2018, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.J.; de Lamas, C.; Sánchez-Pintos, P.; González-Lamuño, D.; Couce, M.L. Bone Status in Patients with Phenylketonuria: A Systematic Review. Nutrients 2020, 12, 2154. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Pinto, A.; Faria, A.; Teixeira, D.; van Wegberg, A.M.J.; Ahring, K.; Feillet, F.; Calhau, C.; MacDonald, A.; Moreira-Rosário, A.; et al. Is the Phenylalanine-Restricted Diet a Risk Factor for Overweight or Obesity in Patients with Phenylketonuria (PKU)? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3443. [Google Scholar] [CrossRef]
- Ilgaz, F.; Pinto, A.; Gökmen-Özel, H.; Rocha, J.C.; Van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Karabulut, E.; Macdonald, A. Long-term growth in phenylketonuria: A systematic review and meta-analysis. Nutrients 2019, 11, 2070. [Google Scholar] [CrossRef]
- Lindegren, M.L.; Krishnaswami, S.; Reimschisel, T.; Fonnesbeck, C.; Sathe, N.A.; McPheeters, M.L. A systematic review of BH4 (Sapropterin) for the adjuvant treatment of phenylketonuria. In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8, pp. 109–119. [Google Scholar]
- Somaraju, U.R.; Merrin, M. Sapropterin dihydrochloride for phenylketonuria. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Qu, J.; Yang, T.; Wang, E.; Li, M.; Chen, C.; Ma, L.; Zhou, Y.; Cui, Y. Efficacy and safety of sapropterin dihydrochloride in patients with phenylketonuria: A meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2019, 85, 893–899. [Google Scholar] [CrossRef]
- Burton, B.K.; Bausell, H.; Katz, R.; LaDuca, H.; Sullivan, C. Sapropterin therapy increases stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU). Mol. Genet. Metab. 2010, 101, 110–114. [Google Scholar] [CrossRef]
- Lotz-Havla, A.S.; Weiß, K.; Schiergens, K.; Regenauer-Vandewiele, S.; Parhofer, K.G.; Christmann, T.; Böhm, L.; Havla, J.; Maier, E.M. Optical Coherence Tomography to Assess Neurodegeneration in Phenylalanine Hydroxylase Deficiency. Front. Neurol. 2021, 12, 2196. [Google Scholar] [CrossRef]
- Ilgaz, F.; Marsaux, C.; Pinto, A.; Singh, R.; Rohde, C.; Karabulut, E.; Gökmen-özel, H.; Kuhn, M.; Macdonald, A. Protein Substitute Requirements of Patients with Phenylketonuria on BH4 Treatment: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1040. [Google Scholar] [CrossRef]
- Garbade, S.F.; Shen, N.; Himmelreich, N.; Haas, D.; Trefz, F.K.; Hoffmann, G.F.; Burgard, P.; Blau, N. Allelic phenotype values: A model for genotype-based phenotype prediction in phenylketonuria. Genet. Med. 2018, 21, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Muntau, A.C.; Röschinger, W.; Habich, M.; Demmelmair, H.; Hoffmann, B.; Sommerhoff, C.P.; Roscher, A.A. Tetrahydrobiopterin as an Alternative Treatment for Mild Phenylketonuria. N. Engl. J. Med. 2002, 347, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Dokoupil, K.; Gokmen-Ozel, H.; Lammardo, A.M.; Motzfeldt, K.; Robert, M.; Rocha, J.C.; van Rijn, M.; Ahring, K.; Bélanger-Quintana, A.; MacDonald, A. Optimising growth in phenylketonuria: Current state of the clinical evidence base. Clin. Nutr. 2012, 31, 16–21. [Google Scholar] [CrossRef]
- Singh, R.H.; Cunningham, A.C.; Mofidi, S.; Douglas, T.D.; Frazier, D.M.; Hook, D.G.; Jeffers, L.; McCune, H.; Moseley, K.D.; Ogata, B.; et al. Updated, web-based nutrition management guideline for PKU: An evidence and consensus based approach. Mol. Genet. Metab. 2016, 118, 72–83. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Côté, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Longo, N.; Siriwardena, K.; Feigenbaum, A.; Dimmock, D.; Burton, B.K.; Stockler, S.; Waisbren, S.; Lang, W.; Jurecki, E.; Zhang, C.; et al. Long-term developmental progression in infants and young children taking sapropterin for phenylketonuria: A two-year analysis of safety and efficacy. Genet. Med. 2015, 17, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, L.; Bueno, M.A.; Couce, M.L.; Lage, S.; Dalmau, J.; Vitoria, I.; Llarena, M.; Andrade, F.; Blasco, J.; Alcalde, C.; et al. 6R-tetrahydrobiopterin treated PKU patients below 4years of age: Physical outcomes, nutrition and genotype. Mol. Genet. Metab. 2015, 115, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Muntau, A.C.; Burlina, A.; Eyskens, F.; Freisinger, P.; De Laet, C.; Leuzzi, V.; Rutsch, F.; Sivri, H.S.; Vijay, S.; Bal, M.O.; et al. Efficacy, safety and population pharmacokinetics of sapropterin in PKU patients <4 years: Results from the SPARK open-label, multicentre, randomized phase IIIb trial. Orphanet J. Rare Dis. 2017, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Aldámiz-Echevarría, L.; Bueno, M.A.; Couce, M.L.; Lage, S.; Dalmau, J.; Vitoria, I.; Andrade, F.; Llarena, M.; Blasco, J.; Alcalde, C.; et al. Tetrahydrobiopterin therapy vs phenylalanine-restricted diet: Impact on growth in PKU. Mol. Genet. Metab. 2013, 109, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Muntau, A.C.; Burlina, A.; Eyskens, F.; Freisinger, P.; Leuzzi, V.; Sivri, H.S.; Gramer, G.; Pazdírková, R.; Cleary, M.; Lotz-Havla, A.S.; et al. Long-term efficacy and safety of sapropterin in patients who initiated sapropterin at <4 years of age with phenylketonuria: Results of the 3-year extension of the SPARK open-label, multicentre, randomised phase IIIb trial. Orphanet J. Rare Dis. 2021, 16, 341. [Google Scholar] [CrossRef]
- Lambruschini, N.; Pérez-Dueñas, B.; Vilaseca, M.A.; Mas, A.; Artuch, R.; Gassió, R.; Gómez, L.; Gutiérrez, A.; Campistol, J. Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol. Genet. Metab. 2005, 86, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.H.; Quirk, M.E.; Douglas, T.D.; Brauchla, M.C. BH4 therapy impacts the nutrition status and intake in children with phenylketonuria: 2-Year follow-up. J. Inherit. Metab. Dis. 2010, 33, 689–695. [Google Scholar] [CrossRef]
- Ziesch, B.; Weigel, J.; Thiele, A.; Mütze, U.; Rohde, C.; Ceglarek, U.; Thiery, J.; Kiess, W.; Beblo, S. Tetrahydrobiopterin (BH4) in PKU: Effect on dietary treatment, metabolic control, and quality of life. J. Inherit. Metab. Dis. 2012, 35, 983–992. [Google Scholar] [CrossRef]
- Demirdas, S.; Maurice-Stam, H.; Boelen, C.C.A.; Hofstede, F.C.; Janssen, M.C.H.; Langendonk, J.G.; Mulder, M.F.; Rubio-Gozalbo, M.E.; van Spronsen, F.J.; de Vries, M.; et al. Evaluation of quality of life in PKU before and after introducing tetrahydrobiopterin (BH4); a prospective multi-center cohort study. Mol. Genet. Metab. 2013, 110, S49–S56. [Google Scholar] [CrossRef]
- Douglas, T.D.; Ramakrishnan, U.; Kable, J.A.; Singh, R.H. Longitudinal quality of life analysis in a phenylketonuria cohort provided sapropterin dihydrochloride. Health Qual. Life Outcomes 2013, 11, 218. [Google Scholar] [CrossRef]
- Scala, I.; Concolino, D.; Della Casa, R.; Nastasi, A.; Ungaro, C.; Paladino, S.; Capaldo, B.; Ruoppolo, M.; Daniele, A.; Bonapace, G.; et al. Long-term follow-up of patients with phenylketonuria treated with tetrahydrobiopterin: A seven years experience. Orphanet J. Rare Dis. 2015, 10, 14. [Google Scholar] [CrossRef]
- Tansek, M.Z.; Groselj, U.; Kelvisar, M.; Kobe, H.; Lampret, B.R.; Battelino, T. Long-term BH4 (sapropterin) treatment of children with hyperphenylalaninemia—Effect on median Phe/Tyr ratios. J. Pediatr. Endocrinol. Metab. 2016, 29, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.; Wolfgart, E.; Weglage, J.; Rutsch, F. Sapropterin treatment does not enhance the health-related quality of life of patients with phenylketonuria and their parents. Acta Paediatr. Int. J. Paediatr. 2017, 106, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Brantley, K.D.; Douglas, T.D.; Singh, R.H. One-year follow-up of B vitamin and Iron status in patients with phenylketonuria provided tetrahydrobiopterin (BH4). Orphanet J. Rare Dis. 2018, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Evers, R.A.F.; van Wegberg, A.M.J.; van Dam, E.; de Vries, M.C.; Janssen, M.C.H.; van Spronsen, F.J. Anthropomorphic measurements and nutritional biomarkers after 5 years of BH 4 treatment in phenylketonuria patients. Mol. Genet. Metab. 2018, 124, 238–242. [Google Scholar] [CrossRef]
- Drug Approval Package: Kuvan (Sapropterin Dihydrochloride) NDA #022181. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022181TOC.cfm (accessed on 11 August 2021).
- Kuvan|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kuvan (accessed on 11 August 2021).
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Walkowiak, D.; Kaluzny, L.; Bukowska-Posadzy, A.; Oltarzewski, M.; Staszewski, R.; Moczko, J.A.; Musielak, M.; Walkowiak, J. Overweight in classical phenylketonuria children: A retrospective cohort study. Adv. Med. Sci. 2019, 64, 409–414. [Google Scholar] [CrossRef]
- Thiele, A.G.; Weigel, J.F.; Ziesch, B.; Rohde, C.; Mütze, U.; Ceglarek, U.; Thiery, J.; Müller, A.S.; Kiess, W.; Beblo, S. Nutritional changes and micronutrient supply in patients with phenylketonuria under therapy with tetrahydrobiopterin (BH4). In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9, pp. 31–40. [Google Scholar]
- Thiele, A.G.; Rohde, C.; Mütze, U.; Arelin, M.; Ceglarek, U.; Thiery, J.; Baerwald, C.; Kiess, W.; Beblo, S. The challenge of long-term tetrahydrobiopterin (BH4) therapy in phenylketonuria: Effects on metabolic control, nutritional habits and nutrient supply. Mol. Genet. Metab. Rep. 2015, 4, 62–67. [Google Scholar] [CrossRef]
- Diener, E.; Lucas, R.E.; Scollon, C.N. Beyond the hedonic treadmill: Revising the adaptation theory of well-being. Am. Psychol. 2006, 61, 305–314. [Google Scholar] [CrossRef]
- Bosch, A.M.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Koledova, E.; Benmedjahed, K.; Regnault, A. Assessment of the impact of phenylketonuria and its treatment on quality of life of patients and parents from seven European countries. Orphanet J. Rare Dis. 2015, 10, 80. [Google Scholar] [CrossRef]
- Huijbregts, S.C.J.; Bosch, A.M.; Simons, Q.A.; Jahja, R.; Brouwers, M.C.G.J.; De Sonneville, L.M.J.; De Vries, M.C.; Hofstede, F.C.; Hollak, C.E.M.; Janssen, M.C.H.; et al. The impact of metabolic control and tetrahydrobiopterin treatment on health related quality of life of patients with early-treated phenylketonuria: A PKU-COBESO study. Mol. Genet. Metab. 2018, 125, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Regnault, A.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Benmedjahed, K.; Bosch, A.M. Development and psychometric validation of measures to assess the impact of phenylketonuria and its dietary treatment on patients’ and parents’ quality of life: The phenylketonuria—Quality of life (PKU-QOL) questionnaires. Orphanet J. Rare Dis. 2015, 10, 59. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Main Result | Patient Number | Included Cohorts | Weighted Means Based on Full-Cohort Data (Means in Individual Cohorts) | ||||
|---|---|---|---|---|---|---|---|---|
| Age (Years) | Follow-Up Time (Months) | Change in Blood Phe Concentrations (%) | Change in Dietary Phe Intake (Fold Increase) | |||||
| Anthropometric measurements | ||||||||
| Weight | Within-subject | p = 0.61 | 67 | Aldámiz-Echevarría 2013 (1) and (2); Evers 2018 | 7.6 (5.0; 5.2; 13.1) | 41 (24; 60; 60) | 28 (43; 42; −3) | 1.4 (1.4; 1.2; 1.5) |
| Between-subject | p = 0.95 | |||||||
| BMI | Within-subject | p = 0.82 | 73 | Singh 2010; Aldámiz-Echevarría 2013 (1) and (2); Evers 2018 | 7.6 (n/r; 5.0; 5.2; 13.1) | 39 (24; 24; 60; 60) | 25 (−10; 43; 42; −3) | 1.6 (3.3; 1.4; 1.2; 1.5) |
| Between-subject | p = 0.02, ↑ | 67 | Aldámiz-Echevarría 2013 (1) and (2); Evers 2018 | 7.6 (5.0; 5.2; 13.1) | 41 (24; 60; 60) | 28 (43; 42; −3) | 1.4 (1.4; 1.2; 1.5) | |
| Height | Within-subject | p = 0.80 | 62 | Singh 2010; Aldámiz-Echevarría 2013 (1) and (2); Evers 2018 | 7.6 (n/r; 5.0; 5.2; 13.1) | 39 (24; 24; 60; 60) | 25 (−10; 43; 42; −3) | 1.6 (3.3; 1.4; 1.2; 1.5) |
| Between-subject | p = 0.43 | 56 | Aldámiz-Echevarría 2013 (1) and (2); Evers 2018 | 7.6 (5.0; 5.2; 13.1) | 41 (24; 60; 60) | 28 (43; 42; −3) | 1.4 (1.4; 1.2; 1.5) | |
| Growth velocity | Within-subject | p = 0.20 | 56 | Aldámiz-Echevarría 2013 (1) and (2); Evers 2018 | 7.6 (5.0; 5.2; 13.1) | 41 (24; 60; 60) | 28 (43; 42; −3) | 1.4 (1.4; 1.2; 1.5) |
| Between-subject | p = 0.73 | |||||||
| Nutritional biomarkers | ||||||||
| Albumin | Within-subject | p = 0.40 | 38 | Lambruschini 2005; Singh 2010; Evers 2018 | 10.3 (5.0; n/r; 13.1) | 40 (12; 24; 60) | −8 (−16; −10; −3) | 1.5 (4.3; 3.3; 1.5) |
| Cholesterol | Within-subject | p = 0.01, ↑ | 27 | Singh 2010; Evers 2018 | 13.1 (n/r; 13.1) | 52 (24; 60) | −5 (−10; −3) | 1.9 (3.3; 1.5) |
| Haemoglobin | Within-subject | p = 0.11 | ||||||
| Quality of life | ||||||||
| HR-QoL (patient-report) | Within-subject | p = 0.86 | 33 | Ziesch 2012; Demirdas 2013; Feldmann 2017 | 12.5 (11.1; 13.8; 12.5) | 5 (3; n/r; 6) | 7 (7; n/r; n/r) | 3.2 (3.4; 4.1; 2.6) |
| Between-subject | p = 0.92 | |||||||
| HR-QoL (proxy-report) | Within-subject | p = 0.14 | 33 | Ziesch 2012; Demirdas 2013; Feldmann 2017 | 12.5 (11.1; 13.8; 12.5) | 5 (3; n/r; 6) | 7 (7; n/r; n/r) | 3.2 (3.4; 4.1; 2.6) |
| Between-subject | p = 0.22 | |||||||
| Results | Cohort Characteristics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study/Analyses | Weight | Body Mass Index | Height | Growth | Head Circumference | Brachial Muscular Area | Brachial Adipose Area | Number of Patients | Age at Baseline (Years) | Follow-Up Time (Months) | Change in Blood Phe Concentration | Fold Increase in Dietary Phe Intake |
| Within-subject analyses | ||||||||||||
| Lambruschini 2005 [27] | = | = | = | ↑ | 11 | 5.0 ± 4.2 | 12 | +16% | 4.3 | |||
| Singh 2010 [28] | = | ↑ | 6 | n/r | n/r | −10% | 3.3 | |||||
| Aldámiz-Echevarría 2013 (1) [25] | = | = | = | = | 36 | 5.0 ± 4.6 | 24 | +43% | 1.4 | |||
| Aldámiz-Echevarría 2013 (2) [25] | = | = | = | = | 10 | 5.2 ± 3.1 | 60 | +42% | 1.2 | |||
| Scala 2015 [32] | ↑ | 17 | n/r | 62 | +53% | 1.7 | ||||||
| Tansek 2016 [33] | = | = | 9 | 6.2 ± 3.1 | 24 | −5% | 3.2 | |||||
| Evers 2018 [36] | ↑ | = | = | = | 21 | 13.1 ± 9.2 | 60 | −3% | 1.5 | |||
| Muntau 2021 (1) [26] | = | = | = | = | 25 | 1.7 ± 1.0 | 36 | −6% | 2.0 | |||
| Muntau 2021 (2) [26] | = | = | = | = | 26 | 1.7 ± 1.0 | 36 | −12% | 1.1 | |||
| Between-subject analyses | ||||||||||||
| Aldámiz-Echevarría 2013 (1) [25] | ↓ | = | = | = | 36 | 5.0 ± 4.6 | 24 | +43% | 1.4 | |||
| Aldámiz-Echevarría 2013 (2) [25] | = | = | = | = | 10 | 5.2 ± 3.1 | 60 | +42% | 1.2 | |||
| Evers 2018 [36] | = | = | = | = | 21 | 13.1 ± 9.2 | 60 | −3% | 1.5 | |||
| Results | Cohort Characteristics | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study/Analyses | Cholesterol | Triglycerides | Iron | Transferrin | Ferritin | Albumin | Total Protein | Transthyretin | Vitamin A | Vitamin B6 | Folate | Vitamin B12 | MMA | Calcifedol | Vitamin E | Calcium | Phsophate | Selenium | Zinc | Haemoglobin | Haematocrit | Number of Patients | Age at Baseline (Years) | Follow-Up Time (Months) | Change in Blood Phe Concentration | Fold increase in Dietary Phe Intake |
| Within-subject analyses | ||||||||||||||||||||||||||
| Lambruschini 2005 [27] | = | = | = | = | = | = | ↑ | = | 11 | 5.0 ± 4.2 | 12 | +16% | 4.3 | |||||||||||||
| Singh 2010 [28] | = | = | = | ↑ | ↑ | ↑ | 6 | n/r | n/r | −10% | 3.3 | |||||||||||||||
| Tansek 2016 [33] | = | = | = | 9 | 6.2 ± 3.1 | 24 | −5% | 3.2 | ||||||||||||||||||
| Brantley 2018 [35] | = | = | = | ↓/= * | 18 | 16.6 ± 10.3 | 12 | −23% | 1.5 | |||||||||||||||||
| Evers 2018 [36] | ↑ | = | = | = | ↓ | = | = | ↓ | ↑ | 21 | 13.1 ± 9.2 | 60 | −3% | 1.5 | ||||||||||||
| Between-subject analyses | ||||||||||||||||||||||||||
| Brantley 2018 [35] | = | = | = | ↓/= * | 18 | 16.6 ± 10.3 | 12 | −23% | 1.5 | |||||||||||||||||
| Evers 2018 [36] | = | = | = | = | = | = | = | = | = | 21 | 13.1 ± 9.2 | 60 | −3% | 1.5 | ||||||||||||
| Results | Cohort Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study/Analyses | Generic HR-QoL of Patients | Specified HR-QoL of Patients | Parental QoL | Number of Patients | Age at Baseline (Years) | Follow-Up Time (Months) | Change in Blood Phe Concentration | Fold increase in Dietary Phe Intake | ||
| Patient Report | Proxy Report | Patient Report | Proxy Report | |||||||
| Within-subject analyses | ||||||||||
| Ziesch 2012 [29] | = | = | 8 | 11.4 ± 4.4 | 3 | +7% | 3.4 | |||
| Demirdas 2013 [30] | = | = | = | = | 10 | 13.8 ± 9.7 | n/r | n/r | 4.1 | |
| Douglas 2013 [31] | ↑ | 11 | n/r | 12 | −33% | 3.8 | ||||
| Between-subject analyses | ||||||||||
| Ziesch 2012 [29] | = | = | 8 | 11.4 ± 4.4 | 3 | +7% | 3.4 | |||
| Demirdas 2013 [30] | = | = | = | = | 10 | 13.8 ± 9.7 | n/r | n/r | 4.1 | |
| Douglas 2013 [31] | = | 11 | n/r | 12 | −33% | 3.8 | ||||
| Feldmann 2017 [34] | = | = | = | 20 | 12.5 | 6 | n/r | 2.6 | ||
| Domain | Data Available | Main Conclusion | Recommendations for Care | Domain-Specific Recommendations for Research |
|---|---|---|---|---|
| Anthropometric measurements | Data from nine cohorts. | No clear effect on most anthropometric measures; possibly a small increase in weight and BMI. | Physicians and dieticians should pay attention to the nutritional status of BH4-treated PKU patients; continued nutritional counselling and education are important to ensure a balanced diet. | Anthropometric measurements should include z-scores for weight, height, and BMI for all patients, and head circumference and growth speed for paediatric patients specifically. |
| Nutritional biomarkers | Data from five cohorts. | No clear effect on most nutritional biomarkers; possibly an increase in blood cholesterol concentrations. | Nutritional biomarkers should at least include homocysteine and/or MMA, haemoglobin, MCV, and ferritin. Other biomarkers, such as cholesterol and 25-hydroxyvitamin D, may be added. | |
| Quality of life | Data from four cohorts. | No clear effect on quality of life. | Issues such as QoL, psychosocial outcomes and mental health, and burden of care should be assessed with the use of PKU-specific (instead of generic) questionnaires, such as the PKU-QoL questionnaire. | |
| Burden of care | No available data. | |||
| Mental health and psychosocial functioning | No available data. | |||
| Bone density | No available data. | Bone density measurements should be investigated (e.g., using DEXA). | ||
| General recommendations for research |
| |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evers, R.A.F.; van Wegberg, A.M.J.; MacDonald, A.; Huijbregts, S.C.J.; Leuzzi, V.; van Spronsen, F.J. Dietary Liberalization in Tetrahydrobiopterin-Treated PKU Patients: Does It Improve Outcomes? Nutrients 2022, 14, 3874. https://doi.org/10.3390/nu14183874
Evers RAF, van Wegberg AMJ, MacDonald A, Huijbregts SCJ, Leuzzi V, van Spronsen FJ. Dietary Liberalization in Tetrahydrobiopterin-Treated PKU Patients: Does It Improve Outcomes? Nutrients. 2022; 14(18):3874. https://doi.org/10.3390/nu14183874
Chicago/Turabian StyleEvers, Roeland A. F., Annemiek M. J. van Wegberg, Anita MacDonald, Stephan C. J. Huijbregts, Vincenzo Leuzzi, and Francjan J. van Spronsen. 2022. "Dietary Liberalization in Tetrahydrobiopterin-Treated PKU Patients: Does It Improve Outcomes?" Nutrients 14, no. 18: 3874. https://doi.org/10.3390/nu14183874
APA StyleEvers, R. A. F., van Wegberg, A. M. J., MacDonald, A., Huijbregts, S. C. J., Leuzzi, V., & van Spronsen, F. J. (2022). Dietary Liberalization in Tetrahydrobiopterin-Treated PKU Patients: Does It Improve Outcomes? Nutrients, 14(18), 3874. https://doi.org/10.3390/nu14183874







