Is Hepatocellular Carcinoma in Fatty Liver Different to Non-Fatty Liver?
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
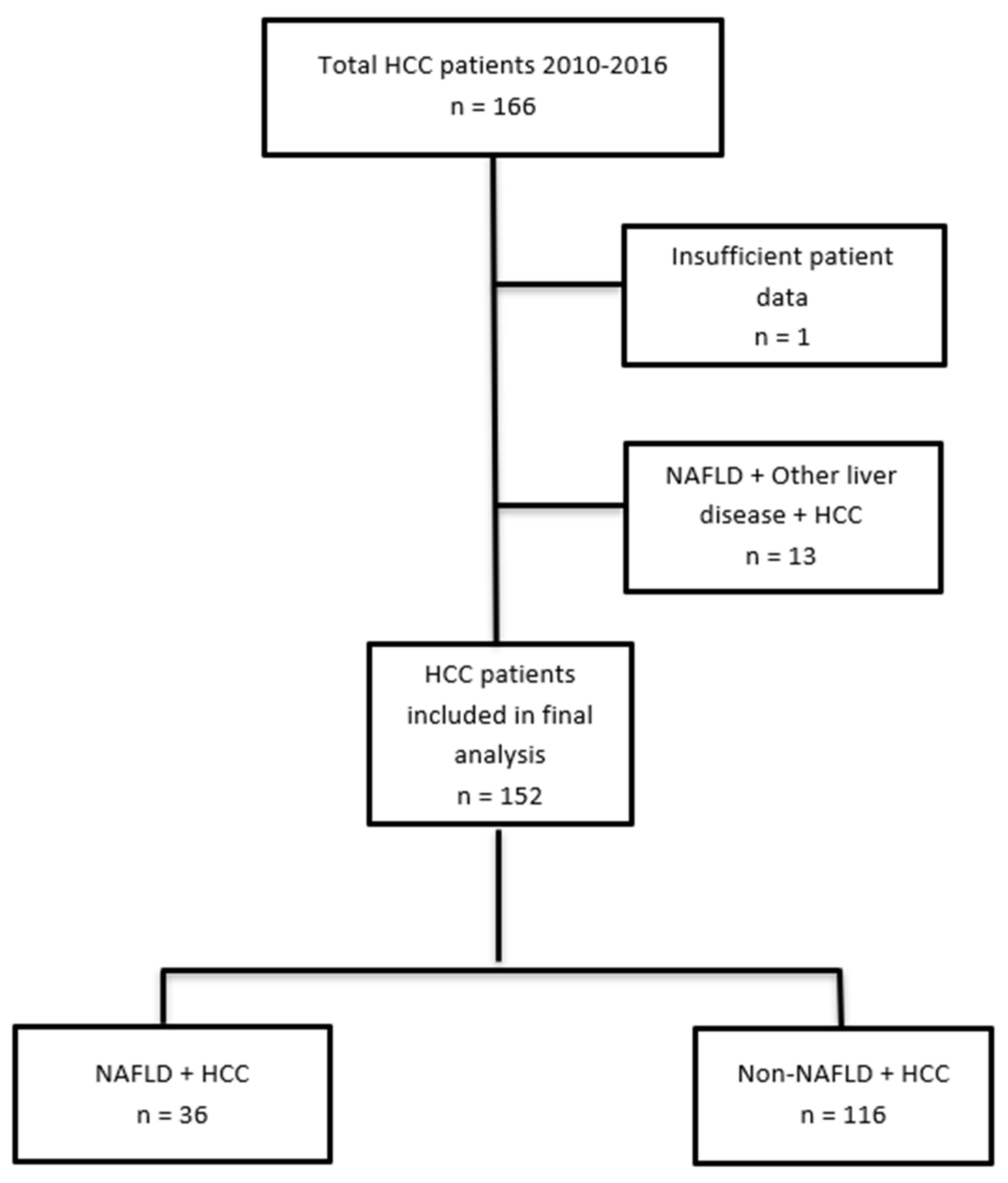
3.1. Study Population
3.2. Treatment of HCC
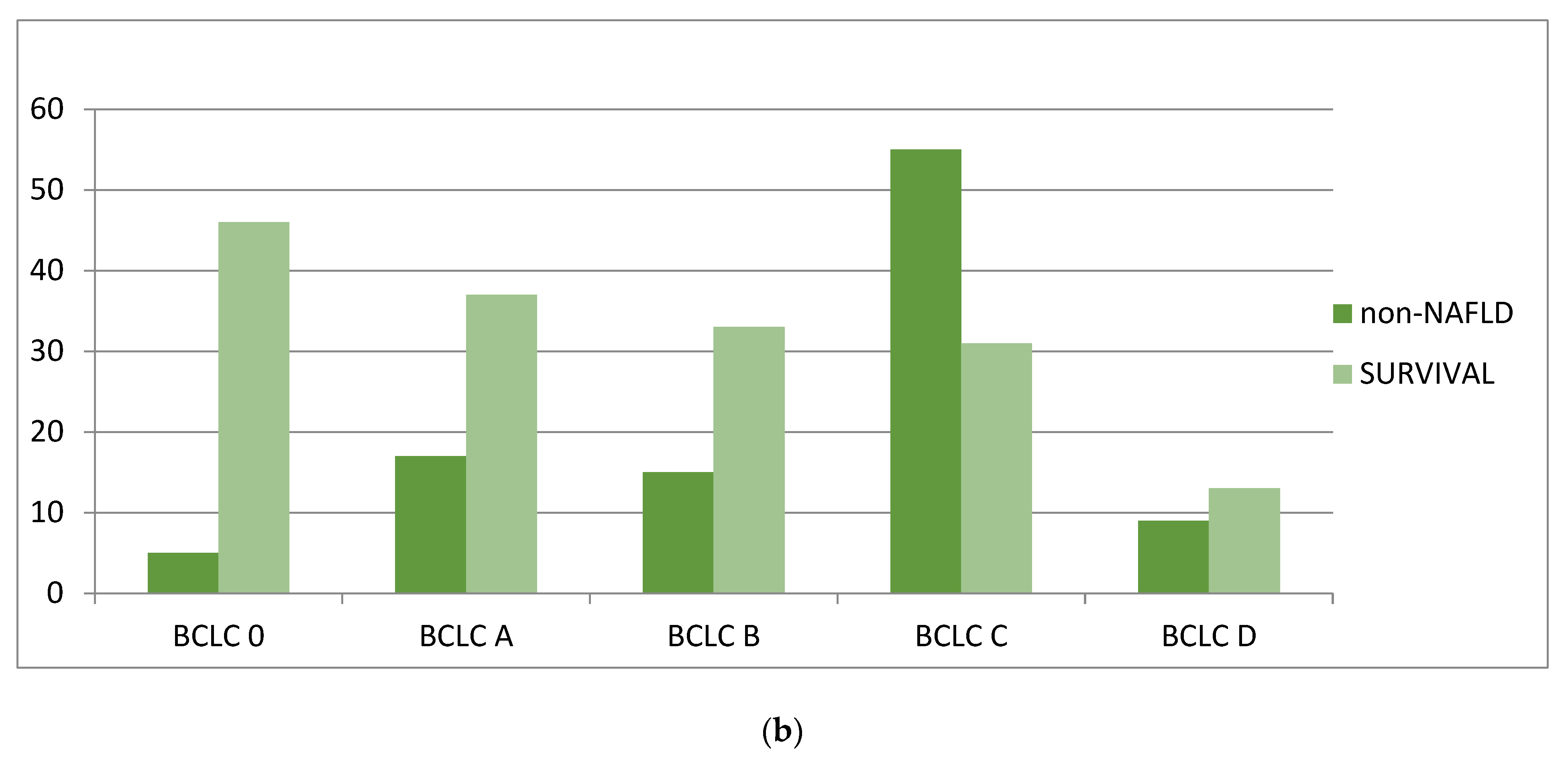
3.3. Overall Survival Analysis
3.4. Predictors of Overall Survival in Patients with NAFLD and Non-NAFLD-HCC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| HCC | Hepatocellular carcinoma |
| HBV | Hepatitis B |
| HBC | Hepatitis C |
| AASLD | American Association of the Study of the Liver |
| BMI | Body mass index |
| BCLC | Barcelona Clinic Liver Cancer |
| mRECIST | Modified Response Evaluation Criteria in Solid Tumours |
| INR | International Normalised Ratio/Prothrombin time |
| AFP | Alpha-fetoprotein |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| GGT | Gamma-glutamyltransferase |
| ALP | Alkaline phosphatase |
| CI | Confidence interval |
| DEB-TACE | Drug-eluting bead transarterial chemoembolization |
| IL-6 | Interleukin-6 |
| TNF-α | Tumour necrosis factor alpha |
| IGF | Insulin-like growth factor |
| CP | Child–Pugh |
| SIRTEX | SIR-Spheres Yttrium-90 resin microspheres |
| RFA | Radiofrequency ablation |
| MWA | Microwave ablation |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- El–Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Flaxman, A.D.; Naghavi, M.; Fitzmaurice, C.; Vos, T.; Abubakar, I.; Abu-Raddad, L.J.; Assadi, R.; Bhala, N.; Cowie, B.; et al. The global burden of viral hepatitis from 1990 to 2013: Findings from the Global Burden of Disease Study 2013. Lancet 2016, 388, 1081–1088. [Google Scholar] [CrossRef]
- Yu, M.C.; Yuan, J.-M.; Lu, S.C. Alcohol, cofactors and the genetics of hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2008, 23 (Suppl. 1), S92–S97. [Google Scholar] [CrossRef]
- Deloitte Access Economics. The economic cost and health burden of liver diseases in Australia. In Gastroenterological Society of Australia; Australian Liver Association: Sydney, Australia, 2013. [Google Scholar]
- Bellentani, S.; Tiribelli, C. The spectrum of liver disease in the general population: Lesson from the Dionysos study. J. Hepatol. 2001, 35, 531–537. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Mahady, S.E.; Adams, L.A. Burden of non-alcoholic fatty liver disease in Australia. J. Gastroenterol. Hepatol. 2018, 33 (Suppl. 1), 1–11. [Google Scholar] [CrossRef]
- Reddy, S.K.; Steel, J.L.; Chen, H.W.; DeMateo, D.J.; Cardinal, J.; Behari, J.; Humar, A.; Marsh, J.W.; Geller, D.A.; Tsung, A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 2012, 55, 1809–1819. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2016, 14, 124–131.e1. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; American Association for the Study of Liver, D. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. NHMRC Australian Guidelines to Reduce Health Risks from Drinking Alcohol. 2009. Available online: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/ds10-alcohol.pdf (accessed on 24 November 2017).
- Desai, A.; Sandhu, S.; Lai, J.-P.; Sandhu, D.S. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J. Hepatol. 2019, 11, 1–18. [Google Scholar] [CrossRef]
- Rahman, R.; Hammoud, G.M.; Almashhrawi, A.A.; Ahmed, K.T.; Ibdah, J.A. Primary hepatocellular carcinoma and metabolic syndrome: An update. World J. Gastrointest. Oncol. 2013, 5, 186–194. [Google Scholar] [CrossRef]
- Ali Kamkar, M.M.; Ahmad, R.; Alsmadi, O.; Behbehani, K. Insight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: Mini-review. J. Diabetes Metab. Disord. 2014, 13, 57. [Google Scholar] [CrossRef]
- Chen, H.P.; Shieh, J.J.; Chang, C.C.; Chen, T.T.; Lin, J.T.; Wu, M.S.; Lin, J.-H.; Wu, C.-Y. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: Population-based and in vitro studies. Gut 2013, 62, 606–615. [Google Scholar] [CrossRef]
- George, A.J.; Thomas, W.G.; Hannan, R.D. The renin–angiotensin system and cancer: Old dog, new tricks. Nat. Rev. Cancer 2010, 10, 745. [Google Scholar] [CrossRef]
- Hester, C.A.; Rich, N.E.; Singal, A.G.; Yopp, A.C. Comparative Analysis of Nonalcoholic Steatohepatitis- Versus Viral Hepatitis- and Alcohol-Related Liver Disease-Related Hepatocellular Carcinoma. J. Natl. Compr. Cancer Netw. JNCCN 2019, 17, 322–329. [Google Scholar] [CrossRef]
- Arnaoutakis, D.J.; Mavros, M.N.; Shen, F.; Alexandrescu, S.; Firoozmand, A.; Popescu, I.; Weiss, M.; Wolfgang, C.L.; Choti, M.A.; Pawlik, T.M. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: A multi-institutional analysis. Ann. Surg. Oncol. 2014, 21, 147–154. [Google Scholar] [CrossRef]
- Than, N.N.; Ghazanfar, A.; Hodson, J.; Tehami, N.; Coldham, C.; Mergental, H.; Manas, D.; Shah, T.; Newsome, P.N.; Reeves, H.; et al. Comparing clinical presentations, treatments and outcomes of hepatocellular carcinoma due to hepatitis C and non-alcoholic fatty liver disease. QJM Mon. J. Assoc. Physicians 2017, 110, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Paradis, V.; Zalinski, S.; Chelbi, E.; Guedj, N.; Degos, F.; Vilgrain, V.; Bedossa, P.; Belghiti, J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology (Baltimore, Md) 2009, 49, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.H.; Lee, L.T.; Hung, S.H.; Lin, W.Y.; Hung, H.F.; Yang, W.S.; Sung, P.-K.; Huang, K.-C. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology 2014, 59, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, S.; Pipitone, R.M.; Pennisi, G.; Celsa, C.; Camma, C.; Di Marco, V.; Barcellona, M.R.; Boemi, R.; Enea, M.; Giannetti, A.; et al. Association Between PNPLA3 rs738409 C>G Variant and Liver-Related Outcomes in Patients with Non-alcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 18, 935–944.e3. [Google Scholar]
- Yoneda, M.; Imajo, K.; Takahashi, H.; Ogawa, Y.; Eguchi, Y.; Sumida, Y.; Yoneda, M.; Kawanaka, M.; Saito, S.; Tokushige, K.; et al. Clinical strategy of diagnosing and following patients with nonalcoholic fatty liver disease based on invasive and noninvasive methods. J. Gastroenterol. 2018, 53, 181–196. [Google Scholar] [CrossRef]
- EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [CrossRef]
- Belghiti, J.; Kianmanesh, R. Surgical treatment of hepatocellular carcinoma. HPB 2005, 7, 42–49. [Google Scholar] [CrossRef]
- Wang, P.; Kang, D.; Cao, W.; Wang, Y.; Liu, Z. Diabetes mellitus and risk of hepatocellular carcinoma: A systematic review and meta-analysis. Diabetes/Metab. Res. Rev. 2012, 28, 109–122. [Google Scholar] [CrossRef]
- Arase, Y.; Kobayashi, M.; Suzuki, F.; Suzuki, Y.; Kawamura, Y.; Akuta, N.; Imai, N.; Kobayashi, M.; Sezaki, H.; Matsumoto, N.; et al. Difference in malignancies of chronic liver disease due to non-alcoholic fatty liver disease or hepatitis C in Japanese elderly patients. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2012, 42, 264–272. [Google Scholar] [CrossRef]
- Leung, C.; Yeoh, S.W.; Patrick, D.; Ket, S.; Marion, K.; Gow, P.; Gow, P.; Angus, P.W. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J. Gastroenterol. 2015, 21, 1189–1196. [Google Scholar] [CrossRef]


| NAFLD (n = 36) | Non-NAFLD (n = 116) | p-Value | |
|---|---|---|---|
| Patient characteristics | |||
| Age at diagnosis, years (mean, 95% CI) | 71.9 (69.4–74.3) | 62.6 (60.7–64.4) | <0.001 |
| Male, % | 24 (66.7) | 106 (91.4) | <0.001 |
| Body mass index (mean, 95% CI) (kg/m2) | 29.1 (26.8–31.4) | 26.2 (25.1–27.4) | 0.02 |
| Hypertension, % | 23 (63.9) | 32 (29.1) | <0.001 |
| Type 2 Diabetes | 28 (78%) | 15 (13%) | <0.01 |
| Hyperlipidaemia | 11 (31%) | 5 (4%) | <0.01 |
| Ethnicity, % | 0.199 | ||
| Asian | 3 (8.3) | 28 (24.1) | 0.199 |
| Caucasian | 32 (88.9) | 84 (72.4) | 0.199 |
| Hispanic | Nil | 1 (0.9) | 0.199 |
| Middle Eastern | 1 (2.8) | 3 (2.6) | 0.199 |
| Diagnosis method, % | 0.11 | ||
| Incidental | 3 (8.6) | 10 (8.9) | 0.99 |
| Screening | 15(42.8) | 69 (61.6) | 0.08 |
| Symptomatic | 17 (48.6) | 33 (29.5) | 0.046 |
| BCLC stage at diagnosis, % | 0.49 | ||
| 0 | Nil | 6 (5.2) | 0.34 |
| A | 8 (22.2) | 19 (16.4) | 0.46 |
| B | 4 (11.1) | 17 (14.6) | 0.78 |
| C | 19 (52.8) | 64 (55.2) | 0.85 |
| D | 5 (13.9) | 10 (8.6) | 0.05 |
| Cirrhosis, % | 29 (80.6) | 103 (90.4) | 0.12 |
| Stage of cirrhosis, % | 0.16 | ||
| Non-cirrhotic | 7 (19.5) | 11 (9.6) | 0.16 |
| CPA | 17 (47.2) | 62 (54.4) | 0.16 |
| CPB | 12 (33.3) | 33 (29.0) | 0.16 |
| CPC | Nil | 8 (7.0) | 0.16 |
| HCC characteristics at diagnosis | |||
| Lesion size (mean, 95% CI) | 5.4 (3.9 to 6.8) | 4.4 (3.7 to 5.1) | 0.20 |
| Number of lesions (mean, 95% CI) | 1.5 (1.2 to 1.9) | 1.7 (1.4 to 1.9) | 0.53 |
| AFP (mean, 95% CI) | 2882.5 (−1859.0 to 7623.9) | 5078.6 (−1204.1 to 11361.3) | 0.72 |
| Liver Disease | Patient Number (%) |
|---|---|
| Alcoholic liver disease | 35 (30%) |
| Hepatitis B | 27 (23%) |
| Hepatitis C | 18 (16%) |
| Hepatitis B + Hepatitis C | 1 (1%) |
| Hepatitis B + alcoholic liver disease | 2 (2%) |
| Hepatitis C + alcoholic liver disease | 29 (25%) |
| Hepatitis B + Hepatitis C + alcoholic liver disease | 1 (1%) |
| Autoimmune hepatitis | 1 (1%) |
| Haemochromatosis + alcoholic liver disease | 1 (1%) |
| Primary biliary cholangitis | 1 (1%) |
| NAFLD | Non-NAFLD | p-Value | |
|---|---|---|---|
| Initial Treatment, % | <0.001 | ||
| Resection | 12 (33.3) | 11 (9.5) | |
| DEB-TACE | 6 (16.7) | 55 (47.4) | |
| SIRTEX | Nil | 6 (5.2) | |
| RFA | 2 (5.6) | 12 (10.3) | |
| MWA | 1 (2.8) | 2 (1.7) | |
| Sorafenib | 1 (2.8) | 9 (7.8) | |
| Best supportive care | 11 (30.6) | 20 (17.2) | |
| Transplant | 1 (2.8) | Nil | |
| DEB-TACE + Resection | Nil | 1 (0.9) | |
| DEB-TACE + RFA | 1 (2.8) | Nil | |
| DEB-TACE + SIRTEX | 1 (2.8) | Nil | |
| Total number of treatments (mean, 95% CI) | 1.57 (1.02–2.13) | 1.84 (1.55–2.14) | 0.192 |
| NAFLD | Non-NAFLD | p-Value | |
|---|---|---|---|
| Deceased, % | 20 (55.6) | 64 (58.2) | 0.78 |
| Cause of death, % | |||
| Liver | 15 (88.2) | 44 (88.0) | 0.98 |
| Non-liver | 2 (11.8) | 6 (12.0) | 0.98 |
| Liver transplant, % | 1 (2.8) | 5 (4.4) | 0.67 |
| Survival from diagnosis, months | |||
| Mean | 25.3 | 30.1 | 0.23 |
| Median | 17.2 | 23.5 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, X.-v.K.; Zhang, J.; Chin, K.L.; Bloom, S.; Nicoll, A.J. Is Hepatocellular Carcinoma in Fatty Liver Different to Non-Fatty Liver? Nutrients 2022, 14, 3875. https://doi.org/10.3390/nu14183875
Nguyen X-vK, Zhang J, Chin KL, Bloom S, Nicoll AJ. Is Hepatocellular Carcinoma in Fatty Liver Different to Non-Fatty Liver? Nutrients. 2022; 14(18):3875. https://doi.org/10.3390/nu14183875
Chicago/Turabian StyleNguyen, Xuan-vinh Kevin, Jason Zhang, Ken Lee Chin, Stephen Bloom, and Amanda J. Nicoll. 2022. "Is Hepatocellular Carcinoma in Fatty Liver Different to Non-Fatty Liver?" Nutrients 14, no. 18: 3875. https://doi.org/10.3390/nu14183875
APA StyleNguyen, X.-v. K., Zhang, J., Chin, K. L., Bloom, S., & Nicoll, A. J. (2022). Is Hepatocellular Carcinoma in Fatty Liver Different to Non-Fatty Liver? Nutrients, 14(18), 3875. https://doi.org/10.3390/nu14183875






