The Impact of Purple-Flesh Potato (Solanum tuberosum L.) cv. “Shadow Queen” on Minor Health Complaints in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
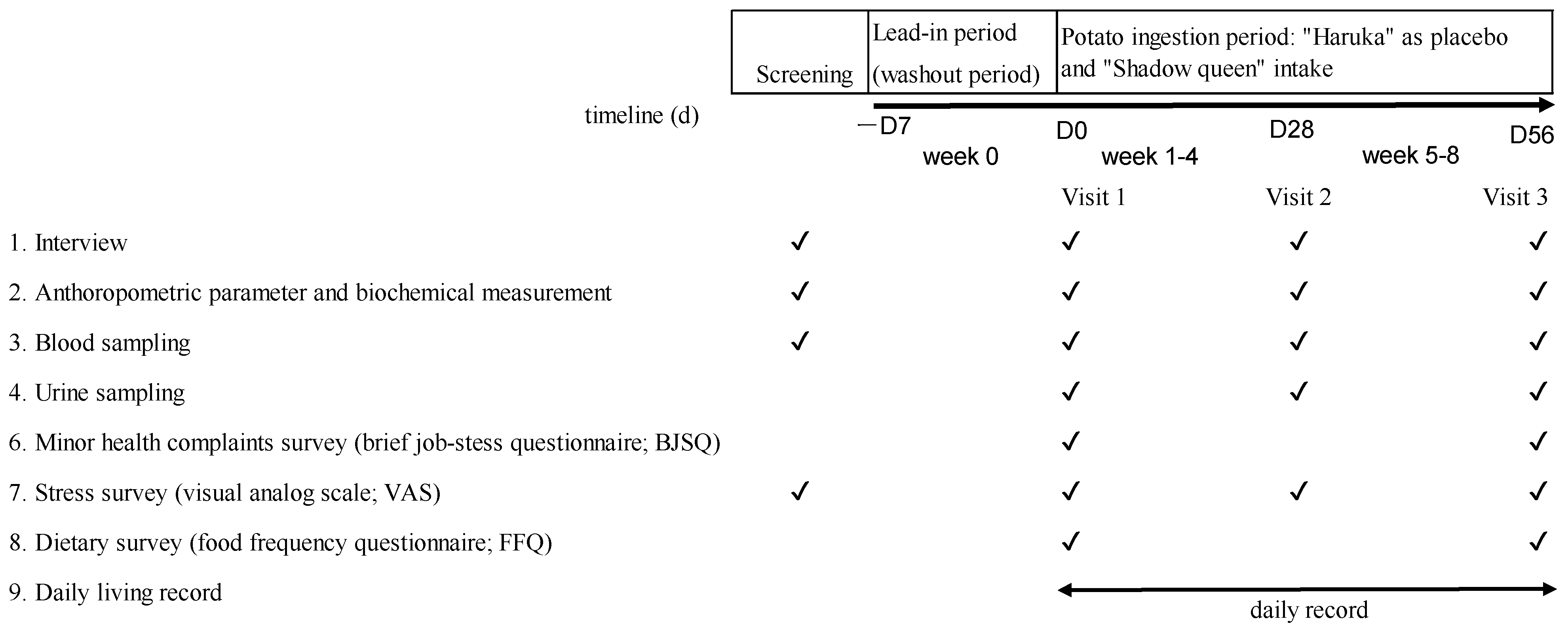
2.1. Study Design
2.2. Subjects
2.3. Test Samples
2.4. Study Outcomes
2.5. Measurement of Peripheral Blood MSC Count
2.6. Measurement of the BJSQ
2.7. Measurement of VAS Questionnaire for Fatigue
2.8. Measurement of Hematological, Biological, and Diet Survey
2.9. Ethics Committee
2.10. Statistical Analysis
3. Results and Discussion
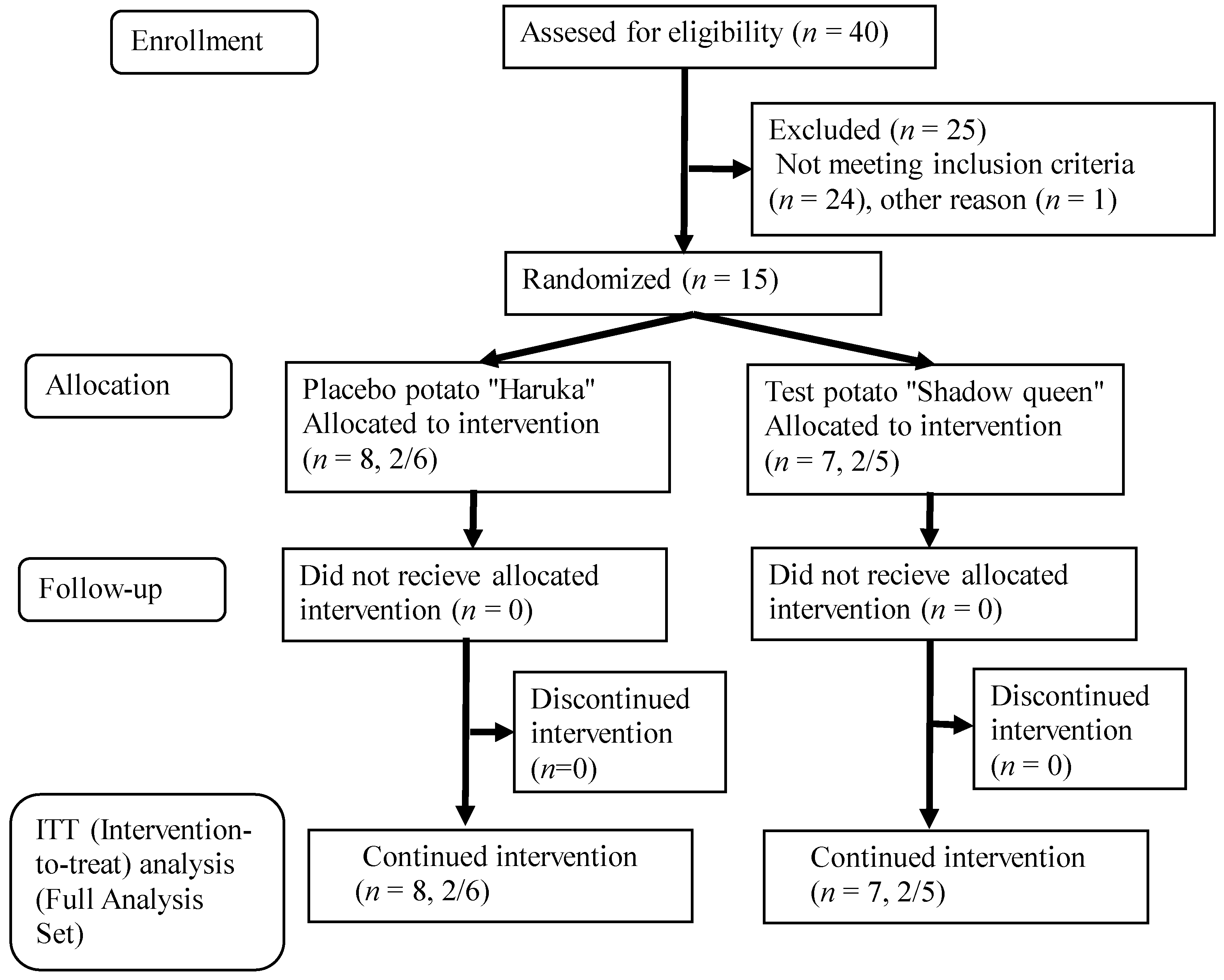
3.1. Number of Subjects and Intake Rate of Test Potatoes
3.2. Physical Characteristics
3.3. Primary Outcomes
3.4. Secondary Outcomes
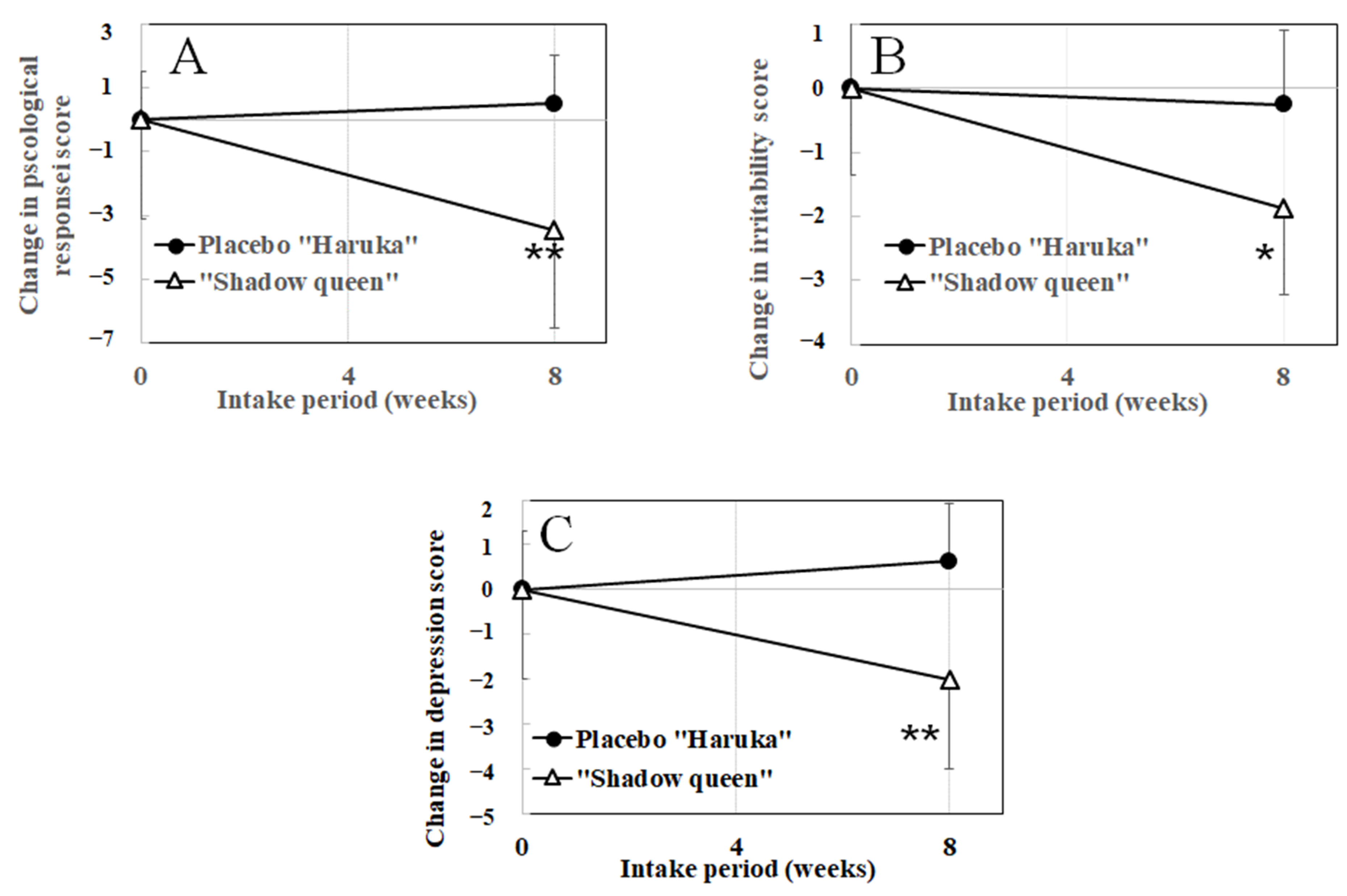
3.4.1. Effect of “Shadow Queen” on Stress Responses Measured Using the BJSQ
3.4.2. The Effect of “Shadow Queen” on the VAS Questionnaire
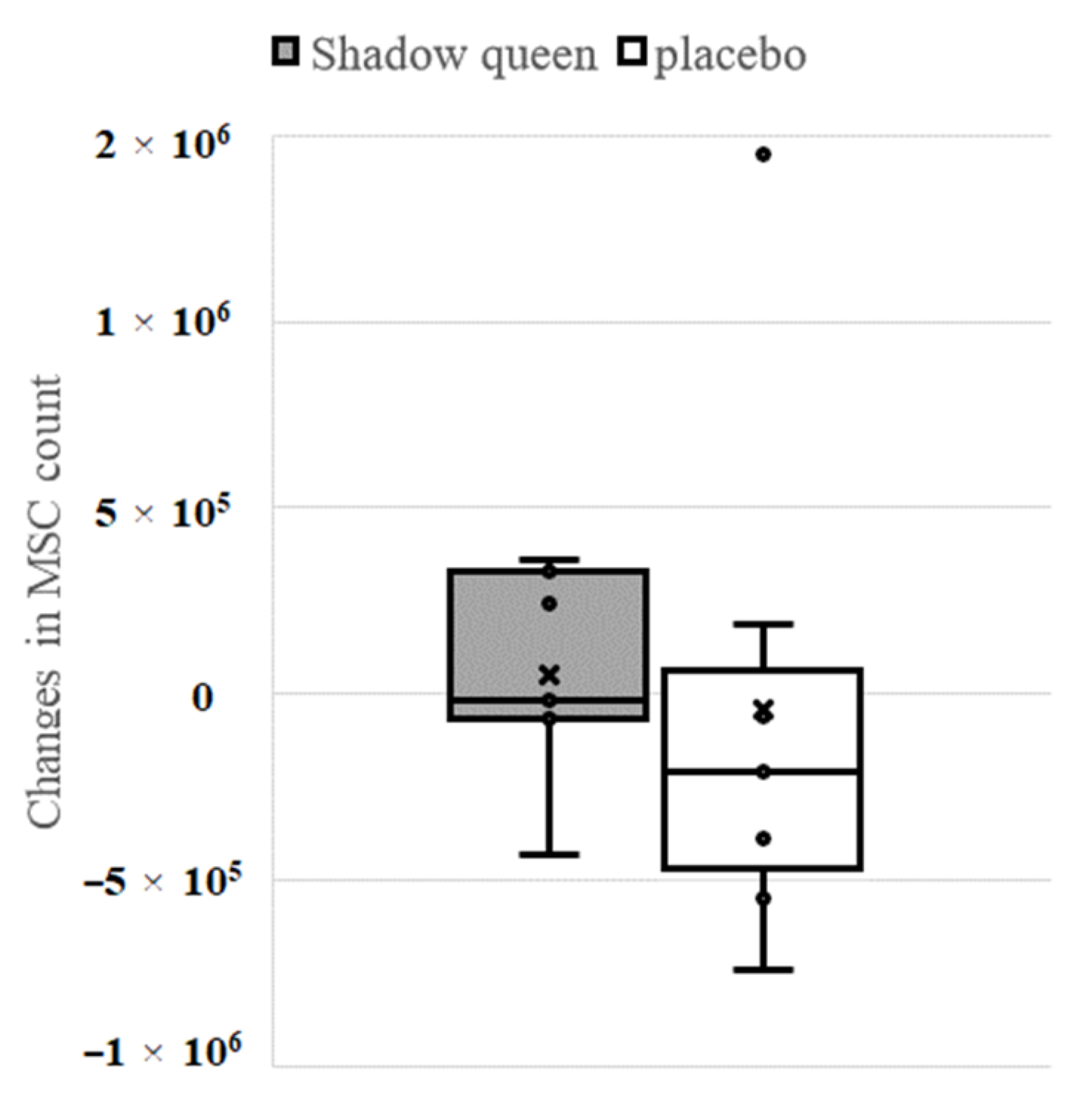
3.4.3. The Effect of “Shadow Queen” on Another Secondary Endpoint
3.5. Safety Assesment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Health, Labor and Welfare. Basic Survey on Industrial Safety and Health. Available online: https://www.mhlw.go.jp/toukei/list/list46-50_an-ji.html (accessed on 25 May 2022). (In Japanese).
- Nagata, T.; Mori, K.; Ohtani, M.; Nagata, M.; Kajiki, S.; Fujino, Y.; Matsuda, S.; Loeppke, R. Total health-related costs due to absenteeism, Presenteeism, and medical and pharmaceutical Eexpenses in Japanese employers. J. Occup. Environ. Med. 2018, 60, e273–e280. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Takenami, E.; Iwasaki-Kurashige, K.; Osada, T.; Katsumura, T.; Hamaoka, T. Effects of blackcurrant anthocyanin intake on peripheral muscle circulation during typing work in humans. Eur. J. Appl. Physiol. 2005, 94, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, O.; Takahashi, R.; Takahashi, T. The effect of a beverage containing Cordyceps sinensis mycelium extract and Ayamurasaki anthocyanin on the improvement of mental function. J. New Rem. Clin. 2000, 49, 21–30. (In Japanese) [Google Scholar]
- Morikubo, K.; Uchida, K.; Kume, A.; Tsunoo, H.; Tajima, T.; Nagata, A. Effect of a mixture of mycelial extract of cultured Coediceps sinensis and ayamurasakianthocyanin on the mental condition of middle-aged people. Jpn. Pharmacol. Ther. 2005, 33, 729–734. (In Japanese) [Google Scholar]
- Chong, E.S.L.; McGhie, T.K.; Heyes, J.A.; Stowell, K.M. Metabolite profiling and quantification of phytochemicals in potato extracts using ultra-high-performance liquid chromatography-mass spectrometry. J. Sci. Food Agric. 2013, 93, 3801–3808. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant, anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J. Funct. Foods 2013, 5, 930–939. [Google Scholar] [CrossRef]
- Sun, X.F.; Du, M.; Navarre, D.A.; Zhu, M.J. Purple potato extract promotes intestinal epithelial differentiation and barrier function by activating amp-activated protein kinase. Mol. Nutr. Food Res. 2018, 62, 1700536. [Google Scholar] [CrossRef]
- Han, K.H.; Hashimoto, N.; Shimada, K.; Sekikawa, M.; Noda, T.; Yamauchi, H.; Hashimoto, M.; Chiji, H.; Topping, D.L.; Fukushima, M. Hepatoprotective effects of purple potato extract against d-galactosamine-induced liver injury in rats. Biosci. Biotechnol. Biochem. 2006, 70, 1432–1437. [Google Scholar] [CrossRef] [Green Version]
- Han, K.H.; Matsumoto, A.; Shimada, K.; Sekikawa, M.; Fukushima, M. Effects of anthocyanin-rich purple potato flakes on antioxidant status in f344 rats fed a cholesterol-rich diet. Br. J. Nutr. 2007, 98, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Han, K.H.; Kim, S.J.; Shimada, K.; Hashimoto, N.; Yamauchi, H.; Fukushima, M. Purple potato flake reduces serum lipid profile in rats fed a cholesterol-rich diet. J. Funct. Foods 2013, 5, 974–980. [Google Scholar] [CrossRef]
- Strugala, P.; Dzydzan, O.; Brodyak, I.; Kucharska, A.Z.; Kuropka, P.; Liuta, M.; Kaleta-Kuratewicz, K.; Przewodowska, A.; Michalowska, D.; Gabrielska, J.; et al. Antidiabetic and antioxidative potential of the blue congo variety of purple potato extract in streptozotocin-induced diabetic rats. Molecules 2019, 24, 3126. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.; Hayashi, K.; Ohara-Takada, K.; Watanuki, H.; Katahira, R.; Ono, H.; Terahara, N. Anthocyanins from skins and fleshes of potato varieties. Food Sci. Technol. Res. 2010, 16, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Hayakawa, K.; Sansawa, H. Involvement of γ-aminobutyric acid (GABA) B receptors in the hypotensive effect of systemically administered GABA in spontaneously hypertensive rats. Jpn. J. Pharmacol. 2002, 89, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Kimura, M.; Kamata, K. Mechanism underlying gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur. J. Pharmacol. 2002, 438, 107–113. [Google Scholar] [CrossRef]
- Nakamura, H.; Takishima, T.; Kometani, T.; Yokogoshi, H. Psychological stress-reducing effect of chocolate enriched with gamma-aminobutyric acid (GABA) in humans: Assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009, 60, 106–113. [Google Scholar] [CrossRef]
- Fujibayashi, M.; Kamiya, T.; Takagaki, K.; Moritani, T. Activation of autonomic nervous system activity by the oral ingestion of GABA. J. Jpn. Soc. Nutr. Food Sci. 2008, 61, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Okita, Y.; Nakamura, H.; Kouda, K.; Takahashi, I.; Takaoka, T.; Kimura, M.; Sugiura, T. Effects of vegetable containing gamma-aminobutyric acid on the cardiac autonomic nervous system in healthy young people. J. Physiol. Anthropol. 2009, 28, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative disease. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Tsang, C.; Smail, N.F.; Almoosawi, S.; McDougall, G.J.M.; Al-Dujaili, E.A.S. Antioxidant rich potato improves arterial stiffness in healthy adults. Plant Foods Hum. Nutr. 2018, 73, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Matsusima, A.; Furuuchi, R.; Sakaguchi, Y.; Goto, H.; Yokoyama, T.; Nishida, H.; Hirayama, M. Acute and chronic flow-mediated dilation and blood pressure responses to daily intake of boysenberry juice: A preliminary study. Int. J. Food Sci. Nutr. 2013, 64, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Baynham, R.; Veldhuijzen van Zanten, J.J.C.S.; Johns, P.W.; Pham, Q.S.; Rendeiro, C. Cocoa Flavanols Improve vascular responses to acute mental stress in young healthy adults. Nutrients 2021, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, K. Vascular action of cocoa flavanols in humans: The roots of the story. J. Cardiovasc. Pharmacol. 2006, 47, S99–S102. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.E.; Netzel, M.; Fanning, K. Foo-based anthocyanin intake and cognitive outcomes in Human Intervention trials: A systematic review. J. Human Nutr. Diet. 2016, 30, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Steves, C.J.; Macgregor, A.; Spector, T.; Cassidy, A. Increased habitual flavonoid intake predicts attenuation of cognitive ageing in twins. BMC Med. 2021, 19, 185. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.W.; Okello, E.J.; Brooker, H.J.; Lester, S.; McDougall, G.J.; Wesnes, K.A. The impact of blackcurrant juice on attention, mood and brain wave spectral activity in young healthy volunteers. Nutr. Neurosci. 2019, 22, 596–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Oh, S.H.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Woo, S.H.; Kim, H.S.; Lee, J.; Jeong, H.S. Antioxidant contents and antioxidant activities of white and colored potatoes (Solanum tuberosum L.). Nutr. Food Sci. 2016, 21, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaspar, K.L.; Park, J.S.; Brown, C.R.; Mathison, B.D.; Navarre, D.A.; Chew, B.P. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J. Nutr. 2010, 141, 108–111. [Google Scholar] [CrossRef] [Green Version]
| Placebo (“Haruka”) | Test (“Shadow Queen”) | |
|---|---|---|
| Anthocyanins as delphinidin equivalents (mg) | 0.0 | 45.0 |
| Water (g) | 60.2 | 58.4 |
| Protein (g) | 1.5 | 1.6 |
| Fat (g) | 0.15 | 0.15 |
| Ash (g) | 0.7 | 0.8 |
| Carbohydrate (g) | 12.5 | 14.1 |
| Dietary fiber (g) | 1.3 | 1.2 |
| Energy (kcal) | 55 kcal | 62 kcal |
| NaCl (mg) | 0.0 | 2.1 |
| Baseline Characteristics | Placebo (“Haruka“) | “Shadow Queen” | p Value |
|---|---|---|---|
| Gender (male/female) (n) | 8 (2/6) | 7 (2/5) | |
| Age (years) | 56.1 ± 4.4 | 55.1 ± 3.8 | 0.653 |
| Body weight (kg) | 54.9 ± 10.3 | 50.9 ± 6.3 | 0.390 |
| Body mass index (kg/m2) | 22.6 ± 5.6 | 20.8 ± 2.2 | 0.199 |
| Body fat ratio (%) | 29.1 ± 5.6 | 25.3 ± 9.3 | 0.355 |
| VAS questionnaires (mm) | 196.1 ± 93.4 | 222.0 ± 97.6 | 0.610 |
| Intake rate (%) | 100.0 ± 0 | 98.7 ± 2.7 | 0.379 |
| Stress Response (Score) | Intervention | Week 0 | Week 8 | Week 0 vs. Week 8 | Changes in Placebo vs. Test Food |
|---|---|---|---|---|---|
| p Value | p Value | ||||
| Psychological stress response | “Haruka” | 4.35 ± 3.77 | 4.75 ± 4.20 | 0.381 | 0.007 ** |
| (0–18) | “Shadow Queen” | 6.57 ± 4.35 | 3.14 ± 3.34 | 0.026# | |
| Physical stress response (0–11) | “Haruka” | 2.50 ± 2.51 | 1.88 ± 2.59 | 0.370 | 0.794 |
| “Shadow Queen” | 1.71 ± 2.43 | 0.86 ± 1.21 | 0.172 | ||
| Vigor (3–12) | “Haruka” | 5.88 ± 2.59 | 6.00 ± 2.14 | 0.802 | 0.367 |
| “Shadow Queen” | 6.00 ± 2.16 | 6.86 ± 3.02 | 0.225 | ||
| Irritability (3–12) | “Haruka” | 6.25 ± 1.04 | 6.00 ± 1.31 | 0.563 | 0.028 * |
| “Shadow Queen” | 7.00 ± 2.77 | 5.14 ± 2.34 | 0.011 # | ||
| Fatigue (3–12) | “Haruka” | 6.25 ± 2.60 | 5.13 ± 3.04 | 0.065 | 0.583 |
| “Shadow Queen” | 7.14 ± 2.67 | 5.57 ± 2.82 | 0.042 # | ||
| Anxiety (3–12) | “Haruka” | 5.13 ± 1.46 | 5.50 ± 2.62 | 0.634 | 0.146 |
| “Shadow Queen” | 6.00 ± 1.63 | 4.86 ± 1.07 | 0.103 | ||
| Depression (6–24) | “Haruka” | 9.38 ± 4.03 | 10.00 ± 4.24 | 0.217 | 0.009 ** |
| “Shadow Queen” | 10.86 ± 2.73 | 8.86 ± 1.57 | 0.038 # | ||
| Physical Complaints (11–44) | “Haruka” | 19.63 ± 6.21 | 18.38 ± 7.67 | 0.454 | 0.519 |
| “Shadow Queen” | 19.43 ± 6.02 | 16.86 ± 3.80 | 0.063 |
| Visual Analog Scale | Intervention | Week 0 | Week 4 | Week 8 | ΔWeek 4 | ΔWeek 8 |
|---|---|---|---|---|---|---|
| Eyestrain (0–100) | “Haruka” | 35.38 ± 23.87 | 42.75 ± 30.58 | 45.50 ± 23.78 | 7.38 ± 24.70 | 10.13 ± 21.52 |
| “Shadow Queen” | 51.29 ± 32.63 | 67.86 ± 25.06 | 74.86 ± 22.66 | 16.57 ± 27.55 | 23.57 ± 29.22 | |
| p value | - | - | - | 0.507 | 0.324 | |
| Eye pain (0–100) | “Haruka” | 70.00 ± 30.74 | 68.75 ± 32.67 | 67.38 ± 29.90 | −1.25 ± 32.06 | −2.63 ± 21.32 |
| “Shadow Queen” | 76.14 ± 31.03 | 79.86 ± 26.12 | 86.14 ± 22.57 | 3.71 ± 29.64 | 10.00 ± 24.67 | |
| p value | - | - | - | 0.762 | 0.307 | |
| Irritability (0–100) | “Haruka” | 66.38 ± 25.14 | 52.00 ± 20.28 | 58.50 ± 23.31 | −14.38 ± 26.10 | −7.88 ± 23.39 |
| “Shadow Queen” | 65.43 ± 26.74 | 74.86 ± 28.35 | 73.00 ± 25.94 | 9.43 ± 27.16 | 7.57 ± 21.75 | |
| p value | - | - | - | 0.107 | 0.210 | |
| Stress (0–100) | “Haruka” | 48.00 ± 31.06 | 43.00 ± 28.06 | 39.50 ± 21.21 | −5.00 ± 23.10 | −8.50 ± 17.31 |
| “Shadow Queen” | 56.43 ± 30.99 | 72.29 ± 29.21 | 69.29 ± 22.76 | 69.29 ± 22.76 | 12.86 ± 22.39 | |
| p value | - | - | - | 0.158 | 0.058 |
| Secondary Outcome | Intervention | Week 0 | Week 8 | Week 0 vs. Week 8 | Changes in Placebo vs. Test Food |
|---|---|---|---|---|---|
| p Value | p Value | ||||
| Blood 1,5-anhydroglucitol(1,5-AG) | “Haruka” | 18.30 ± 7.93 | 19.61 ± 8.84 | 0.012 # | 0.017 * |
| (µg/mL) | “Shadow Queen” | 20.96 ± 3.97 | 20.54 ± 4.46 | 0.447 | |
| Urine 8-hydroxy-2’-deoxyguanisine | “Haruka” | 6.39 ± 1.59 | 5.28 ± 2.02 | 0.059 | 0.520 |
| (8-OHdG) (ng/mg/Creatinine correction value) | “Shadow Queen” | 7.33 ± 3.49 | 5.16 ± 2.93 | 0.194 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda-Yamamoto, M.; Honmou, O.; Sasaki, M.; Haseda, A.; Kagami-Katsuyama, H.; Shoji, T.; Namioka, A.; Namioka, T.; Magota, H.; Oka, S.; et al. The Impact of Purple-Flesh Potato (Solanum tuberosum L.) cv. “Shadow Queen” on Minor Health Complaints in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 2446. https://doi.org/10.3390/nu14122446
Maeda-Yamamoto M, Honmou O, Sasaki M, Haseda A, Kagami-Katsuyama H, Shoji T, Namioka A, Namioka T, Magota H, Oka S, et al. The Impact of Purple-Flesh Potato (Solanum tuberosum L.) cv. “Shadow Queen” on Minor Health Complaints in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2022; 14(12):2446. https://doi.org/10.3390/nu14122446
Chicago/Turabian StyleMaeda-Yamamoto, Mari, Osamu Honmou, Masanori Sasaki, Akane Haseda, Hiroyo Kagami-Katsuyama, Toshihiko Shoji, Ai Namioka, Takahiro Namioka, Hirotoshi Magota, Shinichi Oka, and et al. 2022. "The Impact of Purple-Flesh Potato (Solanum tuberosum L.) cv. “Shadow Queen” on Minor Health Complaints in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 14, no. 12: 2446. https://doi.org/10.3390/nu14122446







