Calcifediol for Use in Treatment of Respiratory Disease
Abstract
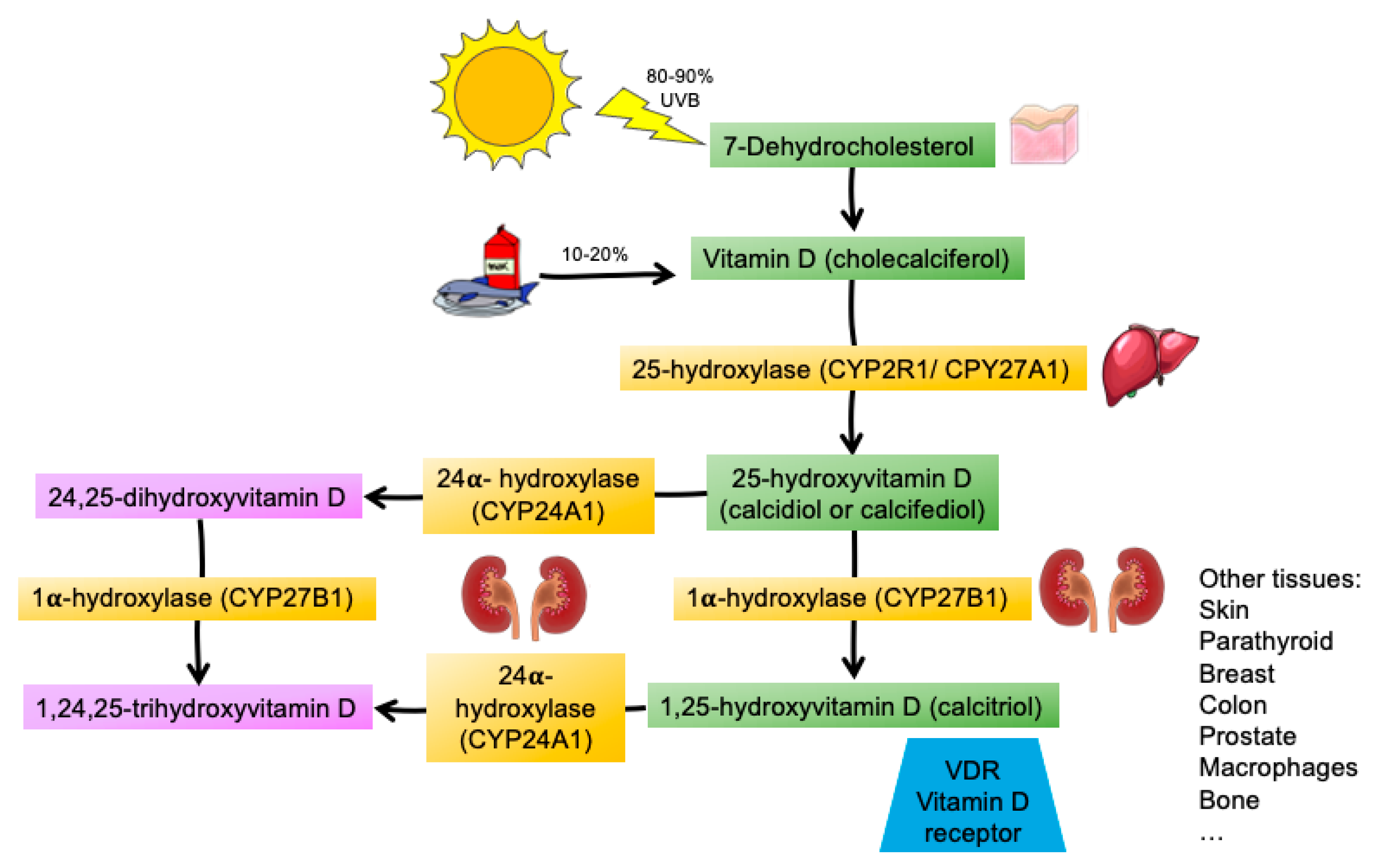
1. Introduction
2. Vitamin D Deficiency and Asthma
3. Vitamin D Deficiency and Chronic Obstructive Pulmonary Disease
4. Calcifediol and COVID-19
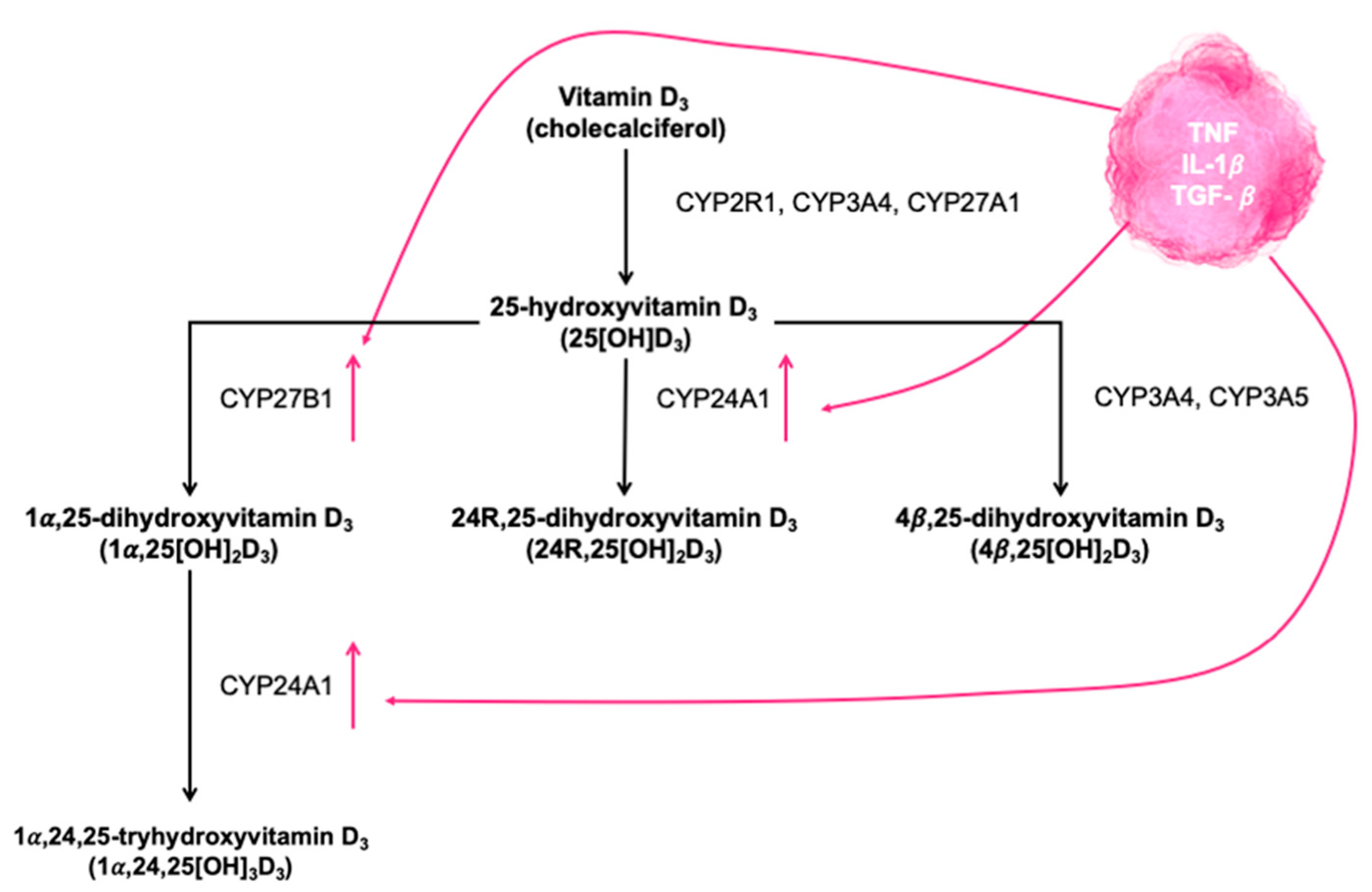
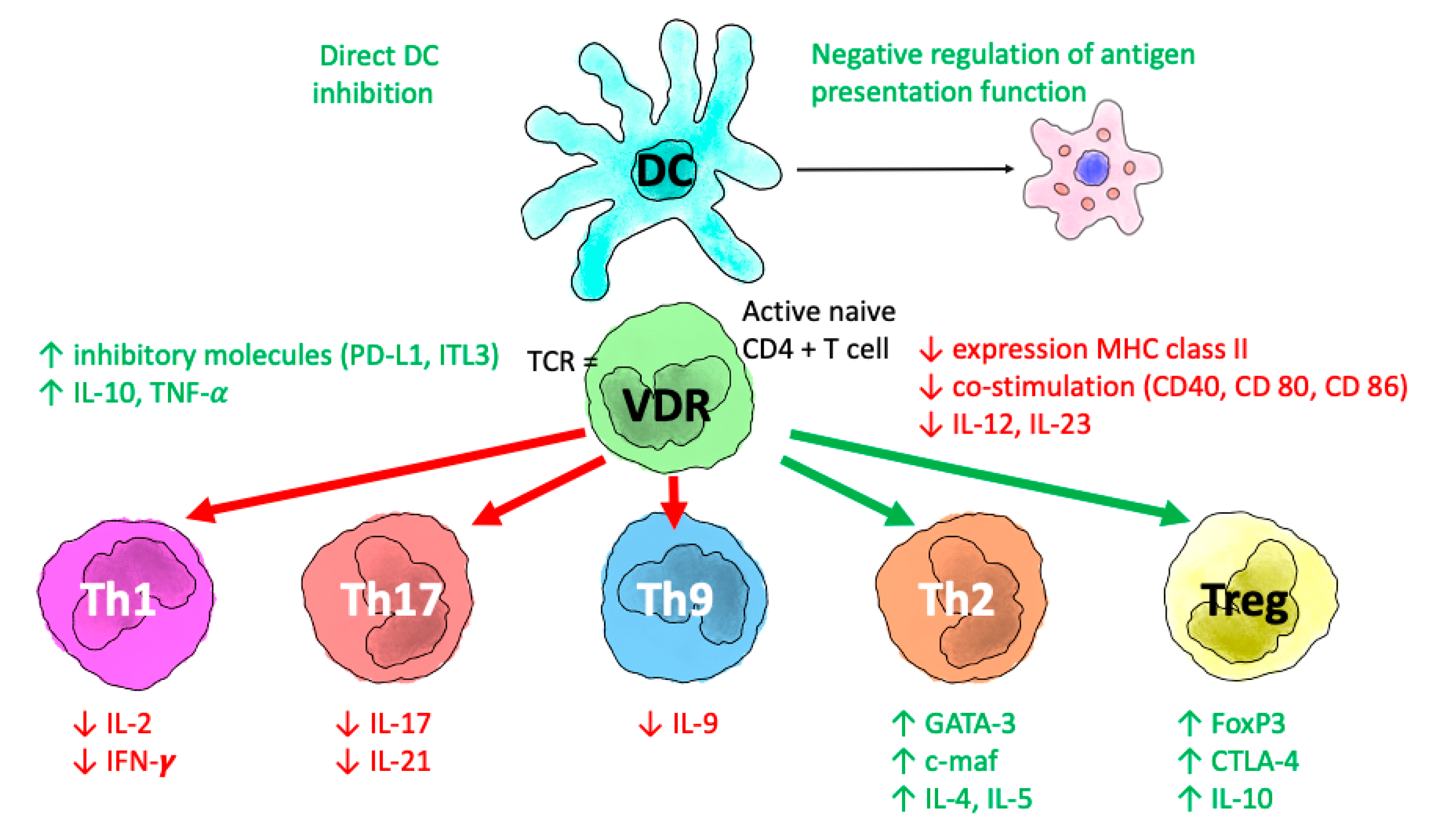
4.1. Potential Mechanisms Linking VDES and COVID-19 Infection
4.2. VDES and the Lungs
4.3. COVID-19 and VDES
- Calcitriol is a potent agent that reduces levels of the renin–angiotensin system, which are increased in COVID-19 infection. It can also generate tissue damage through the angiotensin receptor. If it is not well regulated by calcitriol, the action of renin and the renin–angiotensin axis is inhibited [84].
- Neutrophils are a potent antiviral agent, although they can generate tissue damage by releasing cytokines and chemokines in response to infection. Calcitriol modulates the response of neutrophils, thus helping to reduce tissue damage [65].
- Calcitriol plays a fundamental role as an antithrombotic by reducing the risk of hypercoagulability and pulmonary or systemic thrombosis. Observational data reveal an association between low serum levels of 25(OH)D and the development of thrombotic events in patients with ischemic stroke [85].
- Finally, calcitriol reduces levels the expression of fibronectin and collagen, thus inhibiting the trans-differentiation of epithelial cells into myofibroblasts [86].
4.4. COVID-19 Clinical Data and VDES
5. Vitamin D and Other Respiratory Diseases
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Maretzke, F.; Bechthold, A.; Egert, S.; Ernst, J.B.; van Lent, D.M.; Pilz, S.; Reichrath, J.; Stangl, G.I.; Stehle, P.; Volkert, D.; et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases-An Umbrella Review. Nutrients 2020, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Maestro, M.A.; Molnár, F.; Carlberg, C. Vitamin D and Its Synthetic Analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Vitamin D Supplementation: Cholecalciferol, Calcifediol, and Calcitriol. Eur. J. Clin. Nutr. 2020, 74, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D Bioavailability: State of the Art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Medical Progress: Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Thompson, G.R.; Lewis, B.; Booth, C.C. Absorption of Vitamin D3-3H in Control Subjects and Patients with Intestinal Malabsorption. J. Clin. Investig. 1966, 45, 94–102. [Google Scholar] [CrossRef]
- Davies, M.; Mawer, E.B.; Krawitt, E.L. Comparative Absorption of Vitamin D3 and 25-Hydroxyvitamin D3 in Intestinal Disease. Gut 1980, 21, 287–292. [Google Scholar] [CrossRef]
- DiFranco, K.M.; Mulligan, J.K.; Sumal, A.S.; Diamond, G. Induction of CFTR Gene Expression by 1,25(OH)2 Vitamin D3, 25OH Vitamin D3, and Vitamin D3 in Cultured Human Airway Epithelial Cells and in Mouse Airways. J. Steroid Biochem. Mol. Biol. 2017, 173, 323–332. [Google Scholar] [CrossRef]
- Schrumpf, J.A.; Amatngalim, G.D.; Veldkamp, J.B.; Verhoosel, R.M.; Ninaber, D.K.; Ordonez, S.R.; Van Der Does, A.M.; Haagsman, H.P.; Hiemstra, P.S. Proinflammatory Cytokines Impair Vitamin D-Induced Host Defense in Cultured Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2017, 56, 749–761. [Google Scholar] [CrossRef]
- Bouillon, R. Comparative Analysis of Nutritional Guidelines for Vitamin D. Nat. Rev. Endocrinol. 2017, 13, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D Deficiency: A Worldwide Problem with Health Consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for Health: A Global Perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Vitamin D and Health in the Mediterranean Countries. Hormones 2019, 18, 23–35. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Yakout, S.M.; Alnaami, A.M.; Wani, K.; Alokail, M.S. The Association of Serum 25-OH Vitamin D with Asthma in Saudi Adults. Medicine 2018, 97, e12286. [Google Scholar] [CrossRef]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D Deficiency Is Highly Prevalent in COPD and Correlates with Variants in the Vitamin D-Binding Gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef]
- Christakos, S.; Hewison, M.; Gardner, D.G.; Wagner, C.L.; Sergeev, I.N.; Rutten, E.; Pittas, A.G.; Boland, R.; Ferrucci, L.; Bikle, D.D. Vitamin D: Beyond Bone. Ann. N. Y. Acad. Sci. 2013, 1287, 45–58. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Stöcklin, E.; Sidelnikov, E.; Willett, W.C.; Edel, J.O.; Stähelin, H.B.; Wolfram, S.; Jetter, A.; Schwager, J.; et al. Oral Supplementation with 25(OH)D3 versus Vitamin D3: Effects on 25(OH)D Levels, Lower Extremity Function, Blood Pressure, and Markers of Innate Immunity. J. Bone Miner. Res. 2012, 27, 160–169. [Google Scholar] [CrossRef]
- Gayan-Ramirez, G.; Janssens, W. Vitamin D Actions: The Lung Is a Major Target for Vitamin D, FGF23, and Klotho. JBMR Plus 2021, 5, e10569. [Google Scholar] [CrossRef]
- Nanzer, A.M.; Chambers, E.S.; Ryanna, K.; Richards, D.F.; Black, C.; Timms, P.M.; Martineau, A.R.; Griffiths, C.J.; Corrigan, C.J.; Hawrylowicz, C.M. Enhanced Production of IL-17A in Patients with Severe Asthma Is Inhibited by 1α,25-Dihydroxyvitamin D3 in a Glucocorticoid-Independent Fashion. J. Allergy Clin. Immunol. 2013, 132, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in Asthma: Mechanisms of Action and Considerations for Clinical Trials. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Hawrylowicz, C.M. The Impact of Vitamin D on Regulatory T Cells. Curr. Allergy Asthma Rep. 2011, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabryšová, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The Role of 1α,25-Dihydroxyvitamin D3 and Cytokines in the Promotion of Distinct Foxp3+and IL-10+ CD4+ T Cells. Eur. J. Immunol. 2012, 42, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Urry, Z.; Xystrakis, E.; Richards, D.F.; McDonald, J.; Sattar, Z.; Cousins, D.J.; Corrigan, C.J.; Hickman, E.; Brown, Z.; Hawrylowicz, C.M. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. Am. Soc. Clin. Investig. 2009, 119, 387–398. [Google Scholar]
- Pfeffer, P.E.; Lu, H.; Mann, E.H.; Chen, Y.H.; Ho, T.R.; Cousins, D.J.; Corrigan, C.; Kelly, F.J.; Mudway, I.S.; Hawrylowicz, C.M. Effects of Vitamin D on Inflammatory and Oxidative Stress Responses of Human Bronchial Epithelial Cells Exposed to Particulate Matter. PLoS ONE 2018, 13, e0200040. [Google Scholar] [CrossRef]
- Tolppanen, A.M.; Sayers, A.; Granell, R.; Fraser, W.D.; Henderson, J.; Lawlor, D.A. Prospective Association of 25-Hydroxyvitamin D3 and D2 with Childhood Lung Function, Asthma, Wheezing, and Flexural Dermatitis. Epidemiology 2013, 24, 310–319. [Google Scholar] [CrossRef]
- Camargo, C.A.; Ingham, T.; Wickens, K.; Thadhani, R.; Silvers, K.M.; Epton, M.J.; Town, G.I.; Pattemore, P.; Espinola, J.A.; Crane, J.; et al. Cord-Blood 25-Hydroxyvitamin D Levels and Risk of Respiratory Infection, Wheezing, and Asthma. Pediatrics 2011, 127, e180–e187. [Google Scholar] [CrossRef]
- Pike, K.C.; Inskip, H.M.; Robinson, S.; Lucas, J.S.; Cooper, C.; Harvey, N.C.; Godfrey, K.M.; Roberts, G. Maternal Late-Pregnancy Serum 25-Hydroxyvitamin D in Relation to Childhood Wheeze and Atopic Outcomes. Thorax 2012, 67, 950–956. [Google Scholar] [CrossRef]
- Morales, E.; Romieu, I.; Guerra, S.; Ballester, F.; Rebagliato, M.; Vioque, J.; Tardón, A.; Rodriguez Delhi, C.; Arranz, L.; Torrent, M.; et al. Maternal Vitamin D Status in Pregnancy and Risk of Lower Respiratory Tract Infections, Wheezing, and Asthma in Offspring. Epidemiology 2012, 23, 64–71. [Google Scholar] [CrossRef]
- Bener, A.; Ehlayel, M.S.; Tulic, M.K.; Hamid, Q. Vitamin D Deficiency as a Strong Predictor of Asthma in Children. Int. Arch. Allergy Immunol. 2012, 157, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Van Oeffelen, A.A.M.; Bekkers, M.B.M.; Smit, H.A.; Kerkhof, M.; Koppelman, G.H.; Haveman-Nies, A.; van der A, D.L.; Jansen, E.H.J.M.; Wijga, A.H. Serum Micronutrient Concentrations and Childhood Asthma: The PIAMA Birth Cohort Study. Pediatr. Allergy Immunol. 2011, 22, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Brehm, J.M.; Celedón, J.C.; Soto-Quiros, M.E.; Avila, L.; Hunninghake, G.M.; Forno, E.; Laskey, D.; Sylvia, J.S.; Hollis, B.W.; Weiss, S.T.; et al. Serum Vitamin D Levels and Markers of Severity of Childhood Asthma in Costa Rica. Am. J. Respir. Crit. Care Med. 2009, 179, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Brehm, J.M.; Acosta-Pérez, E.; Klei, L.; Roeder, K.; Barmada, M.; Boutaoui, N.; Forno, E.; Kelly, R.; Paul, K.; Sylvia, J.; et al. Vitamin D Insufficiency and Severe Asthma Exacerbations in Puerto Rican Children. Am. J. Respir. Crit. Care Med. 2012, 186, 140–146. [Google Scholar] [CrossRef]
- Beigelman, A.; Zeiger, R.S.; Mauger, D.; Strunk, R.C.; Jackson, D.J.; Martinez, F.D.; Morgan, W.J.; Covar, R.; Szefler, S.J.; Taussig, L.M.; et al. The Association between Vitamin D Status and the Rate of Exacerbations Requiring Oral Corticosteroids in Preschool Children with Recurrent Wheezing. J. Allergy Clin. Immunol. 2014, 133, 1489–1492. [Google Scholar] [CrossRef]
- Sutherland, E.R.; Goleva, E.; Jackson, L.P.; Stevens, A.D.; Leung, D.Y.M. Vitamin D Levels, Lung Function, and Steroid Response in Adult Asthma. Am. J. Respir. Crit. Care Med. 2010, 181, 699–704. [Google Scholar] [CrossRef]
- Korn, S.; Hübner, M.; Jung, M.; Blettner, M.; Buhl, R. Severe and Uncontrolled Adult Asthma Is Associated with Vitamin D Insufficiency and Deficiency. Respir. Res. 2013, 14, 25. [Google Scholar] [CrossRef]
- Confino-Cohen, R.; Brufman, I.; Goldberg, A.; Feldman, B.S. Vitamin D, Asthma Prevalence and Asthma Exacerbations: A Large Adult Population-Based Study. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 1673–1680. [Google Scholar] [CrossRef]
- Pojsupap, S.; Iliriani, K.; Sampaio, T.Z.A.L.; O’Hearn, K.; Kovesi, T.; Menon, K.; McNally, J.D. Efficacy of High-Dose Vitamin D in Pediatric Asthma: A Systematic Review and Meta-Analysis. J. Asthma 2015, 52, 382–390. [Google Scholar] [CrossRef]
- Jat, K.R.; Goel, N.; Gupta, N.; Gupta, C.P.; Datta, S.; Lodha, R.; Kabra, S.K. Efficacy of Vitamin D Supplementation in Asthmatic Children with Vitamin D Deficiency: A Randomized Controlled Trial (ESDAC Trial). Pediatr. Allergy Immunol. 2021, 32, 479–488. [Google Scholar] [CrossRef]
- Castro, M.; King, T.S.; Kunselman, S.J.; Cabana, M.D.; Denlinger, L.; Holguin, F.; Kazani, S.D.; Moore, W.C.; Moy, J.; Sorkness, C.A.; et al. Effect of Vitamin D3 on Asthma Treatment Failures in Adults with Symptomatic Asthma and Lower Vitamin D Levels: The VIDA Randomized Clinical Trial. JAMA 2014, 311, 2083–2091. [Google Scholar] [CrossRef]
- Arshi, S.; Fallahpour, M.; Nabavi, M.; Bemanian, M.H.; Javad-Mousavi, S.A.; Nojomi, M.; Esmaeilzadeh, H.; Molatefi, R.; Rekabi, M.; Jalali, F.; et al. The Effects of Vitamin D Supplementation on Airway Functions in Mild to Moderate Persistent Asthma. Ann. Allergy Asthma Immunol. 2014, 113, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; MacLaughlin, B.D.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Kilpin, K.; McLaughlin, D.; Fletcher, G.; Mein, C.A.; et al. Double-Blind Randomised Placebo-Controlled Trial of Bolus-Dose Vitamin D3 Supplementation in Adults with Asthma (ViDiAs). Thorax 2015, 70, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between Vitamin D Status and Asthma Control: A Meta-Analysis of Randomized Trials. Respir. Med. 2019, 150, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Andújar-Espinosa, R.; Salinero-González, L.; Illán-Gómez, F.; Castilla-Martínez, M.; Hu-Yang, C.; Ruiz-López, F.J. Effect of Vitamin D Supplementation on Asthma Control in Patients with Vitamin D Deficiency: The ACVID Randomised Clinical Trial. Thorax 2021, 76, 126–133. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, C.; Mei, J.; Zhu, L.; Kong, H. Vitamin D Can Safely Reduce Asthma Exacerbations among Corticosteroid-Using Children and Adults with Asthma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Res. 2021, 92, 49–61. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; De Jongh, R.T. Vitamin d Deficiency in Asthma and Chronic Obstructive Pulmonary Disease: A Chicken-or-Egg Story. Am. J. Respir. Crit. Care Med. 2020, 202, 312–313. [Google Scholar] [CrossRef]
- Mathyssen, C.; Gayan-Ramirez, G.; Bouillon, R.; Janssens, W. Vitamin D Supplementation in Respiratory Diseases: Evidence from Randomized Controlled Trials. Pol. Arch. Intern. Med. 2017, 127, 775–784. [Google Scholar]
- Zhu, M.; Wang, T.; Wang, C.; Ji, Y. The Association between Vitamin D and COPD Risk, Severity, and Exacerbation: An Updated Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2597–2607. [Google Scholar] [CrossRef]
- Lokesh, K.S.; Chaya, S.K.; Jayaraj, B.S.; Praveena, A.S.; Krishna, M.; Madhivanan, P.; Mahesh, P.A. Vitamin D Deficiency Is Associated with Chronic Obstructive Pulmonary Disease and Exacerbation of COPD. Clin. Respir. J. 2021, 15, 389–399. [Google Scholar] [CrossRef]
- Mahesh, P.A.; Lokesh, K.S.; Madhivanan, P.; Chaya, S.K.; Jayaraj, B.S.; Ganguly, K.; Krishna, M. The Mysuru Studies of Determinants of Health in Rural Adults (MUDHRA), India. Epidemiol. Health 2018, 40, e2018027. [Google Scholar] [CrossRef]
- Malinovschi, A.; Masoero, M.; Bellocchia, M.; Ciuffreda, A.; Solidoro, P.; Mattei, A.; Mercante, L.; Heffler, E.; Rolla, G.; Bucca, C. Severe Vitamin D Deficiency Is Associated with Frequent Exacerbations and Hospitalization in COPD Patients. Respir. Res. 2014, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Greiller, C.L.; Suri, R.; Jolliffe, D.A.; Kebadze, T.; Hirsman, A.G.; Griffiths, C.J.; Johnston, S.L.; Martineau, A.R. Vitamin D Attenuates Rhinovirus-Induced Expression of Intercellular Adhesion Molecule-1 (ICAM-1) and Platelet-Activating Factor Receptor (PAFR) in Respiratory Epithelial Cells. J. Steroid Biochem. Mol. Biol. 2019, 187, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D Supplementation to Prevent Asthma Exacerbations: A Systematic Review and Meta-Analysis of Individual Participant Data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; De Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to Prevent Exacerbations of COPD: Systematic Review and Meta-Analysis of Individual Participant Data from Randomised Controlled Trials. Thorax 2019, 74, 337–345. [Google Scholar] [CrossRef]
- Ferrari, R.; Caram, L.M.O.; Tanni, S.E.; Godoy, I.; de Paiva, S.A.R. The Relationship between Vitamin D Status and Exacerbation in COPD Patients—A Literature Review. Respir. Med. 2018, 139, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.; Agusti, A.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Criner, G.; Halpin, D.; Han, M.; Martinez, F.J.; Oca, M.M.; et al. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2022 Report. 2022. Available online: https://goldcopd.org/2022-gold-reports-2/ (accessed on 30 March 2022).
- Jolliffe, D.A.; Stefanidis, C.; Wang, Z.; Kermani, N.Z.; Dimitrov, V.; White, J.H.; McDonough, J.E.; Janssens, W.; Pfeffer, P.; Griffiths, C.J.; et al. Vitamin D Metabolism Is Dysregulated in Asthma and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 202, 371–382. [Google Scholar] [CrossRef]
- Barnes, P.J. Review Series The Cytokine Network in Asthma and Chronic Obstructive Pulmonary Disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2021, 5, e10405. [Google Scholar] [CrossRef]
- Dimitrov, V.; Barbier, C.; Ismailova, A.; Wang, Y.; Dmowski, K.; Salehi-Tabar, R.; Memari, B.; Groulx-Boivin, E.; White, J.H. Vitamin D-Regulated Gene Expression Profiles: Species-Specificity and Cell-Specific Effects on Metabolism and Immunity. Endocrinology 2021, 162, bqaa218. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Entrenas-Castillo, M.; Bouillon, R. Vitamin D Receptor Stimulation to Reduce Acute Respiratory Distress Syndrome (ARDS) in Patients with Coronavirus SARS-CoV-2 Infections: Revised Ms SBMB 2020_166. J. Steroid Biochem. Mol. Biol. 2020, 202, 105719. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Quesada-Gomez, J.M. Vitamin D Endocrine System and COVID-19. JBMR Plus 2021, 5, e10576. [Google Scholar] [CrossRef]
- Martens, P.-J.; Gysemans, C.; Verstuyf, A.; Mathieu, C. Vitamin D’s Effect on Immune Function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Solanki, S.S.; Singh, P.; Kashyap, P.; Sansi, M.S.; Ali, S.A. Promising Role of Defensins Peptides as Therapeutics to Combat against Viral Infection. Microb. Pathog. 2021, 155, 104930. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; van Harten, R.M.; Veldhuizen, E.J.A.; Haagsman, H.P.; Coorens, M. Cathelicidins Modulate TLR-Activation and Inflammation. Front. Immunol. 2020, 11, 1137. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of Inflammatory and Immune Responses by Vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Ødum, N.; Geisler, C. Vitamin D Controls T Cell Antigen Receptor Signaling and Activation of Human T Cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef]
- Vanherwegen, A.S.; Gysemans, C.; Mathieu, C. Vitamin D Endocrinology on the Cross-Road between Immunity and Metabolism. Mol. Cell. Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Geldmeyer-Hilt, K.; Heine, G.; Hartmann, B.; Baumgrass, R.; Radbruch, A.; Worm, M. 1,25-Dihydroxyvitamin D3 Impairs NF-ΚB Activation in Human Naïve B Cells. Biochem. Biophys. Res. Commun. 2011, 407, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zügel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-Dihydroxyvitamin D3 Promotes IL-10 Production in Human B Cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Mathyssen, C.; Aelbrecht, C.; Serré, J.; Everaerts, S.; Maes, K.; Gayan-Ramirez, G.; Vanaudenaerde, B.; Janssens, W. Local Expression Profiles of Vitamin D-Related Genes in Airways of COPD Patients. Respir. Res. 2020, 21, 137. [Google Scholar] [CrossRef]
- Zosky, G.R.; Hart, P.H.; Whitehouse, A.J.O.; Kusel, M.M.; Ang, W.; Foong, R.E.; Chen, L.; Holt, P.G.; Sly, P.D.; Hall, G.L. Vitamin D Deficiency at 16 to 20 Weeks’ Gestation Is Associated with Impaired Lung Function and Asthma at 6 Years of Age. Ann. Am. Thorac. Soc. 2014, 11, 571–577. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Infections: A Systematic Review and Meta-Analysis of Aggregate Data from Randomised Controlled Trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ito, Y.; Uotsu, N.; Yui, K. Intake of 25-Hydroxyvitamin D3 May Reduce the Severity of Upper Respiratory Tract Infection: Post Hoc Analysis of a Randomized, Double-Blind, Placebo-Controlled, Parallel Group Comparison Study. Nutrients 2020, 12, 769. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Mechanisms in Endocrinology Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence That Vitamin d Supplementation Could Reduce Risk of Influenza and Covid-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Roth, A.; Lütke, S.; Meinberger, D.; Hermes, G.; Sengle, G.; Koch, M.; Streichert, T.; Klatt, A.R. LL-37 Fights SARS-CoV-2: The Vitamin D-Inducible Peptide LL-37 Inhibits Binding of SARS-CoV-2 Spike Protein to Its Cellular Receptor Angiotensin Converting Enzyme 2 In Vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- McGregor, R.; Chauss, D.; Freiwald, T.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Zhang, Z.; Teague, H.; West, E.E.; Bibby, J.; et al. An Autocrine Vitamin D-Driven Th1 Shutdown Program Can Be Exploited for COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef]
- Miyata, M.; Lee, J.Y.; Susuki-Miyata, S.; Wang, W.Y.; Xu, H.; Kai, H.; Kobayashi, K.S.; Flavell, R.A.; Li, J.D. Glucocorticoids Suppress Inflammation via the Upregulation of Negative Regulator IRAK-M. Nat. Commun. 2015, 6, 6062. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D Alleviates Lipopolysaccharide-Induced Acute Lung Injury via Regulation of the Renin-Angiotensin System. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.X.; He, D.R. Low Vitamin D Levels Are Associated With the Development of Deep Venous Thromboembolic Events in Patients With Ischemic Stroke. Clin. Appl. Thromb. Hemost. 2018, 24, 69S–75S. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, Y.; Xue, L.; Li, B.; Zhang, Z. 1α,25-Dihydroxyvitamin D3 Attenuates TGF-β-Induced Pro-Fibrotic Effects in Human Lung Epithelial Cells through Inhibition of Epithelial-Mesenchymal Transition. Nutrients 2017, 9, 980. [Google Scholar] [CrossRef]
- Luo, X.; Liao, Q.; Shen, Y.; Li, H.; Cheng, L. Vitamin D Deficiency Is Inversely Associated with COVID-19 Incidence and Disease Severity in Chinese People. J. Nutr. 2021, 151, 98–103. [Google Scholar] [CrossRef]
- Tehrani, S.; Khabiri, N.; Moradi, H.; Mosavat, M.S.; Khabiri, S.S. Evaluation of Vitamin D Levels in COVID-19 Patients Referred to Labafinejad Hospital in Tehran and Its Relationship with Disease Severity and Mortality. Clin. Nutr. ESPEN 2021, 42, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.Z.; Fernandes, A.L.; Sales, L.P.; Santos, M.D.; dos Santos, C.C.; Pinto, A.J.; Goessler, K.F.; Franco, A.S.; Duran, C.S.C.; Silva, C.B.R. Influence of Vitamin D Status on Hospital Length of Stay and Prognosis in Hospitalized Patients with Moderate to Severe COVID-19: A Multicenter Prospective Cohort Study. Am. J. Clin. Nutr. 2021, 114, 598–604. [Google Scholar] [CrossRef]
- Campi, I.; Gennari, L.; Merlotti, D.; Mingiano, C.; Frosali, A.; Giovanelli, L.; Torlasco, C.; Pengo, M.F.; Heilbron, F.; Soranna, D. Vitamin D and COVID-19 Severity and Related Mortality: A Prospective Study in Italy. BMC Infect. Dis. 2021, 21, 566. [Google Scholar] [CrossRef]
- Mariani, J.; Giménez, V.M.M.; Bergam, I.; Tajer, C.; Antonietti, L.; Inserra, F.; Ferder, L.; Manucha, W. Association between Vitamin D Deficiency and COVID-19 Incidence, Complications, and Mortality in 46 Countries: An Ecological Study. Health Secur. 2021, 19, 302–308. [Google Scholar] [CrossRef]
- Sánchez-Zuno, G.A.; González-Estevez, G.; Matuz-Flores, M.G.; Macedo-Ojeda, G.; Hernández-Bello, J.; Mora-Mora, J.C.; Pérez-Guerrero, E.E.; García-Chagollán, M.; Vega-Magaña, N.; Turrubiates-Hernández, F.J. Vitamin d Levels in Covid-19 Outpatients from Western Mexico: Clinical Correlation and Effect of Its Supplementation. J. Clin. Med. 2021, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Lecce, V.; Quaranta, V.N.; Zito, A.; Buonamico, E.; Capozza, E.; Palumbo, A.; Gioia, G.; Valerio, V.N.; Resta, O. Vitamin D Deficiency as a Predictor of Poor Prognosis in Patients with Acute Respiratory Failure Due to COVID-19. J. Endocrinol. Investig. 2021, 44, 765–771. [Google Scholar] [CrossRef]
- Ye, K.; Tang, F.; Liao, X.; Shaw, B.A.; Deng, M.; Huang, G.; Qin, Z.; Peng, X.; Xiao, H.; Chen, C. Does Serum Vitamin D Level Affect COVID-19 Infection and Its Severity?—A Case-Control Study. J. Am. Coll. Nutr. 2021, 40, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M. Treatment With 25-Hydroxyvitamin D3 (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Ohaegbulam, K.C.; Swalih, M.; Patel, P.; Smith, M.A.; Perrin, R. Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series. Am. J. Ther. 2020, 27, 485–490. [Google Scholar] [CrossRef]
- Lakkireddy, M.; Gadiga, S.G.; Malathi, R.D.; Karra, M.L.; Raju, I.S.S.V.P.M.; Ragini; Chinapaka, S.; Baba, K.S.S.S.; Kandakatla, M. Impact of Daily High Dose Oral Vitamin D Therapy on the Inflammatory Markers in Patients with COVID 19 Disease. Sci. Rep. 2021, 11, 10641. [Google Scholar] [CrossRef]
- Annweiler, C.; Hanotte, B.; de l’Eprevier, C.G.; Sabatier, J.-M.; Lafaie, L.; Célarier, T. Vitamin D and Survival in COVID-19 Patients: A Quasi-Experimental Study. J. Steroid Biochem. Mol. Biol. 2020, 204, 105771. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin d Supplementation Associated to Better Survival in Hospitalized Frail Elderly Covid-19 Patients: The Geria-Covid Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F.; Jude, E.B. High-Dose Cholecalciferol Booster Therapy Is Associated with a Reduced Risk of Mortality in Patients with Covid-19: A Cross-Sectional Multi-Centre Observational Study. Nutrients 2020, 12, 3799. [Google Scholar] [CrossRef]
- Giannini, S.; Passeri, G.; Tripepi, G.; Sella, S.; Fusaro, M.; Arcidiacono, G.; Torres, M.O.; Michielin, A.; Prandini, T.; Baffa, V. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid Covid-19 Patients: A Hypothesis-Generating Study. Nutrients 2021, 13, 219. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef] [PubMed]
- Alcala-diaz, J.F.; Limia-perez, L.; Gomez-huelgas, R.; Martin-escalante, M.D.; Cortes-rodriguez, B.; Zambrana-garcia, J.L.; Entrenas-castillo, M.; Perez-caballero, A.I.; López-carmona, M.D.; Garcia-alegria, J. Calcifediol Treatment and Hospital Mortality Due to Covid-19: A Cohort Study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, B.; Fatti, L.M.; Danesi, L.; Gazzano, G.; Croci, M.; Vitale, G.; Gilardini, L.; Bonadonna, S.; Chiodini, I.; Caparello, C.F. Mortality in an Italian Nursing Home during COVID-19 Pandemic: Correlation with Gender, Age, ADL, Vitamin D Supplementation, and Limitations of the Diagnostic Tests. Aging 2020, 12, 24522–24534. [Google Scholar] [CrossRef] [PubMed]
- Vasheghani, M.; Jannati, N.; Baghaei, P.; Rezaei, M.; Aliyari, R.; Marjani, M. The Relationship between Serum 25-Hydroxyvitamin D Levels and the Severity of COVID-19 Disease and Its Mortality. Sci. Rep. 2021, 11, 17594. [Google Scholar] [CrossRef] [PubMed]
- Macaya, F.; Espejo, C.; Valls, A.; Fernández-Ortiz, A.; González Del Castillo, J.; Martín-Sánchez, F.J.; Runkle, I.; Rubio, M.A. Interaction between Age and Vitamin d Deficiency in Severe Covid-19 Infection. Nutr. Hosp. 2020, 37, 1039–1042. [Google Scholar] [CrossRef]
- Mendy, A.; Apewokin, S.; Wells, A.A.; Morrow, A.L. Factors Associated with Hospitalization and Disease Severity in a Racially and Ethnically Diverse Population of COVID-19 Patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M. Cohort Study to Evaluate Effect of Vitamin D, Magnesium and Vitamin B12 in Combination on Severe Outcome Progression in Older Patients with Coronavirus (COVID-19). Nutrition 2020, 79–80, 111017. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short Term, High-Dose Vitamin D Supplementation for COVID-19 Disease: A Randomised, Placebo-Controlled, Study (SHADE Study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Jungreis, I.; Kellis, M. Mathematical Analysis of Córdoba Calcifediol Trial Suggests Strong Role for Vitamin D in Reducing ICU Admissions of Hospitalized COVID-19 Patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Alguwaihes, A.M.; Sabico, S.; Hasanato, R.; Al-Sofiani, M.E.; Megdad, M.; Albader, S.S.; Alsari, M.H.; Alelayan, A.; Alyusuf, E.Y.; Alzahrani, S.H. Severe Vitamin D Deficiency Is Not Related to SARS-CoV-2 Infection but May Increase Mortality Risk in Hospitalized Adults: A Retrospective Case–Control Study in an Arab Gulf Country. Aging Clin. Exp. Res. 2021, 33, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of Vitamin D Level among Asymptomatic and Critically Ill COVID-19 Patients and Its Correlation with Inflammatory Markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R. Low Serum 25-Hydroxyvitamin D (25[OH]D) Levels in Patients Hospitalised with COVID-19 Are Associated with Greater Disease Severity. Clin. Endocrinol. 2020, 93, 508–511. [Google Scholar]
- Barassi, A.; Pezzilli, R.; Mondoni, M.; Rinaldo, R.F.; DavÌ, M.; Cozzolino, M.; D’Eril, M.G.V.; Centanni, S. Vitamin D in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Patients with Non-Invasive Ventilation Support. Panminerva Med. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Loucera, C.; Peña-Chilet, M.; Esteban-Medina, M.; Muñoyerro-Muñiz, D.; Villegas, R.; Lopez-Miranda, J.; Rodriguez-Baño, J.; Túnez, I.; Bouillon, R.; Dopazo, J. Real World Evidence of Calcifediol or Vitamin D Prescription and Mortality Rate of COVID-19 in a Retrospective Cohort of Hospitalized Andalusian Patients. Sci. Rep. 2021, 11, 23380. [Google Scholar] [CrossRef]
- Efird, J.T.; Anderson, E.J.; Jindal, C.; Redding, T.S.; Thompson, A.D.; Press, A.M.; Upchurch, J.; Williams, C.D.; Choi, Y.M.; Suzuki, A. The Interaction of Vitamin D and Corticosteroids: A Mortality Analysis of 26,508 Veterans Who Tested Positive for SARS-CoV-2. Int. J. Environ. Res. Public Health 2021, 19, 447. [Google Scholar] [CrossRef]
- Gönen, M.S.; Alaylıoğlu, M.; Durcan, E.; Özdemir, Y.; Şahin, S.; Konukoğlu, D.; Nohut, O.K.; Ürkmez, S.; Küçükece, B.; Balkan, İ.İ. Rapid and Effective Vitamin d Supplementation May Present Better Clinical Outcomes in Covid-19 (Sars-Cov-2) Patients by Altering Serum Inos1, Il1b, Ifng, Cathelicidin-Ll37, and Icam1. Nutrients 2021, 13, 4047. [Google Scholar] [CrossRef]
- Seal, K.H.; Bertenthal, D.; Carey, E.; Grunfeld, C.; Bikle, D.D.; Lu, C.M. Association of Vitamin D Status and COVID-19-Related Hospitalization and Mortality. J. Gen. Intern. Med. 2022, 37, 853–861. [Google Scholar] [CrossRef]
- Cereda, E.; Bogliolo, L.; Lobascio, F.; Barichella, M.; Zecchinelli, A.L.; Pezzoli, G.; Caccialanza, R. Vitamin D Supplementation and Outcomes in Coronavirus Disease 2019 (COVID-19) Patients from the Outbreak Area of Lombardy, Italy. Nutrition 2021, 82, 111055. [Google Scholar] [CrossRef]
- Cereda, E.; Bogliolo, L.; Klersy, C.; Lobascio, F.; Masi, S.; Crotti, S.; Stefano, L.; Bruno, R.; Corsico, A.G.; Sabatino, A. Vitamin D 25OH Deficiency in COVID-19 Patients Admitted to a Tertiary Referral Hospital. Clin. Nutr. 2021, 40, 2469–2472. [Google Scholar] [CrossRef]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; den Abbeele, K.; Mandal, A.K.J.; Missouris, C.G. Vitamin D Status and Outcomes for Hospitalised Older Patients with COVID-19. Postgrad. Med. J. 2021, 97, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Cosentini, E.; Batori, G.; Marini, E.; Lombardini, R.; Gargaro, M.; Fallarino, F.; Scarponi, A.M. Prevalence of Vitamin D Deficiency and Its Prognostic Impact on Patients Hospitalized with COVID-19. Nutrition 2021, 91–92, 111408. [Google Scholar] [CrossRef] [PubMed]
- Pecina, J.L.; Merry, S.P.; Park, J.G.; Thacher, T.D. Vitamin D Status and Severe COVID-19 Disease Outcomes in Hospitalized Patients. J. Prim. Care Community Health 2021, 12, 21501327211041206. [Google Scholar] [CrossRef]
- Butler-Laporte, G.; Nakanishi, T.; Mooser, V.; Morrison, D.R.; Abdullah, T.; Adeleye, O.; Mamlouk, N.; Kimchi, N.; Afrasiabi, Z.; Rezk, N. Vitamin D and COVID-19 Susceptibility and Severity in the COVID-19 Host Genetics Initiative: A Mendelian Randomization Study. PLoS Med. 2021, 18, e1003605. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.R.; Abdelaziz, T.S.; Fathy, A. Impact of Vitamin D Therapy on the Progress COVID-19: Six Weeks Follow-Up Study of Vitamin D Deficient Elderly Diabetes Patients. Proc. Singap. Healthc. 2021. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; MacEdo, M.B.; Dalmolin, H.H.H. Effect of a Single High Dose of Vitamin D3on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Güven, M.; Gültekin, H. The Effect of High-Dose Parenteral Vitamin D3 on COVID-19-Related Inhospital Mortality in Critical COVID-19 Patients during Intensive Care Unit Admission: An Observational Cohort Study. Eur. J. Clin. Nutr. 2021, 75, 1383–1388. [Google Scholar] [CrossRef]
- Szeto, B.; Zucker, J.E.; LaSota, E.D.; Rubin, M.R.; Walker, M.D.; Yin, M.T.; Cohen, A. Vitamin D Status and COVID-19 Clinical Outcomes in Hospitalized Patients. Endocr. Res. 2021, 46, 66–73. [Google Scholar] [CrossRef]
- Jevalikar, G.; Mithal, A.; Singh, A.; Sharma, R.; Farooqui, K.J.; Mahendru, S.; Dewan, A.; Budhiraja, S. Lack of Association of Baseline 25-Hydroxyvitamin D Levels with Disease Severity and Mortality in Indian Patients Hospitalized for COVID-19. Sci. Rep. 2021, 11, 6258. [Google Scholar] [CrossRef]
- Hernández, J.L.; Nan, D.; Fernandez-Ayala, M.; García-Unzueta, M.; Hernández-Hernández, M.A.; López-Hoyos, M.; Muñoz-Cacho, P.; Olmos, J.M.; Gutiérrez-Cuadra, M.; Ruiz-Cubillán, J.J. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2021, 106, e1343–e1353. [Google Scholar] [CrossRef]
- Lohia, P.; Nguyen, P.; Patel, N.; Kapur, S. Exploring the Link between Vitamin D and Clinical Outcomes in COVID-19. Am. J. Physiol.—Endocrinol. Metab. 2021, 320, E520–E526. [Google Scholar] [CrossRef]
- Bassatne, A.; Basbous, M.; Chakhtoura, M.; El Zein, O.; Rahme, M.; El-Hajj Fuleihan, G. The Link between COVID-19 and Vitamin D (VIVID): A Systematic Review and Meta-Analysis. Metab. Clin. Exp. 2021, 119, 154753. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, A.; Leopold, K.; Jackson, S.; Christensen, S.; Nayfeh, T.; Mohammed, K.; Creo, A.; Tebben, P.; Kumar, S. Association of Vitamin D Deficiency with COVID-19 Infection Severity: Systematic Review and Meta-Analysis. Clin. Endocrinol. 2022, 96, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 Mortality Risk Correlates Inversely with Vitamin D3 Status, and a Mortality Rate Close to Zero Could Theoretically Be Achieved at 50 Ng/ML 25(OH)D3: Results of a Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3596. [Google Scholar] [CrossRef] [PubMed]
- Nogues, X.; Ovejero, D.; Pineda-Moncusí, M.; Bouillon, R.; Arenas, D.; Pascual, J.; Ribes, A.; Guerri-Fernandez, R.; Villar-Garcia, J.; Rial, A.; et al. Calcifediol Treatment and COVID-19–Related Outcomes. J. Clin. Endocrinol. Metab. 2021, 106, E4017–E4027. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Lopez Miranda, J.; Entrenas-Castillo, M.; Nogues y Solans, X.; Mansur, J.; Bouillon, R. Vitamin D Endocrine System and COVID-19. Treatment with Calcifediol. Nutrients 2022. [Google Scholar]
- Oristrell, J.; Oliva, J.C.; Casado, E.; Subirana, I.; Domínguez, D.; Toloba, A.; Balado, A.; Grau, M. Vitamin D Supplementation and COVID-19 Risk: A Population-Based, Cohort Study. J. Endocrinol. Investig. 2022, 45, 167–179. [Google Scholar] [CrossRef]
- Ghelani, D.; Alesi, S.; Mousa, A. Vitamin D and COVID-19: An Overview of Recent Evidence. Int. J. Mol. Sci. 2021, 22, 10559. [Google Scholar] [CrossRef]
- García de Tena, J.; El Hachem Debek, A.; Hernández Gutiérrez, C.; Izquierdo Alonso, J.L. Papel de La Vitamina D En Enfermedad Pulmonar Obstructiva Crónica, Asma y Otras Enfermedades Respiratorias. Arch. Bronconeumol. 2014, 50, 179–184. [Google Scholar] [CrossRef]
- Hewison, M. An Update on Vitamin D and Human Immunity. Clin. Endocrinol. 2012, 76, 315–325. [Google Scholar] [CrossRef]
- Khoo, A.L.; Chai, L.; Koenen, H.; Joosten, I.; Netea, M.; Van Der Ven, A. Translating the Role of Vitamin D 3 in Infectious Diseases. Crit. Rev. Microbiol. 2012, 38, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Baker, I.; Smith, G.D. Meta-Analysis of Vitamin D Receptor Polymorphisms and Pulmonary Tuberculosis Risk. Int. J. Tuberc. Lung Dis. 2005, 9, 1174–1177. [Google Scholar] [PubMed]
- Salahuddin, N.; Ali, F.; Hasan, Z.; Rao, N.; Aqeel, M.; Mahmood, F. Vitamin D Accelerates Clinical Recovery from Tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in Recovery from Tuberculosis]. A Randomized, Placebo-Controlled, Clinical Trial of Vitamin D Supplementation in Patients with Pulmonar. BMC Infect. Dis. 2013, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Amin, Z.; Rumende, C.M. The Effect of Vitamin D as Adjuvant Therapy in Pulmonary Tuberculosis with Moderate-Advance Lesion. Acta Med. Indones. 2006, 38, 1–2. [Google Scholar]
- Ralph, A.P.; Waramori, G.; Pontororing, G.J.; Kenangalem, E.; Wiguna, A.; Tjitra, E.; Sandjaja; Lolong, D.B.; Yeo, T.W.; Chatfield, M.D.; et al. L-Arginine and Vitamin D Adjunctive Therapies in Pulmonary Tuberculosis: A Randomised, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2013, 8, e70032. [Google Scholar] [CrossRef]
- Daley, P.; Jagannathan, V.; John, K.R.; Sarojini, J.; Latha, A.; Vieth, R.; Suzana, S.; Jeyaseelan, L.; Christopher, D.J.; Smieja, M.; et al. Adjunctive Vitamin D for Treatment of Active Tuberculosis in India: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Infect. Dis. 2015, 15, 528–534. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Ganmaa, D.; Wejse, C.; Raqib, R.; Haq, M.A.; Salahuddin, N.; Daley, P.K.; Ralph, A.P.; Ziegler, T.R.; Martineau, A.R. Adjunctive Vitamin D in Tuberculosis Treatment: Meta-Analysis of Individual Participant Data. Eur. Respir. J. 2019, 53, 1802003. [Google Scholar] [CrossRef]
- Stephenson, A.; Brotherwood, M.; Robert, R.; Atenafu, E.; Corey, M.; Tullis, E. Cholecalciferol Significantly Increases 25-Hydroxyvitamin D Concentrations in Adults with Cystic Fibrosis. Am. J. Clin. Nutr. 2007, 85, 1307–1311. [Google Scholar] [CrossRef]
- Ferguson, J.H.; Chang, A.B. Vitamin D Supplementation for Cystic Fibrosis. Cochrane Database Syst. Rev. 2014, 5, CD007298. [Google Scholar] [CrossRef]
- Heist, R.S.; Zhou, W.; Wang, Z.; Liu, G.; Neuberg, D.; Su, L.; Asomaning, K.; Hollis, B.W.; Lynch, T.J.; Wain, J.C.; et al. Circulating 25-Hydroxyvitamin D, VDR Polymorphisms, and Survival in Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2008, 26, 5596–5602. [Google Scholar] [CrossRef]
- Buttigliero, C.; Monagheddu, C.; Petroni, P.; Saini, A.; Dogliotti, L.; Ciccone, G.; Berruti, A. Prognostic Role of Vitamin D Status and Efficacy of Vitamin D Supplementation in Cancer Patients: A Systematic Review. Oncologist 2011, 16, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Parwani, A.V.; Hershberger, P.A.; Lenzner, D.E.; Weissfeld, J.L. Nuclear Vitamin D Receptor Expression Is Associated with Improved Survival in Non-Small Cell Lung Cancer. J. Steroid Biochem. Mol. Biol. 2011, 123, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chen, G.; King, A.N.; Jeon, C.K.; Christensen, P.J.; Zhao, L.; Simpson, R.U.; Thomas, D.G.; Giordano, T.J.; Brenner, D.E.; et al. Characterization of Vitamin D Receptor (VDR) in Lung Adenocarcinoma. Lung Cancer 2012, 77, 265–271. [Google Scholar] [CrossRef]
- Qian, M.; Lin, J.; Fu, R.; Qi, S.; Fu, X.; Yuan, L.; Qian, L. The Role of Vitamin D Intake on the Prognosis and Incidence of Lung Cancer: A Systematic Review and Meta-Analysis. J. Nutr. Sci. Vitaminol. 2021, 67, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Gomez, J.M.; Bouillon, R. Is Calcifediol Better than Cholecalciferol for Vitamin D Supplementation? Osteoporos. Int. 2018, 29, 1697–1711. [Google Scholar] [CrossRef]
- Cesareo, R.; Falchetti, A.; Attanasio, R.; Tabacco, G.; Naciu, A.M.; Palermo, A. Hypovitaminosis D: Is It Time to Consider the Use of Calcifediol? Nutrients 2019, 11, 1016. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Dueñas-Laita, A.; Brandi, M.L.; Jódar, E.; del Pino-Montes, J.; Quesada-Gómez, J.M.; Cereto Castro, F.; Gómez-Alonso, C.; Gallego López, L.; Olmos Martínez, J.M.; et al. Calcifediol Is Superior to Cholecalciferol in Improving Vitamin D Status in Postmenopausal Women: A Randomized Trial. J. Bone Miner. Res 2021, 36, 1967–1978. [Google Scholar] [CrossRef]
- Jetter, A.; Egli, A.; Dawson-Hughes, B.; Staehelin, H.B.; Stoecklin, E.; Goessl, R.; Henschkowski, J.; Bischoff-Ferrari, H.A. Pharmacokinetics of Oral Vitamin D3 and Calcifediol. Bone 2014, 59, 14–19. [Google Scholar] [CrossRef]
- Charoenngam, N.; Kalajian, T.A.; Shirvani, A.; Yoon, G.H.; Desai, S.; McCarthy, A.; Apovian, C.M.; Holick, M.F. A Pilot-Randomized, Double-Blind Crossover Trial to Evaluate the Pharmacokinetics of Orally Administered 25-Hydroxyvitamin D3 and Vitamin D3 in Healthy Adults with Differing BMI and in Adults with Intestinal Malabsorption. Am. J. Clin. Nutr. 2021, 114, 1189–1199. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Entrenas-Castillo, M.; Salinero-González, L.; Entrenas-Costa, L.M.; Andújar-Espinosa, R. Calcifediol for Use in Treatment of Respiratory Disease. Nutrients 2022, 14, 2447. https://doi.org/10.3390/nu14122447
Entrenas-Castillo M, Salinero-González L, Entrenas-Costa LM, Andújar-Espinosa R. Calcifediol for Use in Treatment of Respiratory Disease. Nutrients. 2022; 14(12):2447. https://doi.org/10.3390/nu14122447
Chicago/Turabian StyleEntrenas-Castillo, Marta, Lourdes Salinero-González, Luis M. Entrenas-Costa, and Rubén Andújar-Espinosa. 2022. "Calcifediol for Use in Treatment of Respiratory Disease" Nutrients 14, no. 12: 2447. https://doi.org/10.3390/nu14122447
APA StyleEntrenas-Castillo, M., Salinero-González, L., Entrenas-Costa, L. M., & Andújar-Espinosa, R. (2022). Calcifediol for Use in Treatment of Respiratory Disease. Nutrients, 14(12), 2447. https://doi.org/10.3390/nu14122447





