Effect of Nutrition Education in NAFLD Patients Undergoing Simultaneous Hyperlipidemia Pharmacotherapy: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Randomization and Concealment
2.3. Evaluation of Patients
2.4. Diagnosis of Fatty Liver
2.5. Protocol of NAFLD Specific Nutrition Education (MNT Group) and Nutritional Education Paper Materials (Control Group)
2.6. Primary and Secondary Outcomes
2.7. Sample Size Calculation
2.8. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Comparison of Effects of Nutrition Education between Variables
3.3. Effect of Nutritional Education on Serum ALT Level
3.4. Effect of Nutritional Education on Steatosis, Metabolic and Fibrosis Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Im, H.J.; Ahn, Y.C.; Wang, J.-H.; Lee, M.M.; Son, C.G. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin. Res. Hepatol. Gastroenterol. 2020, 45, 101526. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medić-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, X.; Bian, H.; Xia, M. Metabolic dysfunction associated fatty liver disease and coronavirus disease 2019: Clinical relationship and current management. Lipids Health Dis. 2021, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nasr, P.; Fredrikson, M.; Ekstedt, M.; Kechagias, S. The amount of liver fat predicts mortality and development of type 2 diabetes in non-alcoholic fatty liver disease. Liver Int. 2020, 40, 1069–1078. [Google Scholar] [CrossRef] [Green Version]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-Alcoholic Fatty Liver Disease (NAFLD) and Its Connection with Insulin Resistance, Dyslipidemia, Atherosclerosis and Coronary Heart Disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut 2021, 70, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J. Hepatol. 2018, 68, 335–352. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation. Liver Transplant. 2017, 24, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Lee, H.W.; Yoo, J.-J.; Cho, Y.; Kim, S.U.; Lee, T.H.; Jang, B.K.; Kim, S.G.; Ahn, S.B.; Kim, H.; et al. KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 363–401. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, F.; Pellegrini, E.; Lugari, S.; Mondelli, A.; Bursi, S.; Onfiani, G.; Carubbi, F.; Lonardo, A. Statins and nonalcoholic fatty liver disease in the era of precision medicine: More friends than foes. Atherosclerosis 2019, 284, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, O.; Filozof, C.; Noureddin, M.; Berner-Hansen, M.; Schabel, E.; Omokaro, S.O.; Schattenberg, J.M.; Barradas, K.; Miller, V.; Francque, S.; et al. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: Recommendations from the Liver Forum. J. Hepatol. 2020, 73, 680–693. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020, 44, S53–S72. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.-V.; Fragopoulou, E.; Kontogianni, M. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef]
- Trovato, F.M.; Catalano, D.; Martines, G.F.; Pace, P.; Trovato, G.M. Mediterranean diet and non-alcoholic fatty liver disease: The need of extended and comprehensive interventions. Clin. Nutr. 2015, 34, 86–88. [Google Scholar] [CrossRef]
- Lee, S.-H. Lipid-Lowering Therapy Guidelines. Korean J. Med. 2019, 94, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, S.; Younossi, Z.M.; Remer, E.M.; Gramlich, T.; Ong, J.P.; Hurley, M.; Mullen, K.D.; Cooper, J.N.; Sheridan, M.J. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 745–750. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, H.-Y.; Hwang, J.-Y.; Kwon, S.; Chung, H.R.; Kwak, T.-K.; Kang, M.-H.; Choi, Y.-S. Development of Nutrition Quotient for Korean adults: Item selection and validation of factor structure. J. Nutr. Health 2018, 51, 340–356. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wree, A.; Broderick, L.; Canbay, A.; Hoffman, H.M.; Feldstein, A.E. From NAFLD to NASH to cirrhosis—new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 627–636. [Google Scholar] [CrossRef]
- Hannele, Y. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Kirk, E.; Reeds, D.N.; Finck, B.N.; Mayurranjan, M.S.; Patterson, B.W.; Klein, S. Dietary Fat and Carbohydrates Differentially Alter Insulin Sensitivity during Caloric Restriction. Gastroenterology 2009, 136, 1552–1560. [Google Scholar] [CrossRef] [Green Version]
- Haufe, S.; Engeli, S.; Kast, P.; Böhnke, J.; Utz, W.; Haas, V.; Hermsdorf, M.; Mähler, A.; Wiesner, S.; Birkenfeld, A.L.; et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011, 53, 1504–1514. [Google Scholar] [CrossRef]
- Browning, J.D.; Baker, A.J.; Rogers, T.; Davis, J.; Satapati, S.; Burgess, S.C. Short-term weight loss and hepatic triglyceride reduction: Evidence of a metabolic advantage with dietary carbohydrate restriction. Am. J. Clin. Nutr. 2011, 93, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Hydes, T.J.; Ravi, S.; Loomba, R.; Gray, M.E. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin. Mol. Hepatol. 2020, 26, 383–400. [Google Scholar] [CrossRef]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera, F.; George, J. The Role of Diet and Nutritional Intervention for the Management of Patients with NAFLD. Clin. Liver Dis. 2014, 18, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Gerber, M. Evaluating and adapting the Mediterranean diet for non-Mediterranean populations: A critical appraisal. Nutr. Rev. 2013, 71, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scorletti, E.; West, A.; Bhatia, L.; Hoile, S.P.; McCormick, K.G.; Burdge, G.; Lillycrop, K.; Clough, G.F.; Calder, P.; Byrne, C.D. Treating liver fat and serum triglyceride levels in NAFLD, effects of PNPLA3 and TM6SF2 genotypes: Results from the WELCOME trial. J. Hepatol. 2015, 63, 1476–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | All (n = 48) | Control (n = 21) | MNT (n = 27) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age (years)—mean ± SD | 41 ± 12 | 39 ± 11 | 42 ± 12 | 0.390 |
| Sex (male)—number (percent) | 30 (62.5) | 12 (57) | 18 (66.6) | |
| Laboratory values—mean ± SD | ||||
| White blood cell count (103/µL) | 7.1 ± 2.0 | 7.7 ± 1.9 | 6.6 ± 2.0 | 0.059 |
| Hemoglobin (g/dL) | 15.0 ± 1.1 | 14.9 ± 1.2 | 15.1 ± 1.0 | 0.594 |
| Platelet count (103/µL) | 260 ± 45 | 280 ± 49 | 245 ± 37 | 0.009 |
| AST (IU/L) | 48 ± 28 | 47 ± 35 | 49 ± 23 | 0.891 |
| ALT (IU/L) | 73 ± 42 | 71 ± 34 | 75 ± 48 | 0.713 |
| Total bilirubin (mg/dL) | 0.8 ± 0.3 | 0.8 ± 0.4 | 0.7 ± 0.2 | 0.355 |
| Serum albumin (g/dL) | 4.7 ± 0.3 | 4.72 ± 0.32 | 4.78 ± 0.31 | 0.494 |
| Prothromin time (INR) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.438 |
| Serum creatinine (mg/dL) | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.688 |
| Serum ferritin (ng/mL) | 361 ± 546 | 436 ± 742 | 279 ± 149 | 0.351 |
| Lipid profiles—mean ± SD | ||||
| Total cholesterol (mg/dL) | 239 ± 34 | 243 ± 33 | 235 ± 34 | 0.437 |
| LDL cholesterol (mg/dL) | 168 ± 23 | 171 ± 24 | 167 ± 22 | 0.562 |
| HDL cholesterol (mg/dL) | 45 ± 9 | 45 ± 11 | 45 ± 9.1 | 0.815 |
| Triglyceride (mg/dL) | 188 ± 98 | 163 ± 62 | 207 ± 117 | 0.100 |
| Diabetes related laboratory values—mean ± SD | ||||
| HbA1C (%) | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.7 ± 0.3 | 0.599 |
| Fasting glucose (mg/dL) | 106 ± 12 | 105 ± 10 | 106 ± 13 | 0.673 |
| Serum Insulin (uIU/mL) | 37 ± 62 | 28 ± 21 | 43 ± 81 | 0.361 |
| C-peptide (ng/mL) | 5.2 ± 3.8 | 4.7 ± 2.5 | 5.6 ± 4.6 | 0.370 |
| HOMA-IR | 165 ±257 | 130 ± 92 | 192 ± 333 | 0.362 |
| Anthropometric values—mean ± SD | ||||
| BMI (kg/m2) | 30.1 ± 4.3 | 30.0 ± 3.5 | 30.1 ± 4.8 | 0.933 |
| Muscle mass (kg) | 31.2 ± 8.4 | 29.4 ± 6.3 | 32.6 ± 9.6 | 0.168 |
| Body fat percentage (%) | 34.6 ± 8.2 | 35.3 ± 8.4 | 34.0 ± 8.2 | 0.602 |
| Noninvasive scoring system scores—mean ± SD | ||||
| CAP score (dB/m) | 325 ± 56 | 327 ± 76 | 323 ± 36 | 0.840 |
| Fibrosis score (kPa) | 7.0 ± 4.2 | 6.5 ± 2.5 | 7.4 ± 5.2 | 0.422 |
| FIB-4 | 1.0 ± 0.6 | 0.8 ± 0.4 | 1.1 ± 0.6 | 0.106 |
| Hepatic steatosis index | 43.6 ± 6.0 | 43.7 ± 4.9 | 43.5 ± 6.9 | 0.909 |
| NAFLD fat score | 5.6 ± 9.4 | 4.3 ± 3.4 | 6.7 ± 12.3 | 0.352 |
| NAFLD fibrosis score | −3.1 ± 1.3 | −3.5 ± 1.2 | −2.8 ± 1.3 | 0.063 |
| USG fatty liver grade (%) | 0.013 | |||
| Grade I | 8 (16.6) | 6 (28.5) | 2 (7.41) | |
| Grade II | 20 (41.6) | 4 (19) | 16 (59.2) | |
| Grade III | 20 (41.6) | 11 (52.3) | 9 (33.3) |
| Characteristics | Control (n = 21) | MNT (n = 27) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 48 w | Mean Difference (95% CI) | p | Baseline | 48 w | Mean Difference (95% CI) | p | |
| Primary outcome | ||||||||
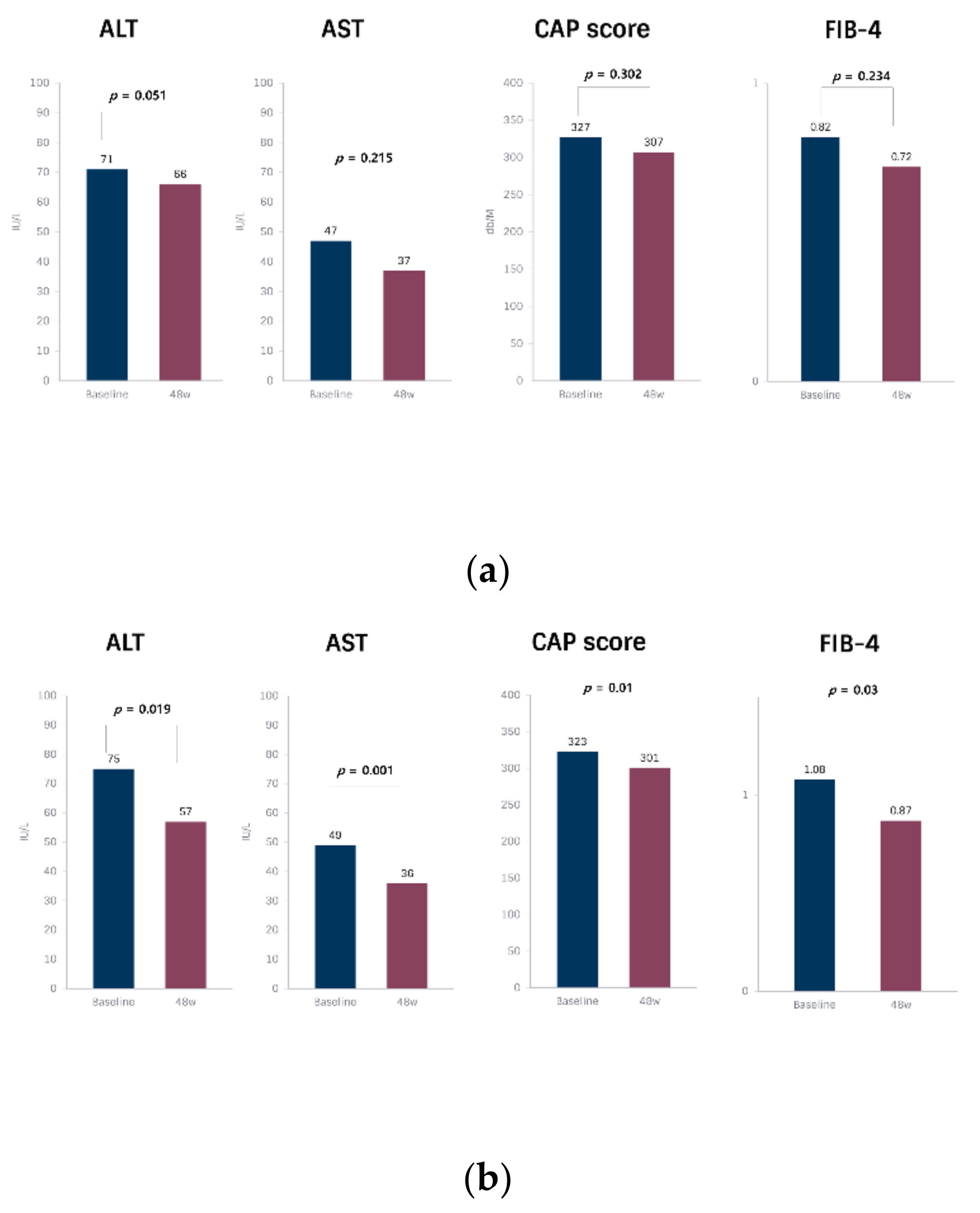
| ALT (IU/L) | 71 ± 34 | 66 ± 37 | −4.8 (−26.8, 17.2) | 0.051 | 75 ± 48 | 57 ± 36 | −18.4 (−33.5, −3.2) | 0.019 |
| Steatosis factors | ||||||||
| AST (IU/L) | 47 ± 35 | 37 ± 17 | −10.3 (−27.1, 0.5) | 0.215 | 49 ± 23 | 36 ± 15 | −12.9 (−20.3, −5.46) | 0.001 |
| CAP score (dB/m) | 327 ± 76 | 307 ± 43 | −20.2 (−60.2, 19.6) | 0.302 | 323 ± 36 | 301 ± 44 | −22.5 (−39.3, −5.8) | 0.010 |
| NAFLD fat score | 4.3 ± 3.38 | 2.9 ± 3.26 | −1.3 (−3.2, 0.5) | 0.159 | 6.6 ± 12.2 | 2.4 ± 1.8 | −4.2 (−8.7, 0.2) | 0.065 |
| Hepatic steatosis index | 43.6 ± 4.8 | 44.5 ± 4.9 | 0.8 (−1.3, 3.04) | 0.428 | 43.5 ± 6.8 | 43 ± 6.1 | −0.4 (−2.2, 1.2) | 0.565 |
| USG fatty liver grade (%) | ||||||||
| Grade 0 | − | − | − | 1 (3.7) | ||||
| Grade I | 6 (28.5) | 6 (28.5) | 2 (7.41) | 11 (40.7) | ||||
| Grade II | 4 (19) | 10 (47.6) | 16 (59.2) | 7 (25.9) | ||||
| Grade III | 11 (52.3) | 5 (23.8) | 9 (33.3) | 8 (29.6) | ||||
| Metabolic factors | ||||||||
| Total cholesterol (mg/dL) | 243 ± 33 | 163 ± 48 | −79.8 (−103, −56) | <0.001 | 235 ± 34 | 152 ± 42 | −83.4 (−103, −63) | <0.001 |
| LDL cholesterol (mg/dL) | 171 ± 24 | 95 ± 36 | −75.2 (−93.7, −56.7) | <0.001 | 167 ± 22 | 88 ± 37 | −78.1 (−95.9, −60.4) | <0.001 |
| HDL cholesterol (mg/dL) | 45 ± 11 | 47 ± 9 | 1.7 (−1.9, 5.5) | 0.336 | 45 ± 9 | 48 ± 9 | 3.3 (0.7, 5.8) | 0.012 |
| Triglyceride (mg/dL) | 163 ± 62 | 166 ± 106 | 2.4 (−38, 2.9) | 0.901 | 207 ± 117 | 145 ± 51 | −62.2 (−107.8, −16.5) | 0.009 |
| BMI (kg/m2) | 29.9 ± 3.5 | 29.8 ± 3.7 | −0.1 (−0.6, 0.2) | 0.450 | 30.0 ± 4.8 | 29.7 ± 4.5 | −0.3 (−0.8, 0.2) | 0.251 |
| Muscle mass (kg) | 29.3 ± 6.2 | 29.5 ± 6.3 | 0.1 (−0.1, 0.4) | 0.332 | 32.5 ± 9.6 | 31.1 ± 7.4 | −1.4 (−3.5, 0.6) | 0.164 |
| Body fat percentage (%) | 35.3 ± 8.3 | 36 ± 7.2 | 0.7 (−2.2, 3.6) | 0.620 | 34 ± 8.2 | 33.4 ± 8.3 | −0.6 (−1.7, 0.4) | 0.236 |
| HOMA-IR | 130 ± 92 | 115 ± 99 | −15.1 (−70.1, 39.9) | 0.573 | 192 ± 333 | 100 ± 56 | −91.5(−212, 29.3) | 0.131 |
| Fasting glucose (mg/dL) | 105 ± 10 | 105 ± 13 | −0.1 (−5.3, 5.1) | 0.970 | 106 ± 13 | 108 ± 11 | 1.4 (−2.1, 5) | 0.402 |
| Fibrosis factors | ||||||||
| NAFLD fibrosis score | −3.4 ± 1.1 | −3.7 ± 1.5 | −0.3 (−0.8, 0.1) | 0.186 | −2.7 ± 1.3 | −2.7 ± 1.3 | 0 (−0.3, 0.3) | 0.998 |
| Fibrosis score (kPa) | 6.4 ± 2.5 | 5.5 ± 1.8 | −0.9 (−1.9, 0.1) | 0.085 | 7.3 ± 5.1 | 5.4 ± 1.4 | −1.9 (−3.9, 0) | 0.053 |
| FIB-4 | 0.8 ± 0.4 | 0.7 ± 0.4 | −0.1 (−0.2, 0) | 0.234 | 1.0 ± 0.6 | 0.8 ± 0.5 | −0.2 (−0.3, 0) | 0.003 |
| Characteristics | Control (n = 21) | MNT (n = 27) | p1 * | p2 * | ||
|---|---|---|---|---|---|---|
| 24 w | 48 w | 24 w | 48 w | |||
| Primary outcome | ||||||
| ALT (IU/L) | 61 ± 25 | 66 ± 37 | 77 ± 57 | 57 ± 36 | 0.246 | 0.400 |
| Steatosis factors | ||||||
| AST (IU/L) | 35 ± 11 | 37 ± 17 | 48 ± 33 | 36 ± 15 | 0.075 | 0.776 |
| CAP score (dB/m) | 299 ± 32 | 307 ± 43 | 297 ± 34 | 301 ± 44 | 0.831 | 0.642 |
| NAFLD fat score | 3.0 ± 2.5 | 2.9 ± 3.2 | 2.8 ± 2.0 | 2.4 ± 1.8 | 0.677 | 0.489 |
| Hepatic steatosis index | 44.5 ± 4.4 | 44.5 ± 4.9 | 43.3 ± 4.8 | 43.0 ± 6.1 | 0.389 | 0.354 |
| USG fatty liver grade (%) | NA | 0.574 | ||||
| Grade 0 | NA | - | NA | 1 (3.7) | ||
| Grade I | NA | 6 (28.5) | NA | 11 (40.7) | ||
| Grade II | NA | 10 (47.6) | NA | 7 (25.9) | ||
| Grade III | NA | 5 (23.8) | NA | 8 (29.6) | ||
| Metabolic factors | ||||||
| Total cholesterol (mg/dL) | 156 ± 43 | 163 ± 48 | 135 ± 28 | 152 ± 42 | 0.051 | 0.392 |
| LDL cholesterol (mg/dL) | 87 ± 31 | 95 ± 36 | 75 ± 24 | 87 ± 37 | 0.140 | 0.456 |
| HDL cholesterol (mg/dL) | 47 ± 9 | 47 ± 9 | 45 ± 7 | 48 ± 9 | 0.408 | 0.767 |
| Triglyceride (mg/dL) | 173 ± 128 | 166 ± 106 | 137 ± 47 | 145 ± 51 | 0.230 | 0.381 |
| BMI (kg/m2) | 29.8 ± 3.9 | 29.8 ± 3.7 | 29.5 ± 4.1 | 29.7 ± 4.5 | 0.845 | 0.974 |
| Muscle mass (kg) | 29.4 ± 6.5 | 29.5 ± 6.3 | 30.9 ± 7.1 | 31.1 ± 7.4 | 0.443 | 0.427 |
| Body fat percentage (%) | 35.9 ± 8.2 | 36.0 ± 7.2 | 33.5 ± 7.7 | 33.4 ± 8.3 | 0.312 | 0.261 |
| HOMA-IR | 121 ± 77 | 115 ± 99 | 101 ± 50 | 100 ± 56 | 0.285 | 0.529 |
| Fasting glucose (mg/dL) | 105 ± 15 | 105 ± 13 | 109 ± 18 | 108 ± 11 | 0.403 | 0.397 |
| HbA1C (%) | NA | 5.9 ± 0.4 | NA | 5.8 ± 0.4 | NA | 0.527 |
| Serum Insulin (uIU/mL) | NA | 24 ± 20 | NA | 20 ± 11 | NA | 0.442 |
| C-peptide (ng/mL) | NA | 3.4 ± 1.1 | NA | 3.1 ± 1.0 | NA | 0.365 |
| Fibrosis factors | ||||||
| NAFLD fibrosis score | −3.6 ± 1.3 | −3.7 ± 1.5 | −2.6 ± 1.1 | −2.7 ± 1.3 | 0.012 | 0.017 |
| Fibrosis score (kPa) | 6.0 ± 2.4 | 5.5 ± 1.8 | 5.7 ± 2.2 | 5.4 ± 1.4 | 0.831 | 0.810 |
| FIB-4 | 0.7 ± 0.3 | 0.7 ± 0.4 | 1.0 ± 0.5 | 0.8 ± 0.5 | 0.039 | 0.345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.M.; Bae, J.H.; Chang, Y.; Lee, S.H.; Moon, J.E.; Jeong, S.W.; Jang, J.Y.; Kim, S.G.; Kim, H.S.; Yoo, J.-J.; et al. Effect of Nutrition Education in NAFLD Patients Undergoing Simultaneous Hyperlipidemia Pharmacotherapy: A Randomized Controlled Trial. Nutrients 2021, 13, 4453. https://doi.org/10.3390/nu13124453
Lee WM, Bae JH, Chang Y, Lee SH, Moon JE, Jeong SW, Jang JY, Kim SG, Kim HS, Yoo J-J, et al. Effect of Nutrition Education in NAFLD Patients Undergoing Simultaneous Hyperlipidemia Pharmacotherapy: A Randomized Controlled Trial. Nutrients. 2021; 13(12):4453. https://doi.org/10.3390/nu13124453
Chicago/Turabian StyleLee, Won Myung, Jea Hurn Bae, Young Chang, Sae Hwan Lee, Ji Eun Moon, Soung Won Jeong, Jae Young Jang, Sang Gyune Kim, Hong Soo Kim, Jeong-Ju Yoo, and et al. 2021. "Effect of Nutrition Education in NAFLD Patients Undergoing Simultaneous Hyperlipidemia Pharmacotherapy: A Randomized Controlled Trial" Nutrients 13, no. 12: 4453. https://doi.org/10.3390/nu13124453






