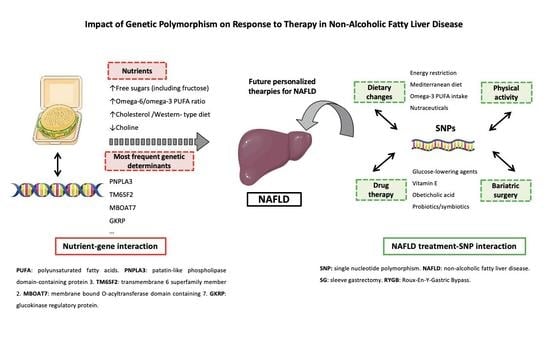
Impact of Genetic Polymorphism on Response to Therapy in Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Nutrigenetics and NAFLD Pathogenesis
2.1. Carbohydrates
2.2. Lipids
2.3. Choline Deficiency in NAFLD
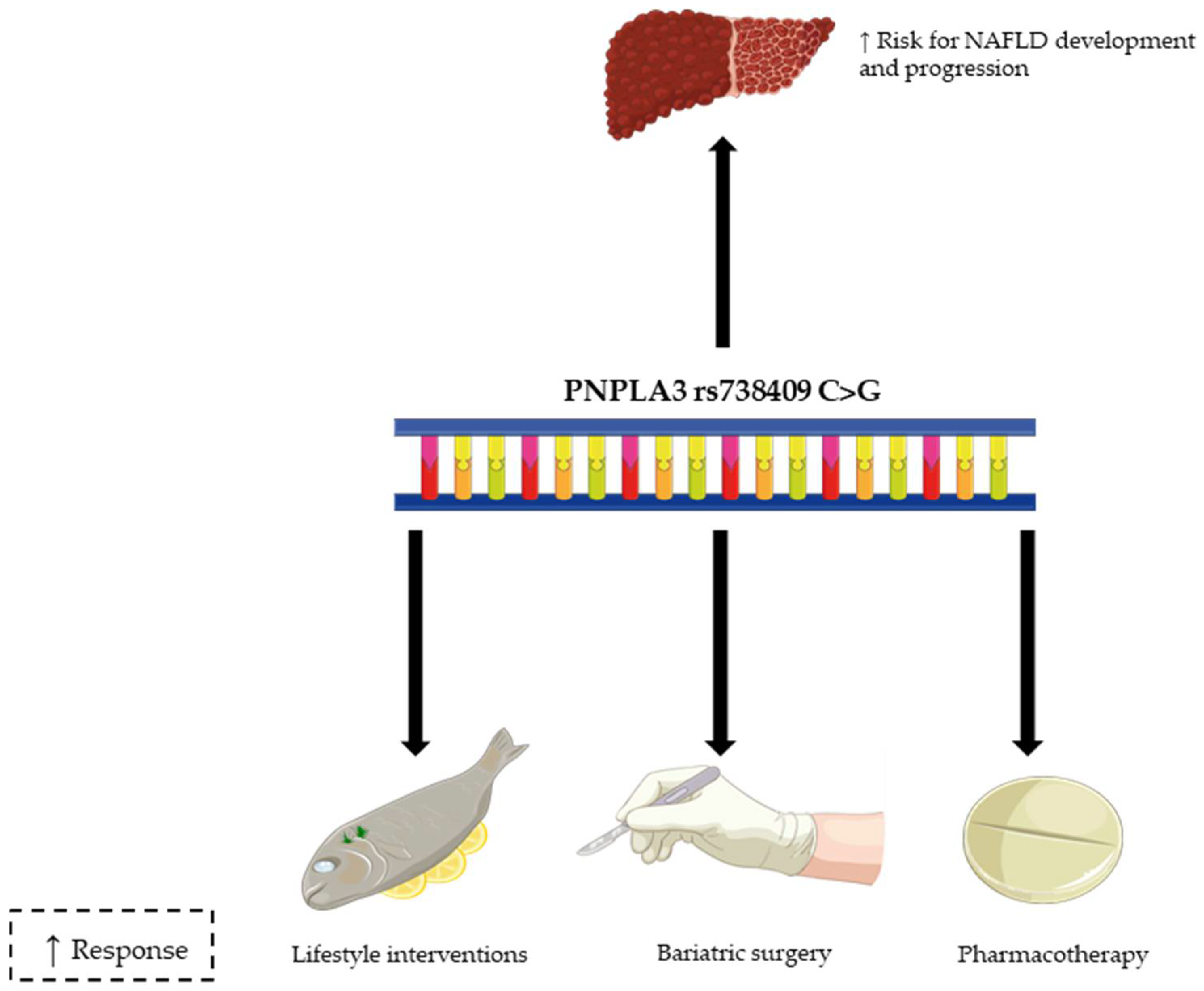
3. Gene Polymorphism and Response to Lifestyle Interventions in NAFLD
3.1. Dietary Changes
3.2. The Role of Omega-3 PUFA
3.3. Specific Nutrients
3.4. Physical Activity
4. Future Perspectives in NAFLD Treatment: Toward Personalized Therapies?
4.1. Bariatric Surgery and NAFLD
4.2. Other Therapies
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2015, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.T.; Kleiner, D.E. Histopathological findings of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. J. Med. Ultrason. 2020, 47, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Tampi, R.; Priyadarshini, M.; Nader, F.; Younossi, I.M.; Racila, A. Burden of Illness and Economic Model for Patients with Nonalcoholic Steatohepatitis in the United States. Hepatology 2019, 69, 564–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.; Kongpakpaisarn, K.; Bohra, C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int. J. Cardiol. 2018, 259, 216–219. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Koshiaris, C.; Henry, J.A.; Noreik, M.; Morris, E.; Manoharan, I.; Tudor, K.; Bodenham, E.; Dunnigan, A.; Jebb, S.A.; et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism 2021, 115, 154455. [Google Scholar] [CrossRef]
- Gonçalves, I.O.; Passos, E.; Rocha-Rodrigues, S.; Torrella, J.R.; Rizo, D.; Santos-Alves, E.; Portincasa, P.; Martins, M.J.; Ascensão, A.; Magalhães, J. Physical exercise antagonizes clinical and anatomical features characterizing Lieber-DeCarli diet-induced obesity and related metabolic disorders. Clin. Nutr. 2015, 34, 241–247. [Google Scholar] [CrossRef]
- Lee, Y.; Doumouras, A.G.; Yu, J.; Brar, K.; Banfield, L.; Gmora, S.; Anvari, M.; Hong, D. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1040–1060.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongiovanni, P.; Valenti, L. Genetics of nonalcoholic fatty liver disease. Metabolism 2016, 65, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Trépo, E.; Romeo, S.; Zucman-Rossi, J.; Nahon, P. PNPLA3 gene in liver diseases. J. Hepatol. 2016, 65, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Li, B.-T.; Sun, M.; Li, Y.-F.; Wang, J.-Q.; Zhou, Z.-M.; Song, B.-L.; Luo, J. Disruption of the ERLIN–TM6SF2–APOB complex destabilizes APOB and contributes to non-alcoholic fatty liver disease. PLoS Genet. 2020, 16, e1008955. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Liu, S.; Zhao, Z.; Yu, X.; Liu, Q.; Xin, Y.; Xuan, S. Association of GCKR Gene Polymorphisms with the Risk of Nonalcoholic Fatty Liver Disease and Coronary Artery Disease in a Chinese Northern Han Population. J. Clin. Transl. Hepatol. 2019, 7, 297. [Google Scholar] [CrossRef]
- Ma, Y.; Belyaeva, O.V.; Brown, P.M.; Fujita, K.; Valles, K.; Karki, S.; De Boer, Y.S.; Koh, C.; Chen, Y.; Du, X.; et al. 17-Beta Hydroxysteroid Dehydrogenase 13 Is a Hepatic Retinol Dehydrogenase Associated with Histological Features of Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 1504–1519. [Google Scholar] [CrossRef]
- Hooper, A.J.; Adams, L.A.; Burnett, J.R. Genetic determinants of hepatic steatosis in man. J. Lipid Res. 2011, 52, 593–617. [Google Scholar] [CrossRef] [Green Version]
- Karkucinska-Wieckowska, A.; Simoes, I.C.M.; Kalinowski, P.; Lebiedzinska-Arciszewska, M.; Zieniewicz, K.; Milkiewicz, P.; Górska-Ponikowska, M.; Pinton, P.; Malik, A.N.; Krawczyk, M.; et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: A complex relationship. Eur. J. Clin. Investig. 2021, e13622. [Google Scholar] [CrossRef] [PubMed]
- Al-Serri, A.; Anstee, Q.M.; Valenti, L.; Nobili, V.; Leathart, J.B.; Dongiovanni, P.; Patch, J.; Fracanzani, A.L.; Fargion, S.; Day, C.P.; et al. The SOD2 C47T polymorphism influences NAFLD fibrosis severity: Evidence from case-control and intra-familial allele association studies. J. Hepatol. 2012, 56, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fares, R.; Petta, S.; Lombardi, R.; Grimaudo, S.; Dongiovanni, P.; Pipitone, R.M.; Rametta, R.; Fracanzani, A.L.; Mozzi, E.; Craxi, A.; et al. The UCP2 -866 G>A promoter region polymorphism is associated with nonalcoholic steatohepatitis. Liver Int. 2015, 35, 1574–1580. [Google Scholar] [CrossRef]
- Lu, M.-Y.; Huang, J.-F.; Liao, Y.-C.; Bai, R.-K.; Trieu, R.B.; Chuang, W.-L.; Yu, M.-L.; Juo, S.-H.H.; Wong, L.-J. Mitochondrial polymorphism 12361A>G is associated with nonalcoholic fatty liver disease. Transl. Res. 2012, 159, 58–59. [Google Scholar] [CrossRef]
- Eslam, M.; George, J. Genetic contributions to NAFLD: Leveraging shared genetics to uncover systems biology. Nat. Rev. Gastroenterol. Hepatol. 2019, 17, 40–52. [Google Scholar] [CrossRef]
- Smagris, E.; BasuRay, S.; Li, J.; Huang, Y.; Lai, K.V.; Gromada, J.; Cohen, J.C.; Hobbs, H.H. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology 2015, 61, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Schröder, T.; Kucharczyk, D.; Bär, F.; Pagel, R.; Derer, S.; Jendrek, S.T.; Sünderhauf, A.; Brethack, A.-K.; Hirose, M.; Möller, S.; et al. Mitochondrial gene polymorphisms alter hepatic cellular energy metabolism and aggravate diet-induced non-alcoholic steatohepatitis. Mol. Metab. 2016, 5, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Rametta, R.; Dongiovanni, P. Genetic and Epigenetic Modifiers of Alcoholic Liver Disease. Int. J. Mol. Sci. 2018, 19, 3857. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.B.; Gunn, P.J.; Fielding, B.A. The Role of Dietary Sugars and de novo Lipogenesis in Non-Alcoholic Fatty Liver Disease. Nutrients 2014, 6, 5679–5703. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.N.; Lê, K.-A.; Walker, R.W.; Vikman, S.; Spruijt-Metz, N.; Weigensberg, M.J.; Allayee, H.; Goran, M.I. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am. J. Clin. Nutr. 2010, 92, 1522–1527. [Google Scholar] [CrossRef] [Green Version]
- Nobili, V.; Liccardo, D.; Bedogni, G.; Salvatori, G.; Gnani, D.; Bersani, I.; Alisi, A.; Valenti, L.; Raponi, M. Influence of dietary pattern, physical activity, and I148M PNPLA3 on steatosis severity in at-risk adolescents. Genes Nutr. 2014, 9, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, N.; Caprio, S.; Pierpont, B.; Van Name, M.; Savoye, M.; Parks, E. Hepatic De Novo Lipogenesis in Obese Youth Is Modulated by a Common Variant in the GCKR Gene. J. Clin. Endocrinol. Metab. 2015, 100, E1125–E1132. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.; Ma, J.; Patel, K.; Berger, S.; Lau, J.; Lichtenstein, A.H. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 833–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todoric, J.; Di Caro, G.; Reibe, S.; Henstridge, D.C.; Green, C.R.; Vrbanac, A.; Ceteci, F.; Conche, C.; McNulty, R.; Shalapour, S.; et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2020, 2, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Genetic-specific Effects of Fructose on Liver Lipogenesis—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03783195?term=Liver+Lipogenesis&cond=Fructose&draw=2&rank=1 (accessed on 26 July 2021).
- Miele, L.; Dall’Armi, V.; Cefalo, C.; Nedovic, B.; Arzani, D.; Amore, R.; Rapaccini, G.; Gasbarrini, A.; Ricciardi, W.; Grieco, A.; et al. A case–control study on the effect of metabolic gene polymorphisms, nutrition, and their interaction on the risk of non-alcoholic fatty liver disease. Genes Nutr. 2014, 9, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.-J. Diet high in fructose promotes liver steatosis and hepatocyte apoptosis in C57BL/6J female mice: Role of disturbed lipid homeostasis and increased oxidative stress. Food Chem. Toxicol. 2017, 103, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Jegatheesan, P.; De Bandt, J. Fructose and NAFLD: The Multifaceted Aspects of Fructose Metabolism. Nutrients 2017, 9, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Berumen, C.I.; Ortiz-Avila, O.; Vargas-Vargas, M.A.; Del Rosario-Tamayo, B.A.; Guajardo-López, C.; Saavedra-Molina, A.; Rodríguez-Orozco, A.R.; Cortés-Rojo, C. The severity of rat liver injury by fructose and high fat depends on the degree of respiratory dysfunction and oxidative stress induced in mitochondria. Lipids Heal. Dis. 2019, 18, 78. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, R.R.; Moreira, L.D.P.D.; Costa, M.A.d.C.; Toledo, R.C.L.; Grancieri, M.; Nascimento, T.P.D.; Ferreira, M.S.L.; da Matta, S.L.P.; Eller, M.R.; Martino, H.S.D.; et al. Kombuchas from green and black teas reduce oxidative stress, liver steatosis and inflammation, and improve glucose metabolism in Wistar rats fed a high-fat high-fructose diet. Food Funct. 2021, 12, 10813–10827. [Google Scholar] [CrossRef]
- Bagul, P.K.; Middela, H.; Mattapally, S.; Padiya, R.; Bastia, T.; Madhusudana, K.; Reddy, B.R.; Chakravarty, S.; Banerjee, S.K. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol. Res. 2012, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Suwannaphet, W.; Meeprom, A.; Yibchok-Anun, S.; Adisakwattana, S. Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem. Toxicol. 2010, 48, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-Y.; Wu, L.-Y.; Hwang, L.S. Effect of a Proanthocyanidin-Rich Extract from Longan Flower on Markers of Metabolic Syndrome in Fructose-Fed Rats. J. Agric. Food Chem. 2008, 56, 11018–11024. [Google Scholar] [CrossRef] [PubMed]
- Polizio, A.H.; Gonzales, S.; Muñoz, M.C.; Peña, C.; Tomaro, M.L. Behaviour of the anti-oxidant defence system and heme oxygenase-1 protein expression in fructose-hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Herath, C.B.; Jia, Z.; Andrikopoulos, S.; Brown, B.E.; Davies, M.; Rivera, L.R.; Furness, J.B.; Forbes, J.; Angus, P.W. Dietary advanced glycation end-products aggravate non-alcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 8026–8040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, N.; Savoye, M.; Kim, G.; Marotto, K.; Shaw, M.M.; Pierpont, B.; Caprio, S. Hepatic Fat Accumulation Is Modulated by the Interaction between the rs738409 Variant in the PNPLA3 Gene and the Dietary Omega6/Omega3 PUFA Intake. PLoS ONE 2012, 7, e37827. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.; Arenaza, L.; Rios, C.; Plows, J.; Berger, P.; Alderete, T.; Fogel, J.; Nayak, K.; Mohamed, P.; Hwang, D.; et al. PNPLA3 Genotype, Arachidonic Acid Intake, and Unsaturated Fat Intake Influences Liver Fibrosis in Hispanic Youth with Obesity. Nutrients 2021, 13, 1621. [Google Scholar] [CrossRef]
- Perez-Diaz-Del-Campo, N.; Abete, I.; Cantero, I.; Marin-Alejandre, B.A.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Riezu-Boj, J.I.; Milagro, F.I.; et al. Association of the SH2B1 rs7359397 Gene Polymorphism with Steatosis Severity in Subjects with Obesity and Non-Alcoholic Fatty Liver Disease. Nutrients 2020, 12, 1260. [Google Scholar] [CrossRef]
- Malhotra, P.; Gill, R.K.; Saksena, S.; Alrefai, W.A. Disturbances in Cholesterol Homeostasis and Non-alcoholic Fatty Liver Diseases. Front. Med. 2020, 7, 467. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Subramanian, S.; Chait, A.; Haigh, W.G.; Yeh, M.M.; Farrell, G.C.; Lee, S.P.; Savard, C. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J. Lipid Res. 2017, 58, 1067–1079. [Google Scholar] [CrossRef] [Green Version]
- Chatrath, H.; Vuppalanchi, R.; Chalasani, N. Dyslipidemia in Patients with Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2012, 32, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, E.; Vergnes, M.-F.; Defoort, C.; Planells, R.; Portugal, H.; Nicolay, A.; Lairon, D. Cholesterol absorption status and fasting plasma cholesterol are modulated by the microsomal triacylglycerol transfer protein −493 G/T polymorphism and the usual diet in women. Genes Nutr. 2010, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouda, W.; Ashour, E.; Shaker, Y.; Ezzat, W. MTP genetic variants associated with non-alcoholic fatty liver in metabolic syndrome patients. Genes Dis. 2017, 4, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cipolla, U.; Cassader, M.; Pinach, S.; Saba, F.; De Michieli, F.; Paschetta, E.; Bongiovanni, D.; Framarin, L.; Leone, N.; et al. TM6SF2 rs58542926 variant affects postprandial lipoprotein metabolism and glucose homeostasis in NAFLD. J. Lipid Res. 2017, 58, 1221–1229. [Google Scholar] [CrossRef] [Green Version]
- Musso, G.; Bo, S.; Cassader, M.; De Michieli, F.; Gambino, R. Impact of sterol regulatory element-binding factor-1c polymorphism on incidence of nonalcoholic fatty liver disease and on the severity of liver disease and of glucose and lipid dysmetabolism. Am. J. Clin. Nutr. 2013, 98, 895–906. [Google Scholar] [CrossRef] [Green Version]
- Guerrerio, A.L.; Colvin, R.M.; Schwartz, A.K.; Molleston, J.P.; Murray, K.F.; Diehl, A.; Mohan, P.; Schwimmer, J.; Lavine, J.E.; Torbenson, M.S.; et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2012, 95, 892–900. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, K.-A.; Kozyreva, O.G.; Song, J.; Galanko, J.A.; Fischer, L.M.; Zeisel, S.H. Common genetic polymorphisms affect the human requirement for the nutrient choline. FASEB J. 2006, 20, 1336–1344. [Google Scholar] [CrossRef]
- Dong, H.; Wang, J.; Li, C.; Hirose, A.; Nozaki, Y.; Takahashi, M.; Ono, M.; Akisawa, N.; Iwasaki, S.; Saibara, T.; et al. The phosphatidylethanolamine N-methyltransferase gene V175M single nucleotide polymorphism confers the susceptibility to NASH in Japanese population. J. Hepatol. 2007, 46, 915–920. [Google Scholar] [CrossRef]
- Kohlmeier, M.; da Costa, K.-A.; Fischer, L.M.; Zeisel, S.H. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 16025–16030. [Google Scholar] [CrossRef] [Green Version]
- EASL–EASD–EASO. Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Marin-Alejandre, B.A.; Abete, I.; Cantero, I.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito, A.; Quiroga, J.; Martinez-Echeverria, A.; Uriz-Otano, J.I.; et al. The Metabolic and Hepatic Impact of Two Personalized Dietary Strategies in Subjects with Obesity and Nonalcoholic Fatty Liver Disease: The Fatty Liver in Obesity (FLiO) Randomized Controlled Trial. Nutrients 2019, 11, 2543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johari, M.I.; Yusoff, K.; Haron, J.; Nadarajan, C.; Ibrahim, K.N.; Wong, M.S.; Hafidz, M.I.A.; Chua, B.E.; Hamid, N.; Arifin, W.N.; et al. A Randomised Controlled Trial on the Effectiveness and Adherence of Modified Alternate-day Calorie Restriction in Improving Activity of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 11232. [Google Scholar] [CrossRef] [PubMed]
- Skytte, M.J.; Samkani, A.; Petersen, A.D.; Thomsen, M.N.; Astrup, A.; Chabanova, E.; Frystyk, J.; Holst, J.J.; Thomsen, H.S.; Madsbad, S.; et al. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: A randomised controlled trial. Diabetologia 2019, 62, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Razavi Zade, M.; Telkabadi, M.H.; Bahmani, F.; Salehi, B.; Farshbaf, S.; Asemi, Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: A randomized clinical trial. Liver Int. 2016, 36, 563–571. [Google Scholar] [CrossRef]
- Perez-Diaz-Del-Campo, N.; Marin-Alejandre, B.A.; Cantero, I.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Riezu-Boj, J.I.; Milagro, F.I.; Tur, J.A.; et al. Differential response to a 6-month energy-restricted treatment depending on SH2B1 rs7359397 variant in NAFLD subjects: Fatty Liver in Obesity (FLiO) Study. Eur. J. Nutr. 2021, 60, 3043–3057. [Google Scholar] [CrossRef]
- Seko, Y.; Yamaguchi, K.; Tochiki, N.; Yano, K.; Takahashi, A.; Okishio, S.; Kataoka, S.; Okuda, K.; Umemura, A.; Moriguchi, M.; et al. The Effect of Genetic Polymorphism in Response to Body Weight Reduction in Japanese Patients with Nonalcoholic Fatty Liver Disease. Genes 2021, 12, 628. [Google Scholar] [CrossRef]
- Sevastianova, K.; Kotronen, A.; Gastaldelli, A.; Perttilä, J.; Hakkarainen, A.; Lundbom, J.; Suojanen, L.; Orho-Melander, M.; Lundbom, N.; Ferrannini, E.; et al. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am. J. Clin. Nutr. 2011, 94, 104–111. [Google Scholar] [CrossRef]
- Shen, J.; Wong, G.L.-H.; Chan, H.L.-Y.; Chan, R.S.; Chan, H.-Y.; Chu, W.; Cheung, B.H.-K.; Yeung, D.K.-W.; Li, L.S.; Sea, M.M.-M.; et al. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2015, 30, 139–146. [Google Scholar] [CrossRef]
- Koot, B.G.P.; Van Der Baan-Slootweg, O.H.; Vinke, S.; Bohte, A.E.; Tamminga-Smeulders, C.L.J.; Jansen, P.L.M.; Stoker, J.; Benninga, M.A. Intensive lifestyle treatment for non-alcoholic fatty liver disease in children with severe obesity: Inpatient versus ambulatory treatment. Int. J. Obes. 2015, 40, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Stachowska, E.; Milkiewicz, P.; Lammert, F.; Milkiewicz, M. Reduction of Caloric Intake Might Override the Prosteatotic Effects of the PNPLA3 p.I148M and TM6SF2 p.E167K Variants in Patients with Fatty Liver: Ultrasound-Based Prospective Study. Digestion 2016, 93, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.Z.; Hennige, A.M.; Peter, A.; Kovarova, M.; Totsikas, C.; Machann, J.; Kröber, S.M.; Sperl, B.; Schleicher, E.; Schick, F.; et al. The Gly385(388)Arg Polymorphism of the FGFR4 Receptor Regulates Hepatic Lipogenesis under Healthy Diet. J. Clin. Endocrinol. Metab. 2019, 104, 2041–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliora, A.C.; Gioxari, A.; Kalafati, I.P.; Diolintzi, A.; Kokkinos, A.; Dedoussis, G.V. The Effectiveness of Mediterranean Diet in Nonalcoholic Fatty Liver Disease Clinical Course: An Intervention Study. J. Med. Food 2019, 22, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Ryterska, K.; Maciejewska, D.; Banaszczak, M.; Milkiewicz, P.; Milkiewicz, M.; Gutowska, I.; Ossowski, P.; Kaczorowska, M.; Jamioł-Milc, D.; et al. Nutritional Strategies for the Individualized Treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) Based on the Nutrient-Induced Insulin Output Ratio (NIOR). Int. J. Mol. Sci. 2016, 17, 1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, H.M.; Johnson, N.A.; Burdon, C.A.; Cohn, J.S.; O’Connor, H.T.; George, J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 56, 944–951. [Google Scholar] [CrossRef] [Green Version]
- He, X.-X.; Wu, X.-L.; Chen, R.-P.; Chen, C.; Liu, X.-G.; Wu, B.-J.; Huang, Z.-M. Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0162368. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Bedogni, G.; Donati, B.; Alisi, A.; Valenti, L. The I148M Variant of PNPLA3 Reduces the Response to Docosahexaenoic Acid in Children with Non-Alcoholic Fatty Liver Disease. J. Med. Food 2013, 16, 957–960. [Google Scholar] [CrossRef] [Green Version]
- Scorletti, E.; West, A.; Bhatia, L.; Hoile, S.P.; McCormick, K.G.; Burdge, G.; Lillycrop, K.; Clough, G.F.; Calder, P.; Byrne, C.D. Treating liver fat and serum triglyceride levels in NAFLD, effects of PNPLA3 and TM6SF2 genotypes: Results from the WELCOME trial. J. Hepatol. 2015, 63, 1476–1483. [Google Scholar] [CrossRef] [Green Version]
- Kuttner, C.-S.; Mancina, R.; Wagenpfeil, G.; Lammert, F.; Stokes, C. Four-Week Omega-3 Supplementation in Carriers of the Prosteatotic PNPLA3 p.I148M Genetic Variant: An Open-Label Study. Lifestyle Genom. 2019, 12, 10–17. [Google Scholar] [CrossRef]
- Oscarsson, J.; Önnerhag, K.; Risérus, U.; Sundén, M.; Johansson, L.; Jansson, P.-A.; Moris, L.; Nilsson, P.M.; Eriksson, J.W.; Lind, L. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled study. J. Clin. Lipidol. 2018, 12, 1390–1403.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentino, P.L.; et al. A Low ω-6 to ω-3 PUFA Ratio (n–6:n–3 PUFA) Diet to Treat Fatty Liver Disease in Obese Youth. J. Nutr. 2020, 150, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cohen, J.C.; Hobbs, H.H. Expression and Characterization of a PNPLA3 Protein Isoform (I148M) Associated with Nonalcoholic Fatty Liver Disease. J. Biol. Chem. 2011, 286, 37085–37093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, J.W.; Lundkvist, P.; Jansson, P.-A.; Johansson, L.; Kvarnström, M.; Moris, L.; Miliotis, T.; Forsberg, G.-B.; Risérus, U.; Lind, L.; et al. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: A double-blind randomised placebo-controlled study. Diabetologia 2018, 61, 1923–1934. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Colletti, A.; Bellentani, S. Nutraceutical Approach to Non-Alcoholic Fatty Liver Disease (NAFLD): The Available Clinical Evidence. Nutrients 2018, 10, 1153. [Google Scholar] [CrossRef] [Green Version]
- Kannt, A.; Papada, E.; Kammermeier, C.; D’Auria, G.; Jiménez-Hernández, N.; Stephan, M.; Schwahn, U.; Madsen, A.N.; Ostergaard, M.V.; Dedoussis, G.; et al. Mastiha (Pistacia lentiscus) Improves Gut Microbiota Diversity, Hepatic Steatosis, and Disease Activity in a Biopsy-Confirmed Mouse Model of Advanced Non-Alcoholic Steatohepatitis and Fibrosis. Mol. Nutr. Food Res. 2019, 63, e1900927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amerikanou, C.; Kanoni, S.; Kaliora, A.C.; Barone, A.; Bjelan, M.; D’Auria, G.; Gioxari, A.; Gosalbes, M.J.; Mouchti, S.; Stathopoulou, M.G.; et al. Effect of Mastiha supplementation on NAFLD: The MAST4HEALTH Randomised, Controlled Trial. Mol. Nutr. Food Res. 2021, 65, 2001178. [Google Scholar] [CrossRef]
- Kanoni, S.; Kumar, S.; Amerikanou, C.; Kurth, M.J.; Stathopoulou, M.G.; Bourgeois, S.; Masson, C.; Kannt, A.; Cesarini, L.; Kontoe, M.-S.; et al. Nutrigenetic Interactions Might Modulate the Antioxidant and Anti-Inflammatory Status in Mastiha-Supplemented Patients with NAFLD. Front. Immunol. 2021, 12, 1688. [Google Scholar] [CrossRef]
- Kalopitas, G.; Antza, C.; Doundoulakis, I.; Siargkas, A.; Kouroumalis, E.; Germanidis, G.; Samara, M.; Chourdakis, M. Impact of Silymarin in individuals with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutrients 2021, 83, 111092. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; Laserna, C.; Rojo, M.Á.; Mora, N.; Sánchez, C.G.; Pina, M.; Sigüenza, R.; Primo, D.; Izaola, O.; De Luis, D. Role of the PNPLA3 polymorphism rs738409 on silymarin + vitamin E response in subjects with non-alcoholic fatty liver disease. Rev. Esp. Enferm. Dig. 2018, 110, 634–640. [Google Scholar] [CrossRef]
- Medina-Urrutia, A.; Lopez-Uribe, A.R.; El Hafidi, M.; González-Salazar, M.D.C.; Posadas-Sánchez, R.; Jorge-Galarza, E.; Del Valle-Mondragón, L.; Juárez-Rojas, J.G. Chia (Salvia hispanica)-supplemented diet ameliorates non-alcoholic fatty liver disease and its metabolic abnormalities in humans. Lipids Health Dis. 2020, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Namazi, N.; Alizadeh, M.; Mirtaheri, E.; Farajnia, S. The Effect of Dried Glycyrrhiza Glabra L. Extract on Obesity Management with Regard to PPAR-γ2 (Pro12Ala) Gene Polymorphism in Obese Subjects Following an Energy Restricted Diet. Adv. Pharm. Bull. 2017, 7, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajiaghamohammadi, A.; Ziaee, A.; Samimi, R. The Efficacy of Licorice Root Extract in Decreasing Transaminase Activities in Non-alcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. Phytother. Res. 2012, 26, 1381–1384. [Google Scholar] [CrossRef] [PubMed]
- Mahamid, M.; Mahroum, N.; Bragazzi, N.L.; Shalaata, K.; Yavne, Y.; Adawi, M.; Amital, H.; Watad, A. Folate and B12 Levels Correlate with Histological Severity in NASH Patients. Nutrients 2018, 10, 440. [Google Scholar] [CrossRef] [Green Version]
- Xin, F.-Z.; Zhao, Z.-H.; Zhang, R.-N.; Pan, Q.; Gong, Z.-Z.; Sun, C.; Fan, J.-G. Folic acid attenuates high-fat diet-induced steatohepatitis via deacetylase SIRT1-dependent restoration of PPARα. World J. Gastroenterol. 2020, 26, 2203–2220. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, X.; Xue, H.; Zhang, P.; Fang, W.; Chen, X.; Ling, W. Coenzyme Q10 attenuates high-fat diet-induced non-alcoholic fatty liver disease through activation of the AMPK pathway. Food Funct. 2019, 10, 814–823. [Google Scholar] [CrossRef]
- Ma, Z.; Chu, L.; Liu, H.; Wang, W.; Li, J.; Yao, W.; Yi, J.; Gao, Y. Beneficial effects of paeoniflorin on non-alcoholic fatty liver disease induced by high-fat diet in rats. Sci. Rep. 2017, 7, srep44819. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Huang, R.; Lin, D.; Wang, Y.; Yang, X.; Huang, X.; Zheng, B.; Chen, Z.; Huang, Y.; Wang, X.; et al. Resveratrol Improves Liver Steatosis and Insulin Resistance in Non-alcoholic Fatty Liver Disease in Association with the Gut Microbiota. Front. Microbiol. 2021, 12, 611323. [Google Scholar] [CrossRef]
- Lee, E.S.; Kwon, M.-H.; Kim, H.M.; Woo, H.B.; Ahn, C.M.; Chung, C.H. Curcumin analog CUR5–8 ameliorates nonalcoholic fatty liver disease in mice with high-fat diet-induced obesity. Metabolism 2020, 103, 154015. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2019, 59, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.-M.; Xia, M.-F.; Wang, Y.; Chang, X.-X.; Yao, X.-Z.; Rao, S.-X.; Zeng, M.-S.; Tu, Y.-F.; Feng, R.; Jia, W.-P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef] [Green Version]
- Katsagoni, C.N.; Georgoulis, M.; Papatheodoridis, G.; Panagiotakos, D.B.; Kontogianni, M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism 2017, 68, 119–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Song, J.; Shang, X.; Chawla, N.; Yang, Y.; Meng, X.; Wang, H.; Ma, J. Physical activity and sedentary behavior can modulate the effect of the PNPLA3 variant on childhood NAFLD: A case-control study in a Chinese population. BMC Med. Genet. 2016, 17, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, N.; Oniki, K.; Kudo, M.; Obata, Y.; Sakamoto, Y.; Tokumaru, N.; Izuka, T.; Watanabe, T.; Otake, K.; Ogata, Y.; et al. A Pilot Study Assessing the Possible Combined Effect of Physical Activity and PNPLA3 rs738409 Polymorphism on the Risk for Non-Alcoholic Fatty Liver Disease in the Japanese Elderly General Population. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, ume 13, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-H.; Stoll, C.R.T.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G. The Effectiveness and Risks of Bariatric Surgery. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicoletti, C.F.; Cortes-Oliveira, C.; Pinhel, M.A.S.; Nonino, C.B. Bariatric Surgery and Precision Nutrition. Nutrients 2017, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.F.; Nonino, C.B.; de Oliveira, B.A.P.; Pinhel, M.A.D.S.; Mansego, M.L.; Milagro, F.I.; Zulet, M.A.; Martinez, J.A. DNA Methylation and Hydroxymethylation Levels in Relation to Two Weight Loss Strategies: Energy-Restricted Diet or Bariatric Surgery. Obes. Surg. 2015, 26, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Vilallonga, R.; Xifra, G.; Sabater-Masdeu, M.; Ricart, W.; Fernández-Real, J.M. Bariatric surgery acutely changes the expression of inflammatory and lipogenic genes in obese adipose tissue. Surg. Obes. Relat. Dis. 2016, 12, 357–362. [Google Scholar] [CrossRef]
- Krawczyk, M.; Jiménez-Agüero, R.; Alustiza, J.M.; Emparanza, J.I.; Perugorria, M.J.; Bujanda, L.; Lammert, F.; Banales, J.M. PNPLA3 p.I148M variant is associated with greater reduction of liver fat content after bariatric surgery. Surg. Obes. Relat. Dis. 2016, 12, 1838–1846. [Google Scholar] [CrossRef]
- Bandstein, M.; Mwinyi, J.; Ernst, B.; Thurnheer, M.; Schultes, B.; Schiöth, H.B. A genetic variant in proximity to the gene LYPLAL1 is associated with lower hunger feelings and increased weight loss following Roux-en-Y gastric bypass surgery. Scand. J. Gastroenterol. 2016, 51, 1050–1055. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, C.F.; Kimura, B.M.; Oliveira, B.; De Pinhel, M.A.S.; Salgado, W.; Marchini, J.S.; Nonino, C.B. Role ofUCP2polymorphisms on dietary intake of obese patients who underwent bariatric surgery. Clin. Obes. 2016, 6, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis. JAMA Intern. Med. 2017, 177, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi-Suzuki, M.; Cusi, K.; Bril, F.; Gong, Y.; Langaee, T.; Frye, R.F. A Genetic Score Associates with Pioglitazone Response in Patients with Non-alcoholic Steatohepatitis. Front. Pharmacol. 2018, 9, 752. [Google Scholar] [CrossRef]
- Kan, H.; Hyogo, H.; Ochi, H.; Hotta, K.; Fukuhara, T.; Kobayashi, T.; Naeshiro, N.; Honda, Y.; Kawaoka, T.; Tsuge, M.; et al. Influence of the rs738409 polymorphism in patatin-like phospholipase 3 on the treatment efficacy of non-alcoholic fatty liver disease with type 2 diabetes mellitus. Hepatol. Res. 2015, 46, E146–E153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, X.; Xu, X.; Yuan, S.; Xu, F.; Liang, H. PNPLA3 I148M is involved in the variability in anti-NAFLD response to exenatide. Endocrine 2020, 70, 517–525. [Google Scholar] [CrossRef]
- Athinarayanan, S.; Wei, R.; Zhang, M.; Bai, S.; Traber, M.; Yates, K.; Cummings, O.W.; Molleston, J.; Liu, W.; Chalasani, N. Genetic Polymorphism of Cytochrome P450 4F2, Vitamin E Level and Histological Response in Adults and Children with Nonalcoholic Fatty Liver Disease Who Participated in PIVENS and TONIC Clinical Trials. PLoS ONE 2014, 9, e95366. [Google Scholar] [CrossRef] [Green Version]
- Fukui, A.; Kawabe, N.; Hashimoto, S.; Murao, M.; Nakano, T.; Shimazaki, H.; Kan, T.; Nakaoka, K.; Ohki, M.; Takagawa, Y.; et al. Vitamin E reduces liver stiffness in nonalcoholic fatty liver disease. World J. Hepatol. 2015, 7, 2749–2756. [Google Scholar] [CrossRef] [Green Version]
- Gawrieh, S.; Guo, X.; Tan, J.; Lauzon, M.; Taylor, K.D.; Loomba, R.; Cummings, O.W.; Pillai, S.; Bhatnagar, P.; Kowdley, K.V.; et al. A Pilot Genome-Wide Analysis Study Identifies Loci Associated with Response to Obeticholic Acid in Patients with NASH. Hepatol. Commun. 2019, 3, 1571–1584. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Maraj, B.; Harding-Theobald, E.; Vittinghoff, E.; Terrault, N.A. Gut microbiome–targeted therapies in nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Am. J. Clin. Nutr. 2019, 110, 139–149. [Google Scholar] [CrossRef]
- Caussy, C.; Tripathi, A.; Humphrey, G.; Bassirian, S.; Singh, S.; Faulkner, C.; Bettencourt, R.; Rizo, E.; Richards, L.; Xu, Z.Z.; et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat. Commun. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062. [Google Scholar] [CrossRef]
- Blasco-Baque, V.; Coupé, B.; Fabre, A.; Handgraaf, S.; Gourdy, P.; Arnal, J.F.; Courtney, M.; Schuster-Klein, C.; Guardiola, B.; Tercé, F.; et al. Associations between hepatic miRNA expression, liver triacylglycerols and gut microbiota during metabolic adaptation to high-fat diet in mice. Diabetologia 2017, 60, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zhu, J.; Zhu, L.; Cheng, M. The Combination of Blueberry Juice and Probiotics Ameliorate Non-Alcoholic Steatohepatitis (NASH) by Affecting SREBP-1c/PNPLA-3 Pathway via PPAR-α. Nutrients 2017, 9, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Design (Sample Size) | Intervention (Time) | Result |
|---|---|---|---|
| Santoro et al., 2012 [47] | Cross-sectional study (127) | - | Higher HFF% and ALT levels in 148M/M variant presenting high dietary n-6/n-3 PUFA consumption |
| Nobili et al., 2013 [79] | RCT (60) | DHA 250–500 mg/day (24 months) | Lower response (steatosis) in I148M variant |
| Scorletti et al., 2015 [80] | RCT (85) | DHA + EPA 4 g/day (15–18 months) | Increased end of study liver fat % in 148M/M variant |
| Eriksson et al., 2018 [85] | RCT (84) | 10 mg dapagliflozin/4 g n-3 PUFA/both (12 weeks) | Combined treatment induced greater response (PDFF) in I148M variant; n-3 PUFA treatment induced decreased response (PDFF) in I148M variant |
| Oscarsson et al., 2018 [82] | RCT (78) | 200 mg fenofibrate/4 g n-3 PUFA (12 weeks) | No influence of I148M on the effects of n-3 PUFA supplementation (PDFF) |
| Kuttner et al., 2019 [81] | Open-label trial (20) | 4 g n-3 PUFA (4 weeks) | No changes in transient elastography (CAP used to quantify liver fat) neither in the control group nor I148M |
| Van Name et al., 2020 [83] | Single-arm unblinded trial (20) | Low n-6/n-3 PUFA ratio (4:1) normocaloric diet (12 weeks) | Significant HFF% reduction in the 148M/M group |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Montoro, J.I.; Cornejo-Pareja, I.; Gómez-Pérez, A.M.; Tinahones, F.J. Impact of Genetic Polymorphism on Response to Therapy in Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 4077. https://doi.org/10.3390/nu13114077
Martínez-Montoro JI, Cornejo-Pareja I, Gómez-Pérez AM, Tinahones FJ. Impact of Genetic Polymorphism on Response to Therapy in Non-Alcoholic Fatty Liver Disease. Nutrients. 2021; 13(11):4077. https://doi.org/10.3390/nu13114077
Chicago/Turabian StyleMartínez-Montoro, José Ignacio, Isabel Cornejo-Pareja, Ana María Gómez-Pérez, and Francisco J. Tinahones. 2021. "Impact of Genetic Polymorphism on Response to Therapy in Non-Alcoholic Fatty Liver Disease" Nutrients 13, no. 11: 4077. https://doi.org/10.3390/nu13114077
APA StyleMartínez-Montoro, J. I., Cornejo-Pareja, I., Gómez-Pérez, A. M., & Tinahones, F. J. (2021). Impact of Genetic Polymorphism on Response to Therapy in Non-Alcoholic Fatty Liver Disease. Nutrients, 13(11), 4077. https://doi.org/10.3390/nu13114077